Search results
Search for "ureas" in Full Text gives 76 result(s) in Beilstein Journal of Organic Chemistry.
Catalytic enantioselective synthesis of selenium-containing atropisomers via C–Se bond formations
- Qi-Sen Gao,
- Zheng-Wei Wei and
- Zhi-Min Chen
Beilstein J. Org. Chem. 2025, 21, 2447–2455, doi:10.3762/bjoc.21.186
- intermediate Int 16 on VQM to form intermediate Int 17. As bifunctional organic catalysts, chiral ureas can synergistically activate both alkynes and selenols, thereby addressing the challenge of overcoming the increased difficulty of racemization caused by the presence of bulky SeR groups (Scheme 5). Summary

Graphical Abstract

Figure 1: Representative examples of chiral selenium-containing compounds.

Scheme 1: Rhodium-catalyzed atroposelective C–H selenylation reported by You’s group [18].

Scheme 2: Rhodium-catalyzed atroposelective C–H selenylation reported by Li et al. [19].

Scheme 3: Organocatalytic asymmetric selenosulfonylation of alkynes.

Scheme 4: Rhodium-catalyzed asymmetric hydroselenation of 1-alkynylindoles. *DCE/DCM 2:1 (v/v), −50 °C.

Scheme 5: Organocatalytic atroposelective hydroselenation of alkynes. *Using cat.3, 4 h.
Electrochemical cyclization of alkynes to construct five-membered nitrogen-heterocyclic rings
- Lifen Peng,
- Ting Wang,
- Zhiwen Yuan,
- Bin Li,
- Zilong Tang,
- Xirong Liu,
- Hui Li,
- Guofang Jiang,
- Chunling Zeng,
- Henry N. C. Wong and
- Xiao-Shui Peng
Beilstein J. Org. Chem. 2025, 21, 2173–2201, doi:10.3762/bjoc.21.166

- -involved ureas, annulation of o-arylalkynylanilines, cyclization of 2-ethynylanilines, selenocyclization of diselenides with 2-ethynylanilines as well as C–H indolization of 2-alkynylanilines with 3-functionalized indoles. Isoindolones were synthesized successfully by electrochemical annulation of
- ureas 1 proceeded smoothly in an undivided cell (reticulated vitreous carbon (RVC) anode, Pt cathode, 5 mA), forming the desired indoles 2 in high yields. The reaction showed good compatibility with various functional groups like phenyl, furyl, alkenyl and alkyl at the acetylene moieties, producing 2a–d
- functional molecules possessing five-membered rings. Electrochemical intramolecular coupling of ureas to form indoles. Electrochemical dehydrogenative annulation of alkynes with anilines. Electrochemical annulations of o-arylalkynylanilines. Electrochemical cyclization of 2-ethynylanilines. Electrochemical

Graphical Abstract

Figure 1: Natural products and functional molecules possessing five-membered rings.

Scheme 1: Electrochemical intramolecular coupling of ureas to form indoles.

Scheme 2: Electrochemical dehydrogenative annulation of alkynes with anilines.

Scheme 3: Electrochemical annulations of o-arylalkynylanilines.

Scheme 4: Electrochemical cyclization of 2-ethynylanilines.

Scheme 5: Electrochemical selenocyclization of diselenides and 2-ethynylanilines.

Scheme 6: Electrochemical cascade approach towards 3-selenylindoles.

Scheme 7: Electrochemical C–H indolization.

Scheme 8: Electrochemical annulation of benzamides and terminal alkynes.

Scheme 9: Electrochemical synthesis of isoindolinone by 5-exo-dig aza-cyclization.

Scheme 10: Electrochemical reductive cascade annulation of o-alkynylbenzamide.

Scheme 11: Electrochemical intramolecular 1,2-amino oxygenation of alkyne.

Scheme 12: Electrochemical multicomponent reaction of nitrile, (thio)xanthene, terminal alkyne and water.

Scheme 13: Electrochemical aminotrifluoromethylation/cyclization of alkynes.

Scheme 14: Electrochemical cyclization of o-nitrophenylacetylene.

Scheme 15: Electrochemical annulation of alkynyl enaminones.

Scheme 16: Electrochemical annulation of alkyne and enamide.

Scheme 17: Electrochemical tandem Michael addition/azidation/cyclization.

Scheme 18: Electrochemical [3 + 2] cyclization of heteroarylamines.

Scheme 19: Electrochemical CuAAC to access 1,2,3-triazole.
Systematic pore lipophilization to enhance the efficiency of an amine-based MOF catalyst in the solvent-free Knoevenagel reaction
- Pricilla Matseketsa,
- Margret Kumbirayi Ruwimbo Pagare and
- Tendai Gadzikwa
Beilstein J. Org. Chem. 2025, 21, 1854–1863, doi:10.3762/bjoc.21.144

- pharmaceutical and agrochemical industries [38][39]. Various types of catalysts are used to enhance the efficiency and selectivity of the Knoevenagel reaction, including Lewis acids, ureas/ thioureas, amino acids, and bases such as alkali metal hydroxides, alkali metal alkoxide, amines, etc. [37][40][41][42][43
- in the rate of reaction was observed from KSU-1 < KSU-1iPr < KSU-1t-Bu < KSU-1n-Hex < KSU-1C14 (Figure 4B) even though roughly 10% of the –NH2 groups in both KSU-1n-Hex and KSU-1C14 had been converted to the less catalytically active alkyl ureas [50]. We should also point out that, despite the lower
- undergoes imine condensation with benzaldehyde. B) Mechanism in which the amine acts as a base, deprotonating malononitrile. Estimated conversions of the reactions of isocyanates with the –OH and –NH2 groups of KSU-1 to form carbamates and ureas, respectively.a Comparison of Knoevenagel catalysis results

Graphical Abstract

Figure 1: Schematic representation of the modulation of MOF pore environments. A) de novo synthesis of severa...

Figure 2: A) Schematic representation of the reaction of KSU-1 with aliphatic isocyanates and the estimated c...

Scheme 1: Probable mechanisms for the Knoevenegel condensation reaction between benzaldehyde and malononitril...

Figure 3: A) Schematic representation of the reaction between benzaldehyde and malononitrile to form benzylid...

Figure 4: Graphical representation of the Knoevenagel catalysis results. A) Comparison of the reaction in tol...

Figure 5: Left: comparison of BMN and HPMM protons in 1H NMR spectra. Note that the peaks corresponding to HP...
Facile synthesis of hydantoin/1,2,4-oxadiazoline spiro-compounds via 1,3-dipolar cycloaddition of nitrile oxides to 5-iminohydantoins
- Juliana V. Petrova,
- Varvara T. Tkachenko,
- Victor A. Tafeenko,
- Anna S. Pestretsova,
- Vadim S. Pokrovsky,
- Maxim E. Kukushkin and
- Elena K. Beloglazkina
Beilstein J. Org. Chem. 2025, 21, 1552–1560, doi:10.3762/bjoc.21.118
- '-disubstituted ureas were initially reacted with oxalyl chloride to form imidazolidinetriones 1a,b, which were then added to an iminophosphorane formed in situ from an aryl azide and triphenylphosphine. As a result of the aza-Wittig reaction, 5-iminohydantoins 2a–i were then used as dipolarophiles in the 32CA

Graphical Abstract

Figure 1: Design and synthetic strategies for the target hydantoin/1,2,4-oxadiazoline spiro-compounds.

Scheme 1: Synthesis of dipolarophiles (5-iminohydantoins 2a–i).

Scheme 2: Preparation of the dipole precursors 4a–d.

Scheme 3: 32CA reactions of nitrile oxides with 5-iminohydantoins (synthesis of spiro-compounds 5a–l). Isolat...

Scheme 4: Cycloaddition of nitrile oxide to 5-iminothiohydantoin 2j. aTriethylamine dropwise addition (2.4 eq...

Figure 2: Atropoisomerism of ortho-substituted spiro-compounds 5b and 5d.

Figure 3: Cytotoxicity investigation of hydantoin/1,2,4-oxadiazolines 5 (MTT test, HCT116 cell line) and sele...
Oxetanes: formation, reactivity and total syntheses of natural products
- Peter Gabko,
- Martin Kalník and
- Maroš Bella
Beilstein J. Org. Chem. 2025, 21, 1324–1373, doi:10.3762/bjoc.21.101
- base [5]. In fact, its hydrogen-bond-accepting ability is even stronger than that of the other 3-, 5- and 6-membered cyclic ethers, as well as the carbonyls of aldehydes, ketones, esters and carbonates [6][7][8]. The only better carbonyl-based hydrogen-bond acceptors are amides, carbamates and ureas [9

Graphical Abstract

Figure 1: Bond lengths and bond angles in oxetane at 140 K [2].

