Search results
Search for "macrocycles" in Full Text gives 206 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239

- . These silver(I) complexes adopted similar structures in solution and in the solid state. As each sulfur atom in the ligand is prochiral, macrocycles L2M2 were obtained as mixtures of diastereoisomers, depending on the configurations of the sulfur atoms coordinated to silver cations. The X-ray structures
- )–1 ligands). The X-ray diffraction of monocrystals 1a revealed the formation of (R,S–1)2·(AgOTf)2 macrocycles driven by silver(I) coordination (Figure 1). The two ligands are facing through the coordination of one syn-thioether group to the same silver cation. Two different crystals were isolated and
- highlighted the two possible arrangements of the ligands that led to different diastereoisomeric macrocycles (Figure 1a,b). In Figure 1a, each silver cation was coordinated to two sulfur atoms with the same configuration (named head-to-head coordination mode for ligands) meanwhile in Figure 1b, each Ag(I
Effect of ring size on photoisomerization properties of stiff stilbene macrocycles
Beilstein J. Org. Chem. 2019, 15, 2408–2418, doi:10.3762/bjoc.15.233

- Sandra Olsson Oscar Benito Perez Magnus Blom Adolf Gogoll Department of Chemistry-BMC, Uppsala University, S-751 23 Uppsala, Sweden Faculty of Chemistry, Universitat de Barcelona, C/ Martí i Franquès 1, 08028 Barcelona, Spain 10.3762/bjoc.15.233 Abstract A series of stiff stilbene macrocycles
- have been studied to investigate the possible impact of the macrocycle ring size on their photodynamic properties. The results show that reducing the ring size counteracts the photoisomerization ability of the macrocycles. However, even the smallest macrocycle studied (stiff stilbene subunits linked by
- lengths of carbon chains were used, with distances between the terminal carbons of 6.4 Å (C6), 8.9 Å (C8), 11.4 Å (C10) and 13.9 Å (C12). The SS-macrocycles have been studied both experimentally and by computational techniques. Results and Discussion Synthesis The synthesis of the macrocycles was based on
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- macrocycles and focus on their application in different areas of supramolecular chemistry. The synthesis is mostly relying on the well-known “click reaction” (CuAAC) leading to 1,4-disubstituted 1,2,3-triazoles that then can be quaternized. Applications of triazolium macrocycles thus prepared include
- -triazolium macrocycles; Review 1. Introduction Supramolecular chemistry – “The chemistry beyond the molecule” [1] – is an ever growing interdisciplinary area has emerged from the early host–guest chemistry to more elaborate bio-inspired supramolecular aggregates by exploiting various noncovalent
- imidazoles [8][9], polypyrroles [10][11], and indole moieties [12][13], as part of supramolecular receptors, triazole heterocycles containing macrocycles have recently been introduced as new host molecules for the selective recognition of ions, mechanically interlocked molecules (MIMs), supramolecular
Multiple threading of a triple-calix[6]arene host
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- Fisciano (Salerno), Italy 10.3762/bjoc.15.207 Abstract The synthesis of the triple-calix[6]arene derivative 6 in which three calix[6]arene macrocycles are linked to a central 1,3,5-trimethylbenzene moiety is reported. Derivative 6 is able to give multiple-threading processes in the presence of
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197

- in the field of crystal engineering [1]. Only few supramolecular capsules were reported so far [29], including the resorcin[4]arene capsules of Diederich and co-workers [21][23], triangular macrocycles assembled by self-complemented halogen bonding [20] and halogen bond templated, polyfluorinated
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- conveniently by means of a macrocyclic condensation reaction between N-functionalized 3,6-dihydroxyphthalimides and 3,6-dichlorotetrazine under mild conditions in a one-pot reaction manner. The novel macrocycles exist as a mixture of rapidly interconvertible conformers in solution while in the solid state they
- noncovalent interactions between anions and the tetrazine rings. Keywords: anion–π interactions; coronarenes; host–guest complexation; N-functionalized phthalimides; O6-corona[3]arene[3]tetrazines; Introduction Synthetic macrocycles [1][2] are always attractive and important because they are unique
- molecular systems to study molecular recognition and the nature of noncovalent interactions. Functional macrocycles also provide essential components for the fabrication or assembly of sophisticated (supra)molecular structures [1][2][3][4], advanced materials [1][2][5][6][7][8][9][10] and machinery systems
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- manifested in chirality transfer. We will also present a crucial and non-intuitive solvent dependence. RSAs (e.g., 1) can be considered as analogues of calix[4]arene sulfonic acids (CSAs) – the class of macrocycles widely studied in the context of various host–guest interactions, especially with various
Nanopatterns of arylene–alkynylene squares on graphite: self-sorting and intercalation
Beilstein J. Org. Chem. 2019, 15, 1848–1855, doi:10.3762/bjoc.15.180
- and non-planar coronoid polycyclic aromatic hydrocarbons (i.e., butyloxy-substituted kekulene and octulene derivatives) are found to be able to intercalate into the intramolecular nanopores. Keywords: macrocycles; scanning tunneling microscopy; self-assembled monolayers; self-sorting; solid/liquid
- , guest molecules can act as alien species that affect the morphologies of intermolecular nanopores [12]. Another approach for the formation of nanopores relies on the physisorption of shape-persistent macrocycles [13][14]. While there are examples for the deposition of organic molecules into rigid
- the solubility limit can be overcome by appropriate substitution. We recently investigated self-assembled nanoporous networks of shape-persistent macrocycles in which dithiophene-based corner building blocks connect linear oligo(phenylene–ethynylene–butadiynylene)s (OPEBs) to form molecular polygons
Novel macrocycles – and old ones doing new tricks
Beilstein J. Org. Chem. 2019, 15, 1838–1839, doi:10.3762/bjoc.15.178

