Search results
Search for "fluorescence emission" in Full Text gives 96 result(s) in Beilstein Journal of Organic Chemistry.
The B & B approach: Ball-milling conjugation of dextran with phenylboronic acid (PBA)-functionalized BODIPY
Beilstein J. Org. Chem. 2020, 16, 2272–2281, doi:10.3762/bjoc.16.188

- with roughly 1 equiv of 1), Dex-1c (ball milling with roughly 0.5 equiv of 1), and Dex-1d (ball milling with roughly 0.1 equiv of 1) expressed as % w/w on the modified dextrans. A) UV–vis absorption and B) fluorescence emission spectra (λexc = 380 nm) of the BODIPY-dextran conjugate Dex-1b solution in
- water. A) Hydrodynamic diameter of (nm) conjugate Dex-1b (at 1 mg/mL in H2O, black curve) and PBS (red curve), respectively. B) TEM image of conjugate Dex-1b in H2O. Fluorescence emission spectra of pyrene (4.4 × 10−8 M) in water and in a water solution in the presence of conjugate Dex-1b. Schematic
Naphthalene diimide bis-guanidinio-carbonyl-pyrrole as a pH-switchable threading DNA intercalator
Beilstein J. Org. Chem. 2020, 16, 2201–2211, doi:10.3762/bjoc.16.185

- chiral properties of the DNA or RNA helical structures [12][13], could also take advantage of induced CD spectrum (ICD) in the visible spectrum range of small achiral dyes, which they show only upon binding to DNA/RNA [14]. Moreover, with recent advances in fluorescence emission-based polarisation
Et3N/DMSO-supported one-pot synthesis of highly fluorescent β-carboline-linked benzothiophenones via sulfur insertion and estimation of the photophysical properties
Beilstein J. Org. Chem. 2020, 16, 1740–1753, doi:10.3762/bjoc.16.146
- measurement of UV–vis absorption and fluorescence emission of the samples, stock solutions of 1.0 mM concentration were prepared using analytical grade CHCl3 as the solvent, and diluted to the final concentration of 4.0 μM. Next, we carefully measured the photophysical properties at room temperature including
One-pot synthesis of dicyclopenta-fused peropyrene via a fourfold alkyne annulation
Beilstein J. Org. Chem. 2020, 16, 791–797, doi:10.3762/bjoc.16.72
- 1.78 eV from the onset of its UV–vis absorption spectrum. Similar to the cyclopenta-fused pyrene derivatives [28][29], compound 1 does not show detectable fluorescence emission. Furthermore, the electrochemical properties of 1 was probed by cyclic voltammetry (CV) in DCM (Figure 3b). According to the
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- dimers and 8 nm (1.25 × 106 cm−1) for the monomer. The difference in properties of the unsymmetric zinc-nickel dimer 16 is observed significantly more in the corresponding fluorescence emission spectrum (Figure 7). The emission observed for the zinc monomer and dimer, 14 and 9, respectively, was
- nickel porphyrin is acting as the acceptor. An electron/energy transfer is occurring between the two porphyrins, therefore, when the molecule is excited at the wavelength of the zinc porphyrin, the fluorescence emission ordinarily observed for the zinc porphyrin does not occur as the energy has been
- fluorescence emission spectra of related dimers (Figure 8), connected via different linker groups, were taken (Figure 9 and Figure 10). Both the triptycene-linked zinc porphyrin dimer 9 and BODIPY dimer 7 were compared with a meso–meso-linked dimer 19 [50] and butadiyne-linked dimer 20 [51]. The UV–vis spectra
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- cause of the activity. Therefore, the interaction of selected compounds, i.e., 11 and 12, with CT DNA was monitored by UV–vis spectroscopy and viscosity measurements. Additionally, the EB−displacing ability of the compounds was evaluated by fluorescence emission spectroscopy. The UV–vis spectra of a CT
- intercalating ability. EB–DNA conjugate exhibits an intense fluorescence emission band at 592 nm, when its solution is excited at 540 nm. Compounds 11 and 12 have not presented any appreciable fluorescence emission either alone in solution or in the co-existence of CT DNA or EB under the same experimental
- conditions (λexcitation = 540 nm at room temperature). Thus, the quenching observed in an EB–DNA solution upon addition of the compounds 11 and 12 may reveal their competition to EB for the DNA-intercalation sites as monitored by fluorescence emission spectroscopy with λexcitation = 540 nm. A significant
Synthesis and circularly polarized luminescence properties of BINOL-derived bisbenzofuro[2,3-b:3’,2’-e]pyridines (BBZFPys)
Beilstein J. Org. Chem. 2020, 16, 325–336, doi:10.3762/bjoc.16.32
- fluorescence emission bands, whereas the (R)-isomers emitted right-handed CPL to produce the mirror images. The calculated luminescence dissymmetry factors [48] glum for the solutions were all within the range of 3.80 × 10−4 to 6.90 × 10−4. On the other hand, in the dispersed solid state in Fomblin® PFPE
Plasma membrane imaging with a fluorescent benzothiadiazole derivative
Beilstein J. Org. Chem. 2019, 15, 2644–2654, doi:10.3762/bjoc.15.257

