Search results
Search for "self-assembly" in Full Text gives 212 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
[2 + 1] Cycloaddition reactions of fullerene C60 based on diazo compounds
Beilstein J. Org. Chem. 2021, 17, 630–670, doi:10.3762/bjoc.17.55
- the starting material for synthesizing adduct 204 (Scheme 39) [123]. A synthesis and a self-assembly of flavin-functionalized fullerene derivative 205 consisting of [60]PCBM and isoalloxazine moieties attached at both ends of a linear 12-carbon aliphatic spacer, based on the same acid (Scheme 40), was
Multiswitchable photoacid–hydroxyflavylium–polyelectrolyte nano-assemblies
Beilstein J. Org. Chem. 2021, 17, 166–185, doi:10.3762/bjoc.17.17

- building blocks for the ternary electrostatic self-assembly, forming well-defined supramolecular assemblies with tunable sizes of 50 to 500 nm. Due to the network of possible chemical reactions for the anthocyanidin and the excited-state dissociation of the photoacid upon irradiation, different ways to
- particles, and information on the molecular structure was gained by UV–vis spectroscopy. Isothermal titration calorimetry (ITC) provided information on the thermodynamics and interaction forces in the supramolecular assembly formation. Keywords: electrostatic self-assembly; hydroxyflavylium
- ; multiswitchable; photoacid; polyelectrolyte; Introduction Supramolecular nanoscale assemblies responding to multiple stimuli are highly desirable in various fields including transport systems, sensors, and optoelectronic applications [1][2][3][4][5][6][7][8]. Self-assembly into supramolecular structures and
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- electron donor, respectively (Figure 3). A benzo-18-crown-6 derivative was connected to the porphyrin ring, which could form a supramolecular complex with fullerene through dipole interactions. The formed supramolecular self-assembly resulted in a high energy-transmission efficiency (over 97%) between the
- ., Orthogonal Supramolecular Assembly Triggered by Inclusion and Exclusion Interactions with Cucurbit[7]uril for Photocatalytic H2 Evolution, ChemSusChem, John Wiley and Sons. Structures of COP-1, CMP-1, and their substrate S-1 and S-2. Supramolecular self-assembly of the light-harvesting system formed by WP5
Tuning the solid-state emission of liquid crystalline nitro-cyanostilbene by halogen bonding
Beilstein J. Org. Chem. 2021, 17, 124–131, doi:10.3762/bjoc.17.13

- of iodofluorobenzene derivatives with nitro-cyanostilbenes is reported. The systematic variation of the fluorination degree and pattern indicates the relevance of the halogen bond strength for the induction of liquid crystalline properties. The modular self-assembly approach enables the efficient
- interactions, which allow for dynamic responses to external stimuli [1]. In addition, the self-assembly of the complementary molecular entities provides an easy access to functional systems and enables recyclability and self-healing properties of the materials [2]. With respect to the formation of
- interactions and packing in the solid state and adds to the shift of the crystallisation temperature. Photophysical studies Recently, our group has shown that self-assembly provides an efficient way to tune fluorescence behaviour of liquid crystalline materials [21]. Phenolic thioethers showing aggregation
Supramolecular polymerization of sulfated dendritic peptide amphiphiles into multivalent L-selectin binders
Beilstein J. Org. Chem. 2021, 17, 97–104, doi:10.3762/bjoc.17.10

- Research Center of Electron Microscopy, Freie Universität Berlin, Fabeckstr. 34a, 14195 Berlin 10.3762/bjoc.17.10 Abstract The synthesis of a sulfate-modified dendritic peptide amphiphile and its self-assembly into one-dimensional rod-like architectures in aqueous medium is reported. The influence of the
- -selectin binders; multivalency; self-assembly in water; supramolecular polymers; Introduction Deciphering the interaction of artificial molecular building blocks with biological components is a key element on the way to understanding and selectively manipulating biological systems. Throughout nature
- self-assembly turns out to be crucial in obtaining supramolecular polymers suitable for interactions with biological targets [16]. Peptide amphiphiles provide access to supramolecular structures in this competitive environment by taking advantage of nature’s versatile toolbox of noncovalent
Control over size, shape, and photonics of self-assembled organic nanocrystals
Beilstein J. Org. Chem. 2021, 17, 42–51, doi:10.3762/bjoc.17.5
- for Nanoscale Science and Nanotechnology, Ben-Gurion University, Beer Sheva 84105, Israel 10.3762/bjoc.17.5 Abstract The facile fabrication of free-floating organic nanocrystals (ONCs) was achieved via the kinetically controlled self-assembly of simple perylene diimide building blocks in aqueous
- ). Convenient control over the structure and function of organic nanocrystals can enhance their utility in new and developed technologies. Keywords: aromatic amphiphiles; exciton diffusion; organic nanocrystals; perylene diimides; self-assembly; Introduction Semiconductor and metal nanoparticles exhibit size
- ]. We have reported on 2D crystalline self-assembled systems based on a hierarchical assembly mode promoted by hydrophobic and π–π interactions [36]. Yet, the size and shape of these systems could not be controlled beyond the 2D morphology. We report herein on the aqueous self-assembly of organic
Molecular basis for protein–protein interactions
Beilstein J. Org. Chem. 2021, 17, 1–10, doi:10.3762/bjoc.17.1