- 10.3762/bjoc.15.178 Keywords: macrocycles; supramolecular chemistry; Macrocycles [1] are the workhorses in supramolecular chemistry. Many basic supramolecular concepts have been developed through studying crown ethers, cryptands, podands and spherands in the 1970s and 1980s. For these contributions
- , Charles Pedersen, Donald J. Cram and Jean-Marie Lehn were awarded the Nobel Prize in Chemistry in 1987. In the 80s and 90s, Jean-Pierre Sauvage and Sir Fraser Stoddart used macrocycles to realize machine-like molecular motion, and they shared the Nobel Prize in Chemistry in 2016 with Ben Feringa. Clearly
- , macrocycles played a central role for the fundamental science that established supramolecular chemistry as an independent field of chemical research as well as for its applications in contemporary research on functional supramolecules and materials. Nowadays, the use of macrocycles has significantly
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- have been synthesized via a threading-followed-by-stoppering approach. Due to the orthogonal binding of CB[6] to ammonium and γ-CD to biphenylene/tetra(ethylene glycol), the [n]rotaxanes display a specific sequence of the interlocked macrocycles. In addition, despite of the asymmetry of γ-CD with
- respect to the orthogonal plane of the axle, only one stereoisomer of the [6]rotaxane was obtained. Keywords: cucurbit[6]uril; cyclodextrin; macrocycles; mechanostereoisomer; rotaxane; Introduction Cyclodextrins (CDs) are macrocycles composed of glucoses linked via α-1,4-glycosidic bonds. CDs of six (α
- orthogonal binding of γ-CD to biphenylene and tetra(ethylene glycol) and CB[6] to ammonium, [n]rotaxanes of only a specific sequence of the interlocked macrocycles were obtained despite of the possibility of other sequence isomers. In addition, the three γ-CDs in the [6]rotaxane were found to adopt only one
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- ; macrocycles; macrocyclic arene; Introduction Macrocyclic host molecules [1][2] play a significant role in host–guest chemistry. Compared with noncyclic molecules, the structures of macrocyclic hosts can greatly enhance the host–guest complexation ability through preorganization. Moreover, cyclic structures
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
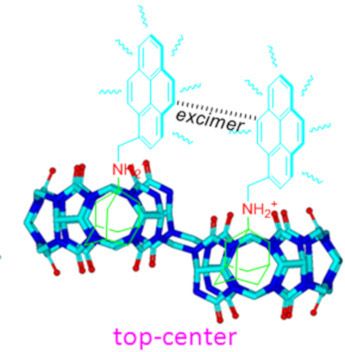
- ]. Keywords: fluorescent; host–guest interaction; macrocycles; molecular recognition; nor-seco-cucurbit[10]uril; pyrene; Introduction Host–guest interactions that trigger molecular recognition are a current topic of interest. For example, understanding the protein–ligand molecular recognition is of paramount
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- : aggregation; circular dichroism; chirality; click chemistry; macrocycles; pillar[5]arenes; Introduction Planar-chiral compounds are structurally appealing and potentially applicable in various functional materials such as chiral discriminators [1][2], chiral polymers, supramolecular sensors [3] and chiral
- that differs from the common macrocycles is the planar chirality resulting from the different orientations of the alkoxy substituents on the rims. Theoretically, eight conformers can be formed including diastereomeric ones: (Sp,Sp,Sp,Sp,Sp), (Rp,Sp,Sp,Sp,Sp), (Rp,Rp,Sp,Sp,Sp), (Rp,Sp,Rp,Sp,Sp) and
Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding
Beilstein J. Org. Chem. 2019, 15, 1592–1600, doi:10.3762/bjoc.15.163