- 2,1,3-benzothiadiazole (BTD) core and its derivatives that are successfully applied in bioimaging experiments. (Left) UV–vis, (center) fluorescence emission and (right) solvatochromic effect (Stokes shift in wavenumbers versus solvent polarity in ETN) of the synthesized BTD-4APTEG (10 μM for all
- microscopy. Arrows indicate the peripherical accumulation of the dyes in the plasma membranes. The letter N indicates the nuclei of the cells (scale bar of 10 μm). Synthesis of the plasma membrane BTD probe (BTD-4APTEG) and its structural features. UV–vis and fluorescence emission data (in different solvents
- probe operating at 400 MHz for 1H and at 100 MHz for 13C NMR. Chemical shifts were expressed in parts per million (ppm) and referenced by the signals of the residual hydrogen atoms of the deuterated solvent, as indicated in the legends. UV–vis absorption (Varian Cary 5000) spectroscopy and fluorescence
α,ß-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
Beilstein J. Org. Chem. 2019, 15, 2524–2533, doi:10.3762/bjoc.15.245
- potential dependent accumulation in mitochondria, indicated by a fluorescence emission shift from red to green. The exposure of 11b to the cells caused remarkable loss of mitochondrial membrane potential, hence the fluorescence gradually shifted from red to green as the membrane potential (Ψm) decreased
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
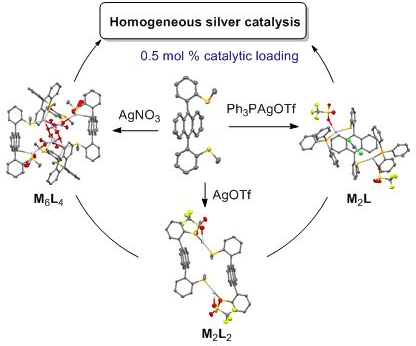
- ). Finally, the photophysical properties of ligand syn-1 (20 μM) and complexes 1a–d (30 μM) were evaluated in dichloromethane (Supporting Information File 1, Figures S4–S8). The UV–visible and fluorescence emission spectra (λexc = 345 nm) of ligand and complexes were similar and correspond to those of 9,10
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- photoinduced Z/E isomerization of hydrazone 1, and accompanied changes in b) UV–vis absorption (1 × 10−5 M; toluene) and c) fluorescence emission spectra (1 × 10−6 M; toluene) before (red) and after (blue) irradiation at 442 nm. Fluorescence decays (dots) in the 500–520 nm spectral region (emission range of
Targeted photoswitchable imaging of intracellular glutathione by a photochromic glycosheet sensor
Beilstein J. Org. Chem. 2019, 15, 2380–2389, doi:10.3762/bjoc.15.230