- , it has been shown that the CCMV capsid formation is driven by electrostatic interactions governed by the pH value and the ionic strength [93]. Here, the positive N terminus of the assembly unit interacts with the negatively charged scaffold and drives the self-assembly process [94]. The advent of
- form binary nanoparticle superlattices. a) Surface engineering of the protein container to yield either positively (right) or negatively (left) charged containers. b) Nanoparticle synthesis. The different nanoparticles are illustrated in different colours. c) Self-assembly of the different charged
Construction of pillar[4]arene[1]quinone–1,10-dibromodecane pseudorotaxanes in solution and in the solid state
Beilstein J. Org. Chem. 2020, 16, 2954–2959, doi:10.3762/bjoc.16.245

- constructing functional materials from the bottom up as well as an important way to create new substances with functions [1][2][3][4][5]. Through ingenious designs and the applications of molecular recognition and self-assembly strategies, many exquisite supramolecular architectures have been fabricated
Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- self-sorting protocols. The present mini review will provide an overview over the latest advancements in this field with a focus on reversibly switchable functions in discrete supramolecular systems. Keywords: copper; fluorescence; self-assembly; self-sorting; zinc porphyrin; Introduction Since self
- self-assembly of the triamines 10 and 11 in the presence of the aldehyde 12 and cobalt(II) ions [52]. When all components were mixed in a single reaction vessel, the 1H NMR spectrum indicated the formation of homoleptic as well as heteroleptic species. In the following, they explored the ability of the
- of various similar molecules. Similarly, Shi controlled a conversion between helicates and a tetrahedral cage by varying the radius of the metal ion (Hg2+ vs Fe2+) [55]. They reported on the self-assembly of the monomer 20, encompassing the quadruple DDAA hydrogen-bonding arrays and 2,2’-bipyridine
Easy access to a carbohydrate-based template for stimuli-responsive surfactants
Beilstein J. Org. Chem. 2020, 16, 2788–2794, doi:10.3762/bjoc.16.229
- distance of the two lipophilic tails resulting in a self-assembly of the molecules into larger aggregates that could be characterized with DLS and TEM [11]. In a previous study by Yuasa et al., it was shown that the distance between the two groups on the anomeric and the 3-position indeed decreased upon a
Encrypting messages with artificial bacterial receptors
Beilstein J. Org. Chem. 2020, 16, 2749–2756, doi:10.3762/bjoc.16.225

- simpler way to modify the bacterial membrane is by adding to X-ODN-1 a complementary strand (Y-ODN-2) that is modified with the desired functionality (Y) (Figure 1A). In this way, the structure of the artificial receptors can be ‘programmed’ by a simple self-assembly process, which provides the means to
Selective recognition of ATP by multivalent nano-assemblies of bisimidazolium amphiphiles through “turn-on” fluorescence response
Beilstein J. Org. Chem. 2020, 16, 2728–2738, doi:10.3762/bjoc.16.223

- to an n-alkyl chain at the other, underwent self-assembly in aqueous media depending on the length of the alkyl segment. The amphiphilic derivatives having n-decyl or longer chains, formed nano-assemblies with cyanic–green emission resulting from the stacked pyrene chromophores in the aggregates. The
- buffer and also in buffer containing 150 mM NaCl at physiological pH value. Furthermore, the multivalent aggregates demonstrated a significant selectivity in ATP detection over ADP, AMP and pyrophosphate. Keywords: amphiphile; ATP; excimer; multivalency; self-assembly; Introduction Supramolecular anion
- ]. In this methodology, multivalent arrays are built from comparatively smaller binding sites through self-assembly. The smaller molecular units are easier to synthesize and moreover, the morphology and the surface functionalities of the resultant multivalent structures can be tuned in a modular fashion
Enzyme-instructed morphological transition of the supramolecular assemblies of branched peptides
Beilstein J. Org. Chem. 2020, 16, 2709–2718, doi:10.3762/bjoc.16.221