- ; hydration; macrocycles; thermodynamic characteristics; Introduction Cyclodextrins (CDs), a family of enzymatically modified starches, are widely used as host macrocycles in forming inclusion complexes with various molecular entities of interest to food industry, pharmacology, cosmetics, catalysis, and
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- Abdulselam Adam Saber Mehrparvar Gebhard Haberhauer Institut für Organische Chemie, Universität Duisburg-Essen, Universitätsstr. 7, D-45117 Essen, Germany 10.3762/bjoc.15.156 Abstract The combination of photo-switchable units with macrocycles is a very interesting field in supramolecular
- , whereas by the use of visible light the stretched trans,trans-isomer is formed. By means of quantum chemical calculations and CD spectroscopy we could show that the trans→cis isomerization is spatially directed; that means that one of the two different macrocycles performs a definite clockwise rotation to
- the high dispersion energy in the compact cis,cis-isomer. Keywords: azobenzene; macrocycles; molecular switch; Introduction In supramolecular chemistry rigid scaffolds are required to arrange different recognition units in predefined distances and spatial orientation to each other [1]. One example
A heteroditopic macrocycle as organocatalytic nanoreactor for pyrroloacridinone synthesis in water
Beilstein J. Org. Chem. 2019, 15, 1505–1514, doi:10.3762/bjoc.15.152
- , makes them highly valuable in view of sustainable chemical applications. Thus, the development of organocatalytic nanoreactors with new features is indeed important to address greener organic syntheses. On the other hand, the rational design of heterotopic macrocycles has attracted intense interest as
- of macrocycles with multiple functional groups has been employed as alternative templates to induce the organic environment for catalysis via multiple weak interactions viz. π–π stacking, hydrogen bonding, etc. [25][26][27][28][29]. At the same time, several efforts have been made to develop
- transformations, in particular, for the synthesis of biologically important highly substituted pyrroloacridines in the ecofriendly solvent water. Results and Discussion Synthesis of the macrocycle Considerable efforts in synthesizing multifunctional macrocycles have been dedicated lately for the construction of
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- Li-Li Wang Yi-Kuan Tu Huan Yao Wei Jiang Shenzhen Grubbs Institute and Department of Chemistry, Southern University of Science and Technology, Xueyuan Boulevard 1088, Shenzhen 518055, China 10.3762/bjoc.15.146 Abstract Two new tetralactam macrocycles with 2,3-dibutoxynaphthalene groups as
- –guest chemistry; macrocycles; molecular recognition; precious metal chloride complexes; Introduction Macrocyclic receptors are the major workhorses in supramolecular chemistry. Design and synthesis of new macrocyclic receptors with new properties is always attractive but is also challenging [1
- ]. Tetralactam macrocycles with four amide NH residues have been studied for ca. three decades and used in a wide range of fields including molecular recognition, optical sensing, molecular machine etc. [2][3][4][5][6][7][8][9][10][11][12][13]. The amide N−H groups directed into the cavity could form hydrogen
Reaction of oxiranes with cyclodextrins under high-energy ball-milling conditions
Beilstein J. Org. Chem. 2019, 15, 1448–1459, doi:10.3762/bjoc.15.145