- with 448 nm light, the fluorescence emission peak of Glyco-DTE was observed at 535 nm (ΦF = 0.263, Table S1 in Supporting Information File 1). Owing to the good overlap between the emission band of the naphthalimide fluorophore and the absorption band of DTE closed isomer, the fluorescence was
- glycosheet The fluorescence emission of Glyco-DTE@MnO2 was efficiently quenched to ca. 15% (ΦF = 0.023, Table S1 in Supporting Information File 1) when increasing concentrations of 2D MnO2 nanosheets were added, and reached saturation around 25 μg/mL (Figure 3A). The quenched fluorescence indicated the
Synthesis of a dihalogenated pyridinyl silicon rhodamine for mitochondrial imaging by a halogen dance rearrangement
Beilstein J. Org. Chem. 2019, 15, 2333–2343, doi:10.3762/bjoc.15.226

- ) Absorption and fluorescence emission spectra of dye 15 measured in PBS buffer pH 7.4. (b, c) Colocalization experiment of dye 15 (red) and MitoTracker® Green FM (cyan) in living HeLa (b) and U2OS (c) cells supporting the application of dye 15 as a specific NIR mito tracker probe. Both cell lines were
Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–)2A fluorescent dyes
Beilstein J. Org. Chem. 2019, 15, 1712–1721, doi:10.3762/bjoc.15.167

- . Thus, the D–π–A dyes exhibit intense photoabsorption and fluorescence emission properties based on the intramolecular charge transfer (ICT) excitation from the D moiety to the A moiety [1][2][3][4]. Moreover, the D–π–A structure possesses considerable structural characteristics: the increase in the
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
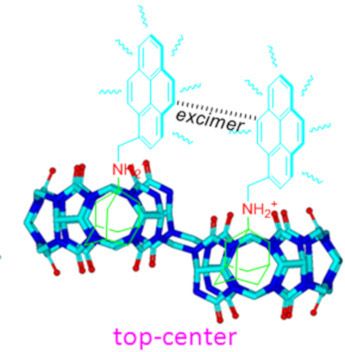
- the fluorescence emission spectra of G2 in the presence of host-1. As can be seen, free G2 produced typical monomer emissions at around 378 and 396 nm in aqueous solution (pH 2) upon excitation of the pyrene fluorophore at 340 nm. When we added host-1 at increasing concentrations to the G2 solution
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- for [C111H104N6KO12]+, 1753.1813; found, 1753.7510. UV–vis absorption (a) and fluorescence emission spectra (b) of Py-6, P5A-Py (λex = 420 nm) and DPA-6, P5A-DPA (λex = 375 nm) in CHCl3, c = 1.0 × 10−5 M, 25 °C. (a) Fluorescence decay curves of Py-6 and P5A-Py at 450 nm and (b) fluorescence decay
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- −@1. Energy-minimized structure of a) AuCl4−@1 and b) AuCl4−@2 at the level of theory of PM3 by using Spartan ’14 (Wavefunction, Inc.). a) Fluorescence emission spectra of 1 (20 µM) upon addition of different amounts of TBA[AuCl4] (concentration range of 0.0−800 µM) and then recorded in
Precious metal-free molecular machines for solar thermal energy storage
Beilstein J. Org. Chem. 2019, 15, 1096–1106, doi:10.3762/bjoc.15.106

- causes the observed fluorescence decrease. The optical parameters of all 4 dyes, including absorption maxima (λmax,abs), fluorescence emission maxima (λmax,f), hypsochromic shift of the absorption maxima (Δλmax), and fluorescence quantum yields (Φf) for different excitation wavelengths, obtained for the
- TDPBE0 spectra in ACN for dye 4b and its Ba2+ complex. Quaternization of 2-methylbenzothiazoles with alkane sultones. Synthesis of 4-(aza-15-crown-5)benzocarbaldehyde (3) [22]. Synthesis of dyes 4a–d. Absorption maxima (λmax,abs), fluorescence emission maxima (λmax,f), shift of the absorption maxima
Synthesis of a water-soluble 2,2′-biphen[4]arene and its efficient complexation and sensitive fluorescence enhancement towards palmatine and berberine
Beilstein J. Org. Chem. 2018, 14, 2236–2241, doi:10.3762/bjoc.14.198