- cellular environment. Keywords: acetylation; branched peptides; enzyme; nanostructures; N-terminal; responsive; self-assembly; Introduction Peptides, being able to self-assemble to exhibit emergent properties and functions [1][2][3][4][5], have received considerable attentions recently. For example
- utilizes nonlinear peptides. For example, nonribosomal peptides exist in other geometries, such as branched (e.g., bleomycin) or cyclic peptides (e.g., vancomycin) [31]. While the understanding of the synthesis of branched peptides is well-developed, the self-assembly and enzymatic conversion of branched
- peptides has received limited attention [2][32][33][34][35][36][37]. For example, Stupp et al. reported that a cell adhesion epitope, RGDS, acts as a branch to peptide amphiphiles for making hydrogels via self-assembly [34][36]. Ulijn et al. connected Fmoc-DAARRGG to a lysine side chain for incorporation
A heterobimetallic tetrahedron from a linear platinum(II)-bis(acetylide) metalloligand
Beilstein J. Org. Chem. 2020, 16, 2701–2708, doi:10.3762/bjoc.16.220

- (acetylide)platinum(II) complex [Pt(L1)2(PBu3)2] as a linear metalloligand. The reaction of this metalloligand with iron(II) cations and pyridine-2-carbaldehyde according to the subcomponent self-assembly approach yielded decanuclear heterobimetallic tetrahedron [Fe4Pt6(L2)12](OTf)8. Thus, combination of
- these two design concepts – the subcomponent self-assembly strategy and the complex-as-a-ligand approach – ensured a fast and easy synthesis of large heterobimetallic coordination cages of tetrahedral shape with a diameter of more than 3 nm as a mixture of all three possible T-, S4- and C3-symmetric
- complexes; pyridylimine ligands; self-assembly; supramolecular chemistry; Introduction The understanding of the general design principles for the self-assembly of metallosupramolecular aggregates [1][2][3][4][5] allowed to access more and more complex and large assemblies over the past decades like
The B & B approach: Ball-milling conjugation of dextran with phenylboronic acid (PBA)-functionalized BODIPY
Beilstein J. Org. Chem. 2020, 16, 2272–2281, doi:10.3762/bjoc.16.188

- their derivatives have been widely used in biomedical applications, especially as drug and gene carriers, both in hydrogels and in nanoparticulate formulations [29][30][31][32]. In this regard, the incorporation of hydrophobic moieties in the branched structure of dextrans induces self-assembly into
- short reaction time, reactant economy, higher degree of functionalization, and solvent-free conditions, compared to solution-based routes are discussed. The dextran mechanochemically conjugated to PBA-BODIPY (Dex-1b, Figure 1) formed nanoparticles through self-assembly retaining the fluorescent
Synthetic approaches to bowl-shaped π-conjugated sumanene and its congeners
Beilstein J. Org. Chem. 2020, 16, 2212–2259, doi:10.3762/bjoc.16.186

- the self-assembly. This was confirmed by single crystal structure and it was noticed that all 15 carbon atoms of the three Cp-types of the ring were interacted with the metal atoms and the six K atoms were sandwiched between the convex faces of two sumanenyl trianions. On the other hand, two years
- potential applications ranging from the chemistry perspective to materials science and technology [1][2][3][4]. As we know that bowled (curved) surfaces are universal in nature for example our planets as well as atomic orbitals possess the curvature which generally affects the charge-transport, redox, self
- -assembly, and optical properties of bowl-shaped π-conjugated systems [5][6][7][8][9]. The synthesis of π-bowls is an extremely challenging job due to the presence of unusual strain in these types of molecules, therefore, the first synthetic breakthrough in this arena came into the picture in the late
pH- and concentration-dependent supramolecular self-assembly of a naturally occurring octapeptide
Beilstein J. Org. Chem. 2020, 16, 2017–2025, doi:10.3762/bjoc.16.168

- drug delivery, tissue engineering and regeneration, and as stimuli-responsive materials. Herein, we report the pH- and concentration-dependent self-assembly and conformational transformation of the newly synthesized octapeptide PEP-1. At pH 7.4, PEP-1 forms β-sheet-rich secondary structures into
- and resulting morphologies due to electrostatic repulsion between charged amino acids. PEP-1 can also form helical or random-coil secondary structures at a relatively low concentration. The obtained pH-sensitive self-assembly behavior of the target octapeptide is expected to contribute to the
- development of novel drug nanocarrier assemblies. Keywords: aqueous self-assembly; pH-responsive systems; secondary structure; self-assembled nanostructures; solid-phase peptide synthesis; Introduction The self-assembly of small molecules is a ubiquitous phenomenon in nature [1] and also has key
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- interaction of the dipeptides with themselves. For example, FF is a popular self-assembly motif, which also explains its poor solubility (≈5 mM) [23]. Hence the thermodynamic data depicts not only the association of a(CFC)2/p(CFC)2 towards FF, but also the dissociation of FF assemblies. The aromatic side
Activated carbon as catalyst support: precursors, preparation, modification and characterization
Beilstein J. Org. Chem. 2020, 16, 1188–1202, doi:10.3762/bjoc.16.104