Figure 2: Analogy of 3-substituted oxetanes to carbonyl and gem-dimethyl groups [12].

Figure 3: Use of oxetanes in drug design – selected examples.

Figure 4: Examples of oxetane-containing natural products.

Scheme 1: Synthetic strategies towards construction of the oxetane ring.

Scheme 2: Overview of intramolecular Williamson etherification and competing Grob fragmentation.

Scheme 3: Synthesis of spiro-oxetanes via 1,4-C–H insertion and Williamson etherification.

Scheme 4: Use of phenyl vinyl selenone in the synthesis of spirooxindole oxetanes.

Scheme 5: Synthesis of bicyclic 3,5-anhydrofuranoses via double epoxide opening/etherification.

Scheme 6: Preparation of spirooxetanes by cycloisomerisation via MHAT/RPC.

Scheme 7: Oxetane synthesis via alcohol C–H functionalisation.

Scheme 8: Access to oxetanes 38 from α-acetyloxy iodides.

Scheme 9: The kilogram-scale synthesis of oxetane intermediate 41.

Scheme 10: Overview of the intramolecular opening of 3-membered rings.

Scheme 11: Synthesis of 4,7-dioxatricyclo[3.2.1.03,6]octane skeletons.

Scheme 12: Silicon-directed electrophilic cyclisation of homoallylic alcohols.

Scheme 13: Hydrosilylation–iodocyclisation of homopropargylic alcohols.

Scheme 14: Cu-catalysed intramolecular O-vinylation of γ-bromohomoallylic alcohols.

Scheme 15: Cu-catalysed intramolecular cross-coupling of hydroxyvinylstannanes.

Scheme 16: Isomerisation of oxiranyl ethers containing weakly carbanion-stabilising groups.

Scheme 17: Cyclisation of diethyl haloalkoxymalonates.

Scheme 18: Synthesis of oxetanes through a 1,5-HAT/radical recombination sequence.

Scheme 19: General approach to oxetanes via [2 + 2] cycloadditions.

Scheme 20: Synthesis of tricyclic 4:4:4 oxetanes through a photochemical triple cascade reaction.

Scheme 21: Iridium-catalysed Paternò–Büchi reaction between α-ketoesters and simple alkenes.

Scheme 22: Three-step synthesis of spirocyclic oxetanes 83 via Paternò–Büchi reaction, nucleophilic ring openi...

Scheme 23: Enantioselective Paternò–Büchi reaction catalysed by a chiral iridium photocatalyst.

Scheme 24: Synthesis of polysubstituted oxetanes 92 via Cu(II)-mediated formal [2 + 2] cycloadditions.

Scheme 25: Synthesis of alkylideneoxetanes via NHC- and DBU-mediated formal [2 + 2] cycloadditions.

Scheme 26: Use of sulphur-stabilised carbanions in ring expansions.

Scheme 27: Synthesis of α,α-difluoro(arylthio)methyl oxetanes.

Scheme 28: Ring expansion in an industrial synthesis of PF-06878031.

Scheme 29: Ring contraction of triflated 2-hydroxy-γ-lactones.

Scheme 30: Ring contraction in an industrial synthesis of PF-06878031.

Scheme 31: Photochemical ring contraction of 2,5-dihydrofurans by aryldiazoacetic acid esters.

Scheme 32: Synthesis of 3-oxetanones via O-H insertion of carbenes.

Scheme 33: Synthesis of phosphonate oxetanones via gold-mediated alkyne oxidation/O–H insertion.

Scheme 34: Syntheses and common derivatisations of 3-oxetanone.

Scheme 35: SN1 substitution of 3-aryloxetan-3-ols by thiols and alcohols.

Scheme 36: Fe–Ni dual-catalytic olefin hydroarylation towards 3-alkyl-3-(hetero)aryloxetanes.

Scheme 37: Synthesis of 3-aryloxetan-3-carboxylic acids.

Scheme 38: Decarboxylative alkylation of 3-aryloxetan-3-carboxylic acids.

Scheme 39: Synthesis of 3-amino-3-aryloxetanes via photoredox/nickel cross-coupling catalysis.

Scheme 40: Intermolecular cross-selective [2 + 2] photocycloaddition towards spirooxetanes.

Scheme 41: Synthesis of 3-aryl-3-aminooxetanes via defluorosulphonylative coupling.

Scheme 42: Two-step synthesis of amide bioisosteres via benzotriazolyl Mannich adducts 170.

Scheme 43: Functionalisation of oxetanyl trichloroacetimidates 172.

Scheme 44: Synthesis of oxetane-amino esters 176.

Scheme 45: Tandem Friedel–Crafts alkylation/intramolecular ring opening of 3-aryloxetan-3-ols.

Scheme 46: Synthesis of polysubstituted furans and pyrroles.

Scheme 47: Synthesis of oxazolines and bisoxazolines.

Scheme 48: Tandem, one-pot syntheses of various polycyclic heterocycles.

Scheme 49: Synthesis of 1,2-dihydroquinolines via skeletal reorganisation of oxetanes.

Scheme 50: Synthesis of benzoindolines and 2,3-dihydrobenzofurans and their derivatisations.

Scheme 51: Synthesis of polysubstituted 1,4-dioxanes.

Scheme 52: Preparation of various lactones via ring opening of oxetane-carboxylic acids 219.

Scheme 53: Tsuji-Trost allylation/ring opening of 3-aminooxetanes.

Scheme 54: Arylative skeletal rearrangement of 3-vinyloxetan-3-ols to 2,5-dihydrofurans.

Scheme 55: Reductive opening of oxetanes using catalytic Mg–H species.

Scheme 56: Opening of oxetanes by silyl ketene acetals.

Scheme 57: Rhodium-catalysed hydroacylation of oxetanes.

Scheme 58: Generation of radicals from oxetanes mediated by a vitamin B12-derived cobalt catalyst.

Scheme 59: Reductive opening of oxetanes by B–Si frustrated Lewis pairs.

Scheme 60: Zirconocene-mediated reductive opening of oxetanes.

Scheme 61: Enantioselective syntheses of small and medium-size rings using chiral phosphoric acids.

Scheme 62: Asymmetric synthesis of 2,3-dihydrobenzo[b]oxepines catalysed by a chiral scandium complex.

Scheme 63: Enantioselective synthesis of 1,3-bromohydrins under a chiral squaramide catalysis.

Scheme 64: Enantioselective opening of 2-aryl-2-ethynyloxetanes by anilines.

Scheme 65: Ru-catalysed insertion of diazocarbonyls into oxetanes.

Scheme 66: Ring expansion of oxetanes by stabilised carbenes generated under blue light irradiation.

Scheme 67: Expansion of oxetanes via nickel-catalysed insertion of alkynyltrifluoroborates.

Scheme 68: Nickel-catalysed expansion of oxetanes into ε-caprolactones.

Scheme 69: Expansion of oxetanes via cobalt-catalysed carbonyl insertion.

Scheme 70: Gold-catalysed intramolecular 1,1-carboalkoxylation of oxetane-ynamides.

Scheme 71: Expansion of oxetanes by stabilised sulphoxonium ylides.

Scheme 72: Cu-catalysed ring expansion of 2-vinyloxetanes by diazoesters.

Scheme 73: Total synthesis of (+)-oxetin.

Scheme 74: Total synthesis of racemic oxetanocin A.

Scheme 75: Total synthesis of (−)-merrilactone A.

Scheme 76: Total synthesis of (+)-dictyoxetane.

Scheme 77: Total synthesis of ent-dichrocephone B.

Scheme 78: Total synthesis of (−)-mitrephorone A.

Scheme 79: Total synthesis of (−)-taxol.
Hypervalent iodine-mediated intramolecular alkene halocyclisation
- Charu Bansal,
- Oliver Ruggles,
- Albert C. Rowett and
- Alastair J. J. Lennox
Beilstein J. Org. Chem. 2024, 20, 3113–3133, doi:10.3762/bjoc.20.258
- oximes 63 and N-hydroxylated ureas 64 depending on the reagent system used. Formation of oxazolidinone oximes 63 occurred using PhI(OCOCF3)2 (PIFA) as an oxidant with pyridine·HBr and the MgO additive. The oxybromocyclisation of a range of unsaturated N-alkoxyureas 62 occurred rapidly in 10 minutes at
- room temperature in acetonitrile with good yields. Formation of the N-hydroxylated ureas 64 occurred using PhI(OPiv)2 and TBABr, with MgO as an additive to trap acetic acid. Aminobromocylization of a range of unsaturated N-alkoxyureas 62 was less successful with longer reactions times up to 1 hours

Graphical Abstract

Figure 1: Example bioactive compounds containing cyclic scaffolds potentially accessible by HVI chemistry.