- , compounds P and B alone only displayed fairly feeble fluorescence emission. Upon addition of 2,2’-CBP4, the fluorescence intensity was remarkably improved more than 600 times (Figure 2 and Supporting Information File 1, Figure S10). This was due to the effect of lowering polar microenvironment when P or B
- delivery, supramolecular amphiphiles, etc. Experimental 2,2’-OEtBP4 was synthesized according to our previously reported method [47]. P and B were purchased from Shanghai Aladdin Bio-Chem Technology Co.,LTD. 1H NMR and 13C NMR spectra were recorded on a Bruker AV500 instrument. The fluorescence emission
- presence of 2,2’-CBP4 in aqueous phosphate buffer solution at pH 7.4 at 298 K. The excitation wavelength is at 352.0 nm. Inset: the nonlinear least-squares analysis to calculate the association constant using the fluorescence emission at 530 nm. Visible emission observed from samples of P and B in the
Rational design of boron-dipyrromethene (BODIPY) reporter dyes for cucurbit[7]uril
Beilstein J. Org. Chem. 2018, 14, 1961–1971, doi:10.3762/bjoc.14.171

- ][30]. They are characterized by narrow absorption and fluorescence emission bands with small Stokes shifts, high molar absorption coefficients, and high quantum yields. Their excitation and emission maxima are in the visible region, usually above 470 nm, and they show high thermal and photochemical
- ) demonstrating the sensing principle based on the pKa of the dye and the complex in the presence and absence of analyte. c) Structures of CB7 and BODIPY derivatives. a) Normalized absorption (solid line) and normalized fluorescence emission spectrum (dotted line) of 0.72 µM 1 in 30% (v/v) ACN in water, pH 7.0
Synthesis and characterization of π–extended “earring” subporphyrins
Beilstein J. Org. Chem. 2018, 14, 1956–1960, doi:10.3762/bjoc.14.170

- tripyrrin moiety are 13.7(1)° and 18.4(1)°, respectively. The difference between the two dihedral angles is much larger than that in 3. The UV–vis/NIR absorption spectra of 3 and 3Pd are shown in Figure 5. However, no fluorescence emission can be observed for 3 and 3Pd. Both 3 and 3Pd display broad Soret
Two novel blue phosphorescent host materials containing phenothiazine-5,5-dioxide structure derivatives
Beilstein J. Org. Chem. 2018, 14, 869–874, doi:10.3762/bjoc.14.73

- . Moreover, their fluorescence emission peaks are in the blue fluorescence region at 408 nm and the fluorescence quantum efficiency (Φ) of CEPDO and CBPDO were 62.5% and 59.7%, respectively. Both CEPDO and CBPDO showed very high thermal stability with decomposition temperatures (Td) of 409 and 396 °C as well
- Table 1. Conclusion In summary, we have designed and synthesized two bipolar host materials CEPDO and CBPDO. CEPDO and CBPDO not only have a high triplet energy but also show a bipolar behavior. Moreover, their fluorescence emission peaks are blue fluorescence at 408 nm and the fluorescence quantum
Fluorogenic PNA probes
Beilstein J. Org. Chem. 2018, 14, 253–281, doi:10.3762/bjoc.14.17

- uracil acted rather like a general fluorescence label. On the other hand, the fluorescent nucleobase 5-(pyren-1-yl)uracil in acpcPNA formed a specific Watson–Crick type base pairing with dA in the DNA strand, and the duplex formation was accompanied by a strong (up to 42-fold) fluorescence emission
Polarization spectroscopy methods in the determination of interactions of small molecules with nucleic acids – tutorial
Beilstein J. Org. Chem. 2018, 14, 84–105, doi:10.3762/bjoc.14.5

- last decades, complementary methods to ECD also were developed, for instance, vibrational CD (VCD) was successfully applied to investigate DNA/ligand interactions [11]. Furthermore, fluorescence detected circular dichroism (FDCD) combines the advantages of both CD and fluorescence emission technique
Development of a fluorogenic small substrate for dipeptidyl peptidase-4
Beilstein J. Org. Chem. 2017, 13, 2690–2697, doi:10.3762/bjoc.13.267
- quantum yield to 0.03. In spectroscopic terms, the fluorescence emission peaks of these compounds red-shifted with increasing TFPE substitution. Additionally, a large Stokes shift was observed for 1, which is important in view of the biomedical use of fluorescent compounds. Next, we changed the solvent














































































































































































































