- , depending on the molar ratio of the CD and epichlorohydrin [40][41][42][43]. As in the HPCD preparations, the aqueous basic solution can crosslink the macrocycles, while the relatively large OH− excess can hydrolyse both the reagent and the simultaneously formed oxirane. In order to prepare bead CDPs
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- ; isoxazoline; macrocycles; nitrile oxide; porphyrin; Introduction In nature, porphyrin-type compounds play a prominent role in life [1]. It is well known that certain vital functions, like O2 transport, photosynthesis etc. depend on the action of porphyrin–metal complexes [2][3][4][5]. Inspired by the natural
- longer than the distance when MeOH coordinated to the Zn2+ ions [49]. This may attribute to the larger volume of the C6H4CO2Me group than MeOH when closing to the Zn2+ center. In the dimer structure, two planes defined by porphyrin macrocycles are parallel with a distance of 7.11 Å. The bulky substituent
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- representative candidate of luminescent transition-metal complexes. We determined the association constants of the GC5A–dye complexes by fluorescence titration and discuss the complexation-induced photophysical changes. In addition, a comparison of the complexation behavior of GC5A with that of other macrocycles
- and potential applications according to the diverse photophysical responses are provided. Keywords: calixarene; host–guest complexation; luminescent dyes; macrocycles; photophysical properties; Introduction Fluorescence sensing represents a powerful detection methodology due to its low cost, ease of
- fluorophores through supramolecular encapsulation using artificial macrocyclic hosts has always been an active research area [10][11][12]. The complexes of macrocycles with luminescent dyes not only act as reporter pairs for sensing [13][14][15], but also offer various applications in bioimaging [16][17
Selective detection of DABCO using a supramolecular interconversion as fluorescence reporter
Beilstein J. Org. Chem. 2019, 15, 1371–1378, doi:10.3762/bjoc.15.137
- compounds. Keywords: copper; detection; fluorescence; interconversion; macrocycles; self-assembly; self-sorting; zinc porphyrin; Introduction Since dynamic multicomponent supramolecular structures are nowadays abundant [1][2], the weak intercomponent binding [3][4][5][6][7][8][9] is often instrumentalized
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- ; cyclotriveratrylenes; HPLC; macrocycles; racemization; saddle isomer; Introduction Cyclotriveratrylenes (CTVs) [1][2][3][4][5][6][7][8] are cyclic bowl-shaped molecules and belong to the most studied concave host molecules in supramolecular chemistry besides, e.g., calixarenes and resorcinarenes [9], cyclodextrins
Host–guest interactions between p-sulfonatocalix[4]arene and p-sulfonatothiacalix[4]arene and group IA, IIA and f-block metal cations: a DFT/SMD study
Beilstein J. Org. Chem. 2019, 15, 1321–1330, doi:10.3762/bjoc.15.131

- formation reactions. Keywords: complex formation; DFT; group IA; IIA and f-block metal cations; macrocycles; p-sulfonatocalix[4]arene; p-sulfonatothiacalix[4]arene; Introduction If macrocycles are pillars of the supramolecular chemistry, then calixarenes (“calix” = vase + “arene”) are the 3rd pillar after
- ., acetaldehyde – ethanal, CH3CHO) are required in condensation reactions with resorcinol and pyrogallol [1]. Thiacalixarenes are macrocycles (or cyclic oligomers) based on a condensation of the same phenol derivatives and sulfur [2]. They are characterized by a larger cavity size than the conventional
- are not as good as those of other common macrocycles. Two positions (phenolic OH groups and p-positions) in the calixarenes’ structure can be easily modified by subsequent reactions [4]. As a result, calixarenes have a great potential as simple scaffolds to build molecular receptors and multivalent
Bambusuril analogs based on alternating glycoluril and xylylene units
Beilstein J. Org. Chem. 2019, 15, 1268–1274, doi:10.3762/bjoc.15.124

- product. Two disastereomers of the macrocycle were separated and characterized by means of NMR spectroscopy and X-ray crystallography. Conformational changes of these diastereomers were investigated using DFT models and variable-temperature NMR. Keywords: bambusurils; conformers; glycolurils; macrocycles
- ; supramolecular chemistry; Introduction Macrocycles consisting of urea building blocks play an important role in supramolecular chemistry [1]. Urea N–H motifs provide macrocycles with the ability to act as anion receptors due to the stabilizing effect of N–H···anion hydrogen bonding [2][3][4]. Furthermore, the
- investigate the synthesis of bambusuril analogs in which glycoluril and chromophoric units alternate. Here we describe the first results of our efforts. Results and Discussion The preparation of the macrocycles was based on a one-pot reaction of 2,4-dimethylglycoluril and m-xylylene dibromide, a structurally
N-doped carbon dots covalently functionalized with pillar[5]arenes for Fe3+ sensing
Beilstein J. Org. Chem. 2019, 15, 1262–1267, doi:10.3762/bjoc.15.123

- stretching vibration of C–N at 1433 cm−1 in CCDs was obviously increased because the abundant amino groups of CN-dots reacted with carboxylic acid groups of the CP[5] macrocycles. Powder X-ray diffraction (PXRD) patterns (Figure 1e) of CCDs show the characteristic peaks of CN-dots and CP[5], further
- was lost until 800 °C. Meanwhile, the weight loss of CCDs reached ca. 35 wt %, indicating that ca. 26 wt % of CP[5] macrocycles were covalently grafted on the surface of CN-dots [17][19]. As shown in Figure 2a, the as-synthesized CN-dots material is soluble in water while the CCDs solid is insoluble
- composites, and the highly selective sensing towards Fe3+ is also related to the binding of Fe3+ with CP[5] ring (Figure 3f) [24][27][28]. Conclusion In summary, a promising fluorescent chemical sensing material (CCDs) was synthesized for the first time by covalently attaching CP[5] macrocycles onto the














































































































































































