Figure 2: A general mechanism for HVI-mediated endo- or exo-halocyclisation.

Scheme 1: Metal-free synthesis of β-fluorinated piperidines 6. Ts = tosyl.

Scheme 2: Intramolecular aminofluorination of unactivated alkenes with a palladium catalyst.

Scheme 3: Aminofluorination of alkenes in the synthesis of enantiomerically pure β-fluorinated piperidines. P...

Scheme 4: Synthesis of β-fluorinated piperidines.

Scheme 5: Intramolecular fluoroaminations of unsaturated amines published by Li.

Scheme 6: Intramolecular aminofluorination of unsaturated amines using 1-fluoro-3,3-dimethylbenziodoxole (12)...

Scheme 7: 3-fluoropyrrolidine synthesis. aDiastereomeric ratio (cis/trans) determined by 19F NMR analysis.

Scheme 8: Kitamura’s synthesis of 3-fluoropyrrolidines. Values in parentheses represent the cis:trans ratio.

Scheme 9: Jacobsen’s enantio- and diastereoselective protocol for the synthesis of syn-β-fluoroaziridines 15.

Scheme 10: Different HVI reagents lead to different diastereoselectivity in aminofluorination competing with c...

Scheme 11: Fluorocyclisation of unsaturated alcohols and carboxylic acids to make tetrahydrofurans, fluorometh...

Scheme 12: Oxyfluorination of unsaturated alcohols.

Scheme 13: Synthesis and mechanism of fluoro-benzoxazepines.

Scheme 14: Intramolecular fluorocyclisation of unsaturated carboxylic acids. Yield of isolated product within ...

Scheme 15: Synthesis of fluorinated tetrahydrofurans and butyrolactone.

Scheme 16: Synthesis of fluorinated oxazolines 32. aReaction time increased to 40 hours. Yields refer to isola...

Scheme 17: Electrochemical synthesis of fluorinated oxazolines.

Scheme 18: Electrochemical synthesis of chromanes.

Scheme 19: Synthesis of fluorinated oxazepanes.

Scheme 20: Enantioselective oxy-fluorination with a chiral aryliodide catayst.

Scheme 21: Catalytic synthesis of 5‑fluoro-2-aryloxazolines using BF3·Et2O as a source of fluoride and an acti...

Scheme 22: Intramolecular carbofluorination of alkenes.

Scheme 23: Intramolecular chlorocyclisation of unsaturated amines.

Scheme 24: Synthesis of chlorinated cyclic guanidines 44.

Scheme 25: Synthesis of chlorinated pyrido[2,3-b]indoles 46.

Scheme 26: Chlorolactonization and chloroetherification reactions.

Scheme 27: Proposed mechanism for the synthesis of chloromethyl oxazolines 49.

Scheme 28: Oxychlorination to form oxazine and oxazoline heterocycles promoted by BCl3.

Scheme 29: Aminobromocyclisation of homoallylic sulfonamides 53. The cis:trans ratios based on the 1H NMR of t...

Scheme 30: Synthesis of cyclic imines 45.

Scheme 31: Synthesis of brominated pyrrolo[2,3-b]indoles 59.

Scheme 32: Bromoamidation of alkenes.

Scheme 33: Synthesis of brominated cyclic guanidines 61 and 61’.

Scheme 34: Intramolecular bromocyclisation of N-oxyureas.

Scheme 35: The formation of 3-bromoindoles.

Scheme 36: Bromolactonisation of unsaturated acids 68.

Scheme 37: Synthesis of 5-bromomethyl-2-oxazolines.

Scheme 38: Synthesis of brominated chiral morpholines.

Scheme 39: Bromoenolcyclisation of unsaturated dicarbonyl groups.

Scheme 40: Brominated oxazines and oxazolines with BBr3.

Scheme 41: Synthesis of 5-bromomethtyl-2-phenylthiazoline.

Scheme 42: Intramolecular iodoamination of unsaturated amines.

Scheme 43: Formation of 3-iodoindoles.

Scheme 44: Iodoetherification of 2,2-diphenyl-4-penten-1-carboxylic acid (47’) and 2,2-diphenyl-4-penten-1-ol (...

Scheme 45: Synthesis of 5-iodomethyl-2-oxazolines.

Scheme 46: Synthesis of chiral iodinated morpholines. aFrom the ʟ-form of the amino acid starting material. Th...

Scheme 47: Iodoenolcyclisation of unsaturated dicarbonyl compounds 74.

Scheme 48: Synthesis of 5-iodomethtyl-2-phenylthiazoline (87).
Ligand effects, solvent cooperation, and large kinetic solvent deuterium isotope effects in gold(I)-catalyzed intramolecular alkene hydroamination
- Ruichen Lan,
- Brock Yager,
- Yoonsun Jee,
- Cynthia S. Day and
- Amanda C. Jones
Beilstein J. Org. Chem. 2024, 20, 479–496, doi:10.3762/bjoc.20.43
- out under a variety of conditions with cationic gold catalysts supported by phosphine ligands. The impact of ligand on gold, protecting group on nitrogen, and solvent and additive on reaction rates was determined. The most effective reactions utilized more Lewis basic ureas, and more electron
- have been masked by the high temperatures and long reaction times of early reports. For example, Widenhoefer engaged carbamates and amides in dioxane at temperatures >80 °C [12][13]. Later work showed that ureas could be engaged at room temperature when a NHC–gold catalyst system was used, outpacing
- nitrogen. Thus, the rate of intramolecular hydroamination is enhanced by a more nucleophilic nitrogen. Another qualitative interpretation is that rates are enhanced by carbonyl basicity. Gas-phase basicity measurements indicate that ureas are more basic than amides [47][50], and here the most Lewis base

Graphical Abstract

Scheme 1: Proposed mechanism and observation of alkylgold intermediates.

Figure 1: First order alkene decay for urea alkene 1a (0.05 M) hydroamination with [JPhosAu(NCCH3)]SbF6 (5, 2...

Figure 2: Cooperative effect of mixed CD2Cl2/MeOH on alkene 1a → 3a conversion with catalyst 5 (2.5 mol %). E...

Figure 3: Different additive impact on rate of 1a → 3a depending upon catalyst and co-solvent. The data for J...

Figure 4: (a) Schematic for synthesis of [L–Au–L]SbF6 where L = JPhos. (b) Perspective drawing of the cation ...

Figure 5: (a) kobs for reaction of urea 1a (0.05 M) in DCM with catalyst 5 and titrated CH3OH/CH3OD. Data for...

Figure 6: Rate of urea 1a (0.05 M) hydroamination with JPhosAu(NCCH3)SbF6 (2.5 mol %) in CH2Cl2 with 5, 25, a...

Figure 7: Observed rates for the reaction of carbamate 1b (0.03–0.24 M) with JackiephosAuNTf2 (0.0013 M, 6a) ...

Figure 8: Influence of catalyst 5 concentration on rate of 1a (0.05 M in CH2Cl2 with 0, 10 μL MeOH). Error ba...

Scheme 2: Proposed alternate mechanism.
Aromatic C–H bond functionalization through organocatalyzed asymmetric intermolecular aza-Friedel–Crafts reaction: a recent update
- Anup Biswas
Beilstein J. Org. Chem. 2023, 19, 956–981, doi:10.3762/bjoc.19.72
- ], amidines [11], isothioureas [12][13], whereas thioureas [14][15], ureas [16], phosphoric acids [17][18], and squaramides [19][20] fall into the second category. The Friedel–Crafts reaction, discovered by Charles Friedel and James Crafts in 1877 allows the aromatic C–H bond functionalization through the

Graphical Abstract

Scheme 1: First organocatalyzed asymmetric aza-Friedel–Crafts reaction.

Scheme 2: Aza-Friedel–Crafts reaction between indoles and cyclic ketimines.

Scheme 3: Aza-Friedel–Crafts reaction utilizing trifluoromethyldihydrobenzoazepinoindoles as electrophiles.

Scheme 4: Aza-Friedel–Crafts reaction utilizing cyclic N-sulfimines as electrophiles.

Scheme 5: Aza-Friedel–Crafts reaction involving N-unprotected imino ester as electrophile.