- aerosol-assisted self-assembly using amphiphilic triblock copolymers as template and low-molecular weight soluble phenol resin as carbon source. The amphiphilic surfactant influences the pore size and mesostructure of the resulting spherical carbons. Finally, the template is removed by calcination [97
Synthesis of new asparagine-based glycopeptides for future scanning tunneling microscopy investigations
Beilstein J. Org. Chem. 2020, 16, 888–894, doi:10.3762/bjoc.16.80

- self-assembly on metal surfaces [16][17]. For such novel MS applications, a new technology, i.e., ES-IBD, has been developed. ES-IBD furthermore allows for the integrity of the material [18]. The application of these new techniques to synthetic glycopeptides is expected to provide new insight into the
- investigation of the self assembly of such glycopeptides on metal surfaces will be performed via pMS and STM in further studies. Description of the starting materials 1a–f and 2a–f. Peptide coupling reactions, including the previous Fmoc cleavage. Cleavage of the fully protected peptides 6 and 7. Yields of the
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
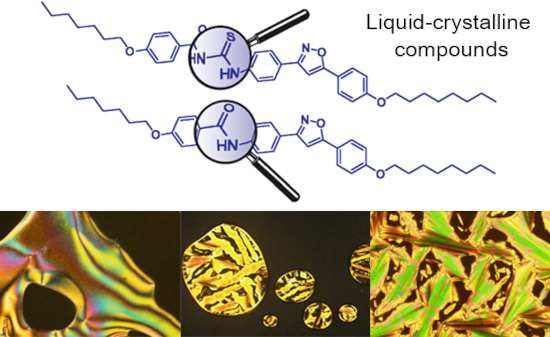
- thioureas is the reaction of carbon disulfide with either one or two different amines [9]. Due to their self-assembly and self-organization through intermolecular hydrogen bonding, thioureas display interesting technological applications to this group of molecules, one of which explores its application in
Influence of the cis/trans configuration on the supramolecular aggregation of aryltriazoles
Beilstein J. Org. Chem. 2019, 15, 2881–2888, doi:10.3762/bjoc.15.282
- - and cis-1,2-glucopyranosyl and cyclohexyl ditriazoles, synthesized by CuAAC "click" chemistry, to form gels was studied, their physical properties determined, and the self-aggregation behavior investigated by SEM, X-ray, and EDC studies. The results revealed that self-assembly was driven mainly by π–π
- , with short and planar films or scales. This could be due to the high proportion of water in the gel and/or the presence of the three free hydroxy groups in its structure, changing its intermolecular self-assembly behavior. Various efforts were made to crystallize the obtained compounds; however, only
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
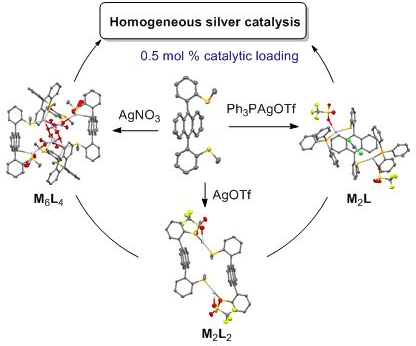
- the stoichiometry of coordination complexes. Atropisomer 1 directed the self-assembly towards the same half-space (regarding the anthracene core). The triflate anion lead to a [2 + 2] macrocycle meanwhile the more coordinating nitrate anion induced the formation of a large globular macrocycle M6L4. A
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- selectivity [35][36][37][38][39][40]. Consequently, arylazopyrazoles have been employed as photoresponsive gelators [41] and adhesives [42] and for controlling antimicrobial response [43][44], cell adhesion to surfaces [45], as well as DNA [46] and microtubule [47] self-assembly using light. Here, we focused
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- template-directed self-assembly procedure via an intramolecular “CuAAC click” reaction in the presence of electron-rich bisnaphtho- or bisbenzo-24-crown-8 ethers. Afterwards, the triazole groups in the catenanes were methylated. The resulting triazolium macrocycle containing catenanes 17a (DN24C8) and 17b































































































































































































































































