Scheme 6: Aza-Friedel–Crafts and lactonization cascade.

Scheme 7: One-pot oxidation and aza-Friedel–Crafts reaction.

Scheme 8: C1 and C2-symmetric phosphoric acids as catalysts.

Scheme 9: Aza-Friedel–Crafts reaction using Nps-iminophosphonates as electrophiles.

Scheme 10: Aza-Friedel–Crafts reaction between indole and α-iminophosphonate.

Scheme 11: [2.2]-Paracyclophane-derived chiral phosphoric acids as catalyst.

Scheme 12: Aza-Friedel–Crafts reaction through ring opening of sulfamidates.

Scheme 13: Isoquinoline-1,3(2H,4H)-dione scaffolds as electrophiles.

Scheme 14: Functionalization of the carbocyclic ring of substituted indoles.

Scheme 15: Aza-Friedel–Crafts reaction between unprotected imines and aza-heterocycles.

Scheme 16: Anilines and α-naphthols as potential nucleophiles.

Scheme 17: Solvent-controlled regioselective aza-Friedel–Crafts reaction.

Scheme 18: Generating central and axial chirality via aza-Friedel–Crafts reaction.

Scheme 19: Reaction between indoles and racemic 2,3-dihydroisoxazol-3-ol derivatives.

Scheme 20: Exploiting 5-aminoisoxazoles as nucleophiles.

Scheme 21: Reaction between unsubstituted indoles and 3-alkynylated 3-hydroxy-1-oxoisoindolines.

Scheme 22: Synthesis of unnatural amino acids bearing an aza-quaternary stereocenter.

Scheme 23: Atroposelective aza-Friedel–Crafts reaction.

Scheme 24: Coupling of 5-aminopyrazole and 3H-indol-3-ones.

Scheme 25: Pyrophosphoric acid-catalyzed aza-Friedel–Crafts reaction on phenols.

Scheme 26: Squaramide-assisted aza-Friedel–Crafts reaction.

Scheme 27: Thiourea-catalyzed aza-Friedel–Crafts reaction.

Scheme 28: Squaramide-catalyzed reaction between β-naphthols and benzothiazolimines.

Scheme 29: Thiourea-catalyzed reaction between β-naphthol and isatin-derived ketamine.

Scheme 30: Quinine-derived molecule as catalyst.

Scheme 31: Cinchona alkaloid as catalyst.

Scheme 32: aza-Friedel–Crafts reaction by phase transfer catalyst.

Scheme 33: Disulfonamide-catalyzed reaction.

Scheme 34: Heterogenous thiourea-catalyzed aza-Friedel–Crafts reaction.

Scheme 35: Total synthesis of (+)-gracilamine.

Scheme 36: Total synthesis of (−)-fumimycin.
A versatile way for the synthesis of monomethylamines by reduction of N-substituted carbonylimidazoles with the NaBH4/I2 system
- Lin Chen,
- Xuan Zhou,
- Zhiyong Chen,
- Changxu Wang,
- Shunjie Wang and
- Hanbing Teng
Beilstein J. Org. Chem. 2022, 18, 1032–1039, doi:10.3762/bjoc.18.104
- attacked by a nucleophile the imidazole group readily dissociates. The N-substituted carbonylimidazoles have favorable reactivity and can be widely used in the synthesis of various valuable products such as ureas [63][64][65][66][67][68][69][70], carbamates [66][71][72][73][74], thiocarbamates [66], and

Graphical Abstract

Scheme 1: The synthesis of formamides and monomethylamines.

Scheme 2: The possible reaction mechanism. RDS = rate determining step.
New advances in asymmetric organocatalysis
- Radovan Šebesta
Beilstein J. Org. Chem. 2022, 18, 240–242, doi:10.3762/bjoc.18.28

- possibilities in combination of covalent and noncovalent activation, our group designed and synthesized N-sulfinylpyrrolidine-containing ureas and thioureas and applied them in Michael additions of aldehydes to heterocycle containing nitroalkenes [25]. Dubey and Chowdhury showed that 1,4-conjugate additions of
N-Sulfinylpyrrolidine-containing ureas and thioureas as bifunctional organocatalysts
- Viera Poláčková,
- Dominika Krištofíková,
- Boglárka Némethová,
- Renata Górová,
- Mária Mečiarová and
- Radovan Šebesta
Beilstein J. Org. Chem. 2021, 17, 2629–2641, doi:10.3762/bjoc.17.176
- )-C1 as well as N-sulfinylureas (S,R)- and (S,S)-C2 in excellent yields (Scheme 2). Application of thioureas C1 and ureas C2 in the Michael addition of aldehydes to nitroalkenes Michael addition in solution As the first benchmark transformation, we opted for the Michael addition of butanal (6a) to β
- nitroalkene 9 using catalyst (S,R)-C2. Synthesis of isothiocyanate 3a and isocyanate 3b. Synthesis of sulfinylthioureas C1 and ureas C2. Synthesis of adducts 8a,d,f in solution. Michael additions of aldehydes 6b–d with nitroalkene 9. Michael addition of 3-phenylpropanal (6c) to nitroalkene 11. Optimization of

Graphical Abstract

Figure 1: Catalyst design principles.

Scheme 1: Synthesis of isothiocyanate 3a and isocyanate 3b.

Scheme 2: Synthesis of sulfinylthioureas C1 and ureas C2.

Scheme 3: Synthesis of adducts 8a,d,f in solution.

Figure 2: DFT-calculated (PBEh-3c/def2-SV(P)//M06-2X/def2-TZVP) structures of catalyst (S,R) and (S,S)-C2, en...

Figure 3: a) Arrangements of reactants in the transition states; b) DFT-calculated (PBEh-3c/def2-SV(P)//M06-2...

Figure 4: DFT-calculated (PBEh-3c/def2-SV(P)//M06-2X/def2-TZVP) reaction profile for the Michael addition of ...
Base-free enantioselective SN2 alkylation of 2-oxindoles via bifunctional phase-transfer catalysis
- Mili Litvajova,
- Emiliano Sorrentino,
- Brendan Twamley and
- Stephen J. Connon
Beilstein J. Org. Chem. 2021, 17, 2287–2294, doi:10.3762/bjoc.17.146
- to the ability of squaramides to bind anionic species more strongly than ureas. The moderate enantiocontrol observed thus far prompted us to posit that the nitrogen atom on the quinoline moiety of the catalyst could participate to the deprotonation of 5a, therefore, leading to less selective

Graphical Abstract

Figure 1: The importance of the 3,3-spirooxindole core and its access through enantioselective enolate alkyla...

Scheme 1: A) SN2 alkylation of 3-subtituted-2-oxindoles not readily functionalisable; B) Previous work: enant...

Figure 2: Substrate scope. aIsolated yield. bDetermined by CSP-HPLC. cValue in brackets refers to reaction co...

Scheme 2: Enantioselective synthesis of a CRTH2 receptor antagonist.
Synthetic accesses to biguanide compounds
- Oleksandr Grytsai,
- Cyril Ronco and
- Rachid Benhida
Beilstein J. Org. Chem. 2021, 17, 1001–1040, doi:10.3762/bjoc.17.82


Graphical Abstract

Figure 1: Tautomeric forms of biguanide.

Figure 2: Illustrations of neutral, monoprotonated, and diprotonated structures biguanide.

Figure 3: The main approaches for the synthesis of biguanides. The core structure is obtained via the additio...

Scheme 1: The three main preparations of biguanides from cyanoguanidine.

Scheme 2: Synthesis of butylbiguanide using CuCl2 [16].

Scheme 3: Synthesis of biguanides by the direct fusion of cyanoguanidine and amine hydrochlorides [17,18].

Scheme 4: Synthesis of ethylbiguanide and phenylbiguanide as reported by Smolka and Friedreich [14].

Scheme 5: Synthesis of arylbiguanides through the reaction of cyanoguanidine with anilines in water [19].

Scheme 6: Synthesis of aryl- and alkylbiguanides by adaptations of Cohn’s procedure [20,21].

Scheme 7: Microwave-assisted synthesis of N1-aryl and -dialkylbiguanides [22,23].

Scheme 8: Synthesis of aryl- and alkylbiguanides by trimethylsilyl activation [24,26].

Scheme 9: Synthesis of phenformin analogs by TMSOTf activation [27].

Scheme 10: Synthesis of N1-(1,2,4-triazolyl)biguanides [28].

Scheme 11: Synthesis of 2-guanidinobenzazoles by addition of ortho-substituted anilines to cyanoguanidine [30,32] and...

Scheme 12: Synthesis of 2,4-diaminoquinazolines by the addition of 2-cyanoaniline to cyanoguanidine and from 3...

Scheme 13: Reactions of anthranilic acid and 2-mercaptobenzoic acid with cyanoguanidine [24,36,37].

Scheme 14: Synthesis of disubstituted biguanides with Cu(II) salts [38].

Scheme 15: Synthesis of an N1,N2,N5-trisubstituted biguanide by fusion of an amine hydrochloride and 2-cyano-1...

Scheme 16: Synthesis of N1,N5-disubstituted biguanides by the addition of anilines to cyanoguanidine derivativ...

Scheme 17: Microwave-assisted additions of piperazine and aniline hydrochloride to substituted cyanoguanidines ...

Scheme 18: Synthesis of N1,N5-alkyl-substituted biguanides by TMSOTf activation [27].

Scheme 19: Additions of oxoamines hydrochlorides to dimethylcyanoguanidine [49].

Scheme 20: Unexpected cyclization of pyridylcyanoguanidines under acidic conditions [50].

Scheme 21: Example of industrial synthesis of chlorhexidine [51].

Scheme 22: Synthesis of symmetrical N1,N5-diarylbiguanides from sodium dicyanamide [52,53].

Scheme 23: Synthesis of symmetrical N1,N5-dialkylbiguanides from sodium dicyanamide [54-56].

Scheme 24: Stepwise synthesis of unsymmetrical N1,N5-trisubstituted biguanides from sodium dicyanamide [57].

Scheme 25: Examples for the synthesis of unsymmetrical biguanides [58].

Scheme 26: Examples for the synthesis of an 1,3-diaminobenzoquinazoline derivative by the SEAr cyclization of ...

Scheme 27: Major isomers formed by the SEAr cyclization of symmetric biguanides derived from 2- and 3-aminophe...

Scheme 28: Lewis acid-catalyzed synthesis of 8H-pyrrolo[3,2-g]quinazoline-2,4-diamine [63].

Scheme 29: Synthesis of [1,2,4]oxadiazoles by the addition of hydroxylamine to dicyanamide [49,64].

Scheme 30: Principle of “bisamidine transfer” and analogy between the reactions with N-amidinopyrazole and N-a...

Scheme 31: Representative syntheses of N-amidino-amidinopyrazole hydrochloride [68,69].

Scheme 32: First examples of biguanide syntheses using N-amidino-amidinopyrazole [66].

Scheme 33: Example of “biguanidylation” of a hydrazide substrate [70].

Scheme 34: Example for the synthesis of biguanides using S-methylguanylisothiouronium iodide as “bisamidine tr...

Scheme 35: Synthesis of N-substituted N1-cyano-S-methylisothiourea precursors.

Scheme 36: Addition routes on N1-cyano-S-methylisothioureas.

Scheme 37: Synthesis of an hydroxybiguanidine from N1-cyano-S-methylisothiourea [77].

Scheme 38: Synthesis of an N1,N2,N3,N4,N5-pentaarylbiguanide from the corresponding triarylguanidine and carbo...

Scheme 39: Reactions of N,N,N’,N’-tetramethylguanidine (TMG) with carbodiimides to synthesize hexasubstituted ...

Scheme 40: Microwave-assisted addition of N,N,N’,N’-tetramethylguanidine to carbodiimides [80].

Scheme 41: Synthesis of N1-aryl heptasubstituted biguanides via a one-pot biguanide formation–copper-catalyzed ...

Scheme 42: Formation of 1,2-dihydro-1,3,5-triazine derivatives by the reaction of guanidine with excess carbod...

Scheme 43: Plausible mechanism for the spontaneous cyclization of triguanides [82].

Scheme 44: a) Formation of mono- and disubstituted (iso)melamine derivatives by the reaction of biguanides and...

Scheme 45: Reactions of 2-aminopyrimidine with carbodiimides to synthesize 2-guanidinopyrimidines as “biguanid...

Scheme 46: Non-catalyzed alternatives for the addition of 2-aminopyrimidine derivatives to carbodiimides. A) h...

Scheme 47: Addition of guanidinomagnesium halides to substituted cyanamides [90].

Scheme 48: Microwave-assisted synthesis of [11C]metformin by the reaction of 11C-labelled dimethylcyanamide an...

Scheme 49: Formation of 4-amino-6-dimethylamino[1,3,5]triazin-2-ol through the reaction of Boc-guanidine and d...

Scheme 50: Formation of 1,3,5-triazine derivatives via the addition of guanidines to substituted cyanamides [92].

Scheme 51: Synthesis of biguanide by the reaction of O-alkylisourea and guanidine [93].

Scheme 52: Aromatic nucleophilic substitution of guanidine on 2-O-ethyl-1,3,5-triazine [95].

Scheme 53: Synthesis of N1,N2-disubstituted biguanides by the reaction of guanidine and thioureas in the prese...

Scheme 54: Cyclization reactions involving condensations of guanidine(-like) structures with thioureas [97,98].

Scheme 55: Condensations of guanidine-like structures with thioureas [99,100].

Scheme 56: Condensations of guanidines with S-methylisothioureas [101,102].

Scheme 57: Addition of 2-amino-1,3-diazaaromatics to S-alkylisothioureas [103,104].

Scheme 58: Addition of guanidines to 2-(methylsulfonyl)pyrimidines [105].

Scheme 59: An example of a cyclodesulfurization reaction to a fused 3,5-diamino-1,2,4-triazole [106].

Scheme 60: Ring-opening reactions of 1,3-diaryl-2,4-bis(arylimino)-1,3-diazetidines [107].

Scheme 61: Formation of 3,5-diamino-1,2,4-triazole derivatives via addition of hydrazines to 1,3-diazetidine-2...

Scheme 62: Formation of a biguanide via the addition of aniline to 1,2,4-thiadiazol-3,5-diamines, ring opening...

Figure 4: Substitution pattern of biguanides accessible by synthetic pathways a–h.
Valorisation of plastic waste via metal-catalysed depolymerisation
- Francesca Liguori,
- Carmen Moreno-Marrodán and
- Pierluigi Barbaro
Beilstein J. Org. Chem. 2021, 17, 589–621, doi:10.3762/bjoc.17.53


Graphical Abstract

Figure 1: Potential classification of plastic recycling processes. The area covered by the present review is ...

Figure 2: EG produced during glycolytic depolymerisation of PET using DEG + DPG as solvent and titanium(IV) n...

Scheme 1: Simplified representation of the conversion of 1,4-PBD to C16–C44 macrocycles using Ru metathesis c...

Figure 3: Main added-value monomers obtainable by catalytic depolymerisation of PET via chemolytic methods.

Scheme 2: Hydrogenolytic depolymerisation of PET by ruthenium complexes.

Scheme 3: Depolymerisation of PET via catalytic hydrosilylation by Ir(III) pincer complex.

Scheme 4: Catalytic hydrolysis (top) and methanolysis (bottom) reactions of PET.

Scheme 5: Depolymerisation of PET by glycolysis with ethylene glycol.

Figure 4: Glycolysis of PET: evolution of BHET yield over time, with and without zinc acetate catalyst (196 °...

Scheme 6: Potential activated complex for the glycolysis reaction of PET catalysed by metallated ILs and evol...

Scheme 7: One-pot, two-step process for PET repurposing via chemical recycling.

Scheme 8: Synthetic routes to PLA.

Scheme 9: Structures of the zinc molecular catalysts used for PLA-methanolysis in various works. a) See [265], b) ...

Scheme 10: Depolymerisation of PLLA by Zn–N-heterocyclic carbene complex.

Scheme 11: Salalen ligands.

Scheme 12: Catalytic hydrogenolysis of PLA.

Scheme 13: Catalytic hydrosilylation of PLA.

Scheme 14: Hydrogenative depolymerisation of PBT and PCL by molecular Ru catalysts.

Scheme 15: Glycolysis reaction of PCT by diethylene glycol.

Scheme 16: Polymerisation–depolymerisation cycle of 3,4-T6GBL.

Scheme 17: Polymerisation–depolymerisation cycle of 2,3-HDB.

Scheme 18: Hydrogenative depolymerisation of PBPAC by molecular Ru catalysts.

Scheme 19: Catalytic hydrolysis (top), alcoholysis (middle) and aminolysis (bottom) reactions of PBPAC.

Scheme 20: Hydrogenative depolymerisation of PPC (top) and PEC (bottom) by molecular Ru catalysts.

Scheme 21: Polymerisation-depolymerisation cycle of BEP.

Scheme 22: Hydrogenolysis of polyamides using soluble Ru catalysts.

Scheme 23: Catalytic depolymerisation of epoxy resin/carbon fibres composite.

Scheme 24: Depolymerisation of polyethers with metal salt catalysts and acyl chlorides.

Scheme 25: Proposed mechanism for the iron-catalysed depolymerisation reaction of polyethers. Adapted with per...
Synthesis of legonmycins A and B, C(7a)-hydroxylated bacterial pyrrolizidines
- Wilfred J. M. Lewis,
- David M. Shaw and
- Jeremy Robertson
Beilstein J. Org. Chem. 2021, 17, 334–342, doi:10.3762/bjoc.17.31
- pyrrolizidines; cyanoketones; legonmycin; vinylogous ureas; Introduction At least 40 members of the large class of pyrrolizidine alkaloids [1][2][3][4] have so far been characterized from bacterial cultures. Of these ‘bacterial pyrrolizidines’, those of the vinylogous urea type are particularly intriguing from
- vinylogous ureas, since they lack the C(1) carbonyl group, their structural relationship with bohemamine, the first genuine member of the group, qualifies them as honorary members. The structure of bohemamine, named by its discoverers at Bristol–Myers Company and Cornell University after the opera La Bohème

Graphical Abstract

Figure 1: The clazamycins, and selected bacterial pyrrolizidines of the vinylogous urea type. For consistency...

Figure 2: Key species in the biosynthesis of legonmycins A (3) and B (4), and the pyrrolizixenamides A–D (9–12...

Scheme 1: Preparation of the legonmycin core.

Scheme 2: Hypothesis for the oxidative hydrolysis of diacylated pyrrolizidinone 18 (R = iBu).

Scheme 3: Preparation of C(7a)-functionalized pyrrolizinone derivatives and synthesis of legonmycins A and B.
Recent developments in enantioselective photocatalysis
- Callum Prentice,
- James Morrisson,
- Andrew D. Smith and
- Eli Zysman-Colman
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197


Graphical Abstract

Scheme 1: Amine/photoredox-catalysed α-alkylation of aldehydes with alkyl bromides bearing electron-withdrawi...

Scheme 2: Amine/HAT/photoredox-catalysed α-functionalisation of aldehydes using alkenes.

Scheme 3: Amine/cobalt/photoredox-catalysed α-functionalisation of ketones and THIQs.

Scheme 4: Amine/photoredox-catalysed α-functionalisation of aldehydes or ketones with imines. (a) Using keton...

Scheme 5: Bifunctional amine/photoredox-catalysed enantioselective α-functionalisation of aldehydes.

Scheme 6: Bifunctional amine/photoredox-catalysed α-functionalisation of aldehydes using amine catalysts via ...

Scheme 7: Amine/photoredox-catalysed RCA of iminium ion intermediates. (a) Synthesis of quaternary stereocent...

Scheme 8: Bifunctional amine/photoredox-catalysed RCA of enones in a radical chain reaction initiated by an i...

Scheme 9: Bifunctional amine/photoredox-catalysed RCA reactions of iminium ions with different radical precur...

Scheme 10: Bifunctional amine/photoredox-catalysed radical cascade reactions between enones and alkenes with a...

Scheme 11: Amine/photocatalysed photocycloadditions of iminium ion intermediates. (a) External photocatalyst u...

Scheme 12: Amine/photoredox-catalysed addition of acrolein (94) to iminium ions.

Scheme 13: Dual NHC/photoredox-catalysed acylation of THIQs.

Scheme 14: NHC/photocatalysed spirocyclisation via photoisomerisation of an extended Breslow intermediate.

Scheme 15: CPA/photoredox-catalysed aza-pinacol cyclisation.

Scheme 16: CPA/photoredox-catalysed Minisci-type reaction between azaarenes and α-amino radicals.

Scheme 17: CPA/photoredox-catalysed radical additions to azaarenes. (a) α-Amino radical or ketyl radical addit...

Scheme 18: CPA/photoredox-catalysed reduction of azaarene-derived substrates. (a) Reduction of ketones. (b) Ex...

Scheme 19: CPA/photoredox-catalysed radical coupling reactions of α-amino radicals with α-carbonyl radicals. (...

Scheme 20: CPA/photoredox-catalysed Povarov reaction.

Scheme 21: CPA/photoredox-catalysed reactions with imines. (a) Decarboxylative imine generation followed by Po...

Scheme 22: Bifunctional CPA/photocatalysed [2 + 2] photocycloadditions.

Scheme 23: PTC/photocatalysed oxygenation of 1-indanone-derived β-keto esters.

Scheme 24: PTC/photoredox-catalysed perfluoroalkylation of 1-indanone-derived β-keto esters via a radical chai...

Scheme 25: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 26: Bifunctional hydrogen bonding/photocatalysed intramolecular RCA cyclisation of a quinolone.

Scheme 27: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 28: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloaddition reactions. (a) First use of...

Scheme 29: Bifunctional hydrogen bonding/photocatalysed deracemisation of allenes.

Scheme 30: Bifunctional hydrogen bonding/photocatalysed deracemisation reactions. (a) Deracemisation of sulfox...

Scheme 31: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloaddition of coumarins....

Scheme 32: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloadditions of quinolones. (a) Intramo...

Scheme 33: Hydrogen bonding/photocatalysed formal arylation of benzofuranones.

Scheme 34: Hydrogen bonding/photoredox-catalysed dehalogenative protonation of α,α-chlorofluoro ketones.

Scheme 35: Hydrogen bonding/photoredox-catalysed reductions. (a) Reduction of 1,2-diketones. (b) Reduction of ...

Scheme 36: Hydrogen bonding/HAT/photocatalysed deracemisation of cyclic ureas.

Scheme 37: Hydrogen bonding/HAT/photoredox-catalysed synthesis of cyclic sulfonamides.

Scheme 38: Hydrogen bonding/photoredox-catalysed reaction between imines and indoles.

Scheme 39: Chiral cation/photoredox-catalysed radical coupling of two α-amino radicals.

Scheme 40: Chiral phosphate/photoredox-catalysed hydroetherfication of alkenols.

Scheme 41: Chiral phosphate/photoredox-catalysed synthesis of pyrroloindolines.

Scheme 42: Chiral anion/photoredox-catalysed radical cation Diels–Alder reaction.

Scheme 43: Lewis acid/photoredox-catalysed cycloadditions of carbonyls. (a) Formal [2 + 2] cycloaddition of en...

Scheme 44: Lewis acid/photoredox-catalysed RCA reaction using a scandium Lewis acid between α-amino radicals a...

Scheme 45: Lewis acid/photoredox-catalysed RCA reaction using a copper Lewis acid between α-amino radicals and...

Scheme 46: Lewis acid/photoredox-catalysed synthesis of 1,2-amino alcohols from aldehydes and nitrones using a...

Scheme 47: Lewis acid/photocatalysed [2 + 2] photocycloadditions of enones and alkenes.

Scheme 48: Meggers’s chiral-at-metal catalysts.

Scheme 49: Lewis acid/photoredox-catalysed α-functionalisation of ketones with alkyl bromides bearing electron...

Scheme 50: Bifunctional Lewis acid/photoredox-catalysed radical coupling reaction using α-chloroketones and α-...

Scheme 51: Lewis acid/photocatalysed RCA of enones. (a) Using aldehydes as acyl radical precursors. (b) Other ...

Scheme 52: Bifunctional Lewis acid/photocatalysis for a photocycloaddition of enones.

Scheme 53: Lewis acid/photoredox-catalysed RCA reactions of enones using DHPs as radical precursors.

Scheme 54: Lewis acid/photoredox-catalysed functionalisation of β-ketoesters. (a) Hydroxylation reaction catal...

Scheme 55: Bifunctional copper-photocatalysed alkylation of imines.

Scheme 56: Copper/photocatalysed alkylation of imines. (a) Bifunctional copper catalysis using α-silyl amines....

Scheme 57: Bifunctional Lewis acid/photocatalysed intramolecular [2 + 2] photocycloaddition.

Scheme 58: Bifunctional Lewis acid/photocatalysed [2 + 2] photocycloadditions (a) Intramolecular cycloaddition...

Scheme 59: Bifunctional Lewis acid/photocatalysed rearrangement of 2,4-dieneones.

Scheme 60: Lewis acid/photocatalysed [2 + 2] cycloadditions of cinnamate esters and styrenes.

Scheme 61: Nickel/photoredox-catalysed arylation of α-amino acids using aryl bromides.

Scheme 62: Nickel/photoredox catalysis. (a) Desymmetrisation of cyclic meso-anhydrides using benzyl trifluorob...

Scheme 63: Nickel/photoredox catalysis for the acyl-carbamoylation of alkenes with aldehydes using TBADT as a ...

Scheme 64: Bifunctional copper/photoredox-catalysed C–N coupling between α-chloro amides and carbazoles or ind...

Scheme 65: Bifunctional copper/photoredox-catalysed difunctionalisation of alkenes with alkynes and alkyl or a...

Scheme 66: Copper/photoredox-catalysed decarboxylative cyanation of benzyl phthalimide esters.

Scheme 67: Copper/photoredox-catalysed cyanation reactions using TMSCN. (a) Propargylic cyanation (b) Ring ope...

Scheme 68: Palladium/photoredox-catalysed allylic alkylation reactions. (a) Using alkyl DHPs as radical precur...

Scheme 69: Manganese/photoredox-catalysed epoxidation of terminal alkenes.

Scheme 70: Chromium/photoredox-catalysed allylation of aldehydes.

Scheme 71: Enzyme/photoredox-catalysed dehalogenation of halolactones.

Scheme 72: Enzyme/photoredox-catalysed dehalogenative cyclisation.

Scheme 73: Enzyme/photoredox-catalysed reduction of cyclic imines.

Scheme 74: Enzyme/photocatalysed enantioselective reduction of electron-deficient alkenes as mixtures of (E)/(Z...

Scheme 75: Enzyme/photoredox catalysis. (a) Deacetoxylation of cyclic ketones. (b) Reduction of heteroaromatic...

Scheme 76: Enzyme/photoredox-catalysed synthesis of indole-3-ones from 2-arylindoles.

Scheme 77: Enzyme/HAT/photoredox catalysis for the DKR of primary amines.

Scheme 78: Bifunctional enzyme/photoredox-catalysed benzylic C–H hydroxylation of trifluoromethylated arenes.
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- of amines. The latter then aminolyzed the carbamates 149 to generate ureas 151 and γ-halo-β-hydroxyalkanethiols 150. The intermediates 150 further underwent an intramolecular cyclization to produce the thietane-3-ols 145 in low to good yields [58] (Scheme 30). Paclitaxel (Taxol®) and docetaxel

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- good yields of 145h–l (Scheme 32). Additionally, products derived from ureas (i.e., 146), N,N-dimethylaniline (i.e., 147), amides (i.e., 148), and lactams (i.e., 149a) were obtained. Moreover, for N-methylpyrrolidinone, α-arylation was achieved in a good yield and regioselectivity for the substitution

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
- Itamar L. Gonçalves,
- Rafaela R. da Rosa,
- Vera L. Eifler-Lima and
- Aloir A. Merlo
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
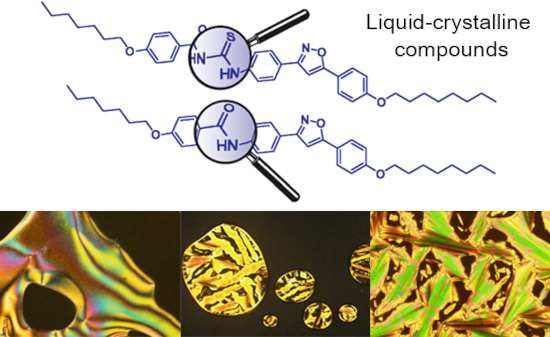
- liquid crystal design [10][11]. N,N′-Bis(3,4,5-trialkoxylphenyl)ureas were identified as columnar liquid crystalline compounds presenting ferroelectric properties [12]. Another study reported cyclic disubstituted ureas with liquid crystalline ferroelectric and antiferroelectric phases [13]. Relating to
- the thiourea moiety, there is only one recent report, in which some derivatives of N-benzoyl-N’-arylthiourea with liquid crystalline properties have been investigated [14]. One aspect that compounds forming hydrogen bonds, such as ureas, thioureas, amides etc. for the use in electronic devices is the
- the thiocyanate salt, following the same experimental protocol. However, when a cyanate salt was used, only the amides 19–22 were isolated as the main products. The target ureas were not obtained, according to Scheme 3, part A. To test the hypothesis of intermediate formation of acyl cation [ArCO

Graphical Abstract

Scheme 1: Amines 3, 4, 8, 9, 12 and 13 installed on 5-membered isoxazoline and isoxazole rings.

Scheme 2: Synthesis of acylisoxazolinylthioureas 17a–c and acylisoxazolylthioureas 18a–c. (i) SOCl2, reflux, ...

Scheme 3: Synthesis of amides. Part A: (i) SOCl2, reflux; (ii) KOCN, acetone, reflux; (iii) amines 3, 4, 8 an...

Figure 1: Optical textures observed on POM for thioureas 17a (a), 17b (b), 18a (c), 18b (d) and 18c (e,f). Al...

Figure 2: Optical textures observed on POM of amide 19. (a) Fan-shaped focal conic texture of the SmA mesopha...

Figure 3: DSC curves for the thiourea 17a (A), amide 19 (B) and 20 (C) upon the first heating and cooling cur...

Figure 4: TGA analysis for thiourea 18c; and amides 20 and 22.
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
- Keishiro Tahara,
- Tetsufumi Nakakita,
- Alyona A. Starikova,
- Takashi Ikeda,
- Masaaki Abe and
- Jun-ichi Kikuchi
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- were investigated to realize electrochemically controlled H-bonding [23][24][25][26][27][28][29][30][31][32][33][34][35][36]. Especially, oxidation-active ureas are an important class of such compounds [25][26]. In the neutral state, ureas can provide two NH protons for multiple H-bonding, as often
- ][34][35][36][37][38][39][40]. In contrast to the vast majority of oxidation-active ureas, those having two redox centers at both ends have not received attention except for 1,3-bis(ferrocenyl)urea FcFc [41][42], a cyclometalated diruthenium complex [43], and bis(NAr3) counterparts, including 1a
- substituents R at the para-position of the benzene adjacent to the nitrogen centers were synthesized to tune the oxidation potential of the nitrogen centers (Figure 1). According to a previously reported method for the synthesis of ureas [45], symmetric ureas 1 were synthesized from the corresponding amines

Graphical Abstract

Figure 1: Structures of target compounds 1 and reference compound Ph1b.

Figure 2: Cyclic voltammograms (left) and differential pulse voltammograms (right) of (top) 1a, (middle) Ph1b,...

Figure 3: Key frontier orbitals (isosurface values 0.02 au) (top), DFT-optimized structures with Mullliken ch...

Figure 4: UV–vis-NIR spectral changes of CH2Cl2/n-Bu4NPF6 (0.10 M) solutions containing (a) 1b (4.5 × 10−4 M)...

Figure 5: Vis-NIR spectra of 1b+ (green line) obtained by bulk electrolysis, with Gaussian deconvolutions (bl...
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165


Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.
Bambusuril analogs based on alternating glycoluril and xylylene units
- Tomáš Lízal and
- Vladimír Šindelář
Beilstein J. Org. Chem. 2019, 15, 1268–1274, doi:10.3762/bjoc.15.124

- urea groups can participate in intermolecular hydrogen bonding resulting in the formation of tubular [5][6][7] or gel-like [8] structures. Ureas lacking of N–H hydrogen atoms such as ethyleneureas, glycolurils, and biotin are also important building blocks of macrocyclic receptors such as cucurbiturils

Graphical Abstract

Scheme 1: Reaction of 2,4-dimethylglycoluril and m-xylylene dibromide.

Figure 1: Molecular structures of 1a and 1b.

Figure 2: Conformers of macrocycles 1a, 1b optimized at the CAM-B3LYP/6-31G(d) level of theory.

Figure 3: 1H NMR spectrum of 1a in MeCN-d3 measured at −40 °C. #Signal of water. *Signal of acetonitrile.

Figure 4: 2D EXSY spectra (ROESY cross-peaks in blue, EXSY cross-peaks in red) measured in MeCN-d3 at −40 °C....

Figure 5: X-ray crystal structure of 1b-1 (left), layered structure of crystal packing (right).
Steroid diversification by multicomponent reactions
- Leslie Reguera,
- Cecilia I. Attorresi,
- Javier A. Ramírez and
- Daniel G. Rivera
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- , etc. The urea component has the main structural restrictions, since monosubstituted alkyl ureas work well but thioureas have provided much lower yields. Wang et al. produced a library of steroidal [17,16-d]pyrimidine derivatives such as 40 employing a particular extension of the Biginelli reaction

Graphical Abstract

Figure 1: Structures of natural steroids of A) animal and B) plant origin.

Scheme 1: Synthesis of a steroidal β-lactam by Ugi reaction of a cholanic aldehyde [14].

Scheme 2: Synthetic route to steroidal 2,5-diketopiperazines based on a diastereoselective Ugi-4CR with an an...

Scheme 3: Multicomponent synthesis of a heterocycle–steroid hybrid using a ketosteroid as carbonyl component [18]....

Scheme 4: Synthesis of peptidomimetic–steroid hybrids using the Ugi-4CR with spirostanic amines and carboxyli...

Scheme 5: Synthesis of azasteroids using the Ugi-4CR with androstanic and pregnanic carboxylic acids [22].

Figure 2: Ugi-4CR-derived library of androstanic azasteroids with diverse substitution patterns at the phenyl...

Scheme 6: Synthesis of 4-azacholestanes by an intramolecular Ugi-4C-3R [26].

Scheme 7: Synthesis of amino acid–steroid hybrid by multiple Ugi-4CR using steroidal isocyanides [29].

Scheme 8: Synthesis of ecdysteroid derivatives by Ugi-4CR using a steroidal isocyanide [30].

Scheme 9: Stereoselective multicomponent synthesis of a steroid–tetrahydropyridine hybrid using a chiral bifu...

Scheme 10: Pd(II)-catalyzed three-component reaction with an alkynyl seco-cholestane [34].

Scheme 11: Multicomponent synthesis of steroid–thiazole hybrids from a steroidal ketone [36].

Scheme 12: Synthesis of cholanic pseudo-peptide derivatives by novel MCRs based on the reactivity of ynamide [37,38].

Scheme 13: Synthesis of steroid-fused pyrimidines and pyrimidones using the Biginelli-3CR [39,42,43].

Scheme 14: Synthesis of steroidal pyridopyrimidines by a reaction sequence comprising a 4CR followed by a post...

Scheme 15: Synthesis of steroid-fused pyrimidines by MCR of 2-hydroxymethylene-3-ketosteroids [46].

Scheme 16: Synthesis of steroid-fused naphthoquinolines by the Kozlov–Wang MCR using ketosteroids [50,51].

Scheme 17: Conjugation of steroids to carbohydrates and peptides by the Ugi-4CR [62,63].

Scheme 18: Solid-phase multicomponent conjugation of peptides to steroids by the Ugi-4CR [64].

Scheme 19: Solid-phase multicomponent conjugation of peptides to steroids by the Petasis-3CR [68].

Scheme 20: Synthesis of steroidal macrobicycles (cages) by multiple multicomponent macrocyclizations based on ...

Scheme 21: One-pot synthesis of steroidal cages by double Ugi-4CR-based macrocyclizations [76].
A three-component, Zn(OTf)2-mediated entry into trisubstituted 2-aminoimidazoles
- Alexei Lukin,
- Anna Bakholdina,
- Anna Kryukova,
- Alexander Sapegin and
- Mikhail Krasavin
Beilstein J. Org. Chem. 2019, 15, 1061–1064, doi:10.3762/bjoc.15.103
- possibility to incorporate of an external primary amine into the cyclization process, which would lead to trisubstituted 2-aminoimidazoles 5 has not been explored (Figure 1). 2-Aminoimidazoles have a remarkably broad utility in medicinal chemistry [7]. Moreover, ureas 4 can, in principle, be generated in situ
- preparation of ureas 4 could, in principle, enable a three-component entry to imidazoles 5 (an attractive option from the standpoint of library array synthesis), we compared the isolated yield of the above reaction (with the ready-made urea 4a) with the yield obtained in the three-component format. To our
- synthesis of trisubstituted 2-aminoimidazoles. This is the first example illustrating the utility of such ureas in the synthesis of imidazoles as previously reported syntheses only involved base-promoted cyclization into 2-imidazolones. The reaction can be conveniently conducted in a three-component format

Graphical Abstract

Figure 1: Examples of imidazole (1 and 2) and oxazole (3) syntheses from propargylamides previously reported ...

Figure 2: Substrate scope for the three-component synthesis of 5.

Figure 3: Plausible mechanism for the formation of 5.
Silanediol versus chlorosilanol: hydrolyses and hydrogen-bonding catalyses with fenchole-based silanes
- Falco Fox,
- Jörg M. Neudörfl and
- Bernd Goldfuss
Beilstein J. Org. Chem. 2019, 15, 167–186, doi:10.3762/bjoc.15.17

- ], (thio)ureas [30][31] and phosphoric acid derivatives [27]. Cyclodiphosph(V)azanes [32][33][34][35] and silanediols [1][28][36] are two new hydrogen bonding scaffolds for anion recognition [37] and ion-pair catalysis [6][38]. Since Kondo et al. established silanediol 1 [39] as HBD for anion recognition

Graphical Abstract

Figure 1: Hydrogen-bonding silanediols, i.e., di(1-naphthyl)silanediol (1) [39], silanediols 2 [41-43], binaphthylsilane...

Scheme 1: Hydrogen-bond-catalyzed N-acyl Mannich reaction of in situ-generated isoquinolin derivative 10 with...

Scheme 2: Synthesis of BIFOXSiCl2, starting with BIFOL (5) [52,54] yielding dichlorosilane 7.

Scheme 3: Hydrolysis of BIFOXSiCl2 (7) yielding the corresponding silanediol 9 and controlled hydrolysis of B...

Scheme 4: Hydrolysis of dichlorosilanes 13 and 14 to their corresponding silanediols 1 and 15 [51,60].

Figure 2: Hydrolyses of dichlorosilane 7 and 14 to BIFOXSi(OH)2 (9, green circle) and bis(2,4,6-tri-tert-buty...

Figure 3: Hydrolyses of BIFOXSiCl2 (7) to BIFOXSi(OH)2 (9, green circle), bis(2,4,6-tri-tert-butylphenoxy)dic...

Scheme 5: Two investigated pathways for the hydrolysis of the dichlorosilanes. Front attack mechanism (front)...

Figure 4: Three transition structures each, for the hydrolysis of BIFOXSiCl2 (7) and BIFOXSiCl(OH) (8) consid...

Figure 5: Computed hydrolyses of BIFOXSiCl2 (7) to BIFOXSiCl(OH) 8ax and BIFOXSiCl(OH) 8eq and subsequent com...

Figure 6: Transition state leading to 8eq following front1 attack (Ea = 32.6 kcal mol−1, Figure 5, Table 3, entry 1). Breaki...

Figure 7: Transition state leading to 8ax following front2 attack (Ea = 33.2 kcal mol−1, Figure 5, Table 3, entry 2). Breaki...

Figure 8: Transition state leading to 8eq following side attack (Ea = 37.4 kcal mol−1, Figure 5, Table 3, entry 3). Breaking...

Figure 9: Transition state leading to 9 following side attack (Ea = 31.4 kcal mol−1, Figure 5, Table 3, entry 6). Breaking a...

Figure 10: Transition state leading to 9 following front1 attack (Ea = 33.4 kcal mol−1, Figure 5, Table 3, entry 4). Breaking...

Figure 11: Transition state leading to 9 following front2 attack (Ea = 40.2 kcal mol−1, Figure 5, Table 3, entry 5). Breaking...

Figure 12: X-ray crystal structure of BIFOXSiCl2 (7). H atoms on the chiral backbone are omitted for clarity i...

Figure 13: X-ray crystal structure of BIFOXSiCl(OH) (8). H atoms on the chiral backbone are omitted for clarit...

Figure 14: X-ray crystal structure ofrac-BIFOXSi(OH)2 (9) forming dimers. H atoms on the chiral backbone are o...

Figure 15: X-ray crystal structure of BIFOXSi(OH)2 (9) forming a tetramer. H atoms on the chiral backbone are ...

Figure 16: X-ray crystal structure of BIFOXSi(OH)2 (9) forming a dimeric structure with two bridged acetone mo...

Figure 17: X-ray crystal structure of BIFOXSiCl(OH) (8), binding an acetone molecule. H atoms on the chiral ba...

Scheme 6: Hydrogen-bond-catalyzed N-acyl Mannich reaction of in situ-generated 10 with different silyl ketene...

Scheme 7: Hydrogen-bond-catalyzed nucleophilic substitution of 18 with BIFOXSi(OH)2 (9) and nucleophile silyl...

Scheme 8: Nucleophilic substitution of 20 with BIFOXSi(OH)2 (9) and nucleophile silyl ketene acetals 11, 20 a...






