Search results
Search for "sugars" in Full Text gives 191 result(s) in Beilstein Journal of Organic Chemistry.
Double-headed nucleosides: Synthesis and applications
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98
- of deoxyribonucleic acids (DNA) or ribonucleic acids (RNA), which contain either a purine or pyrimidine nucleobase and a furanosyl moiety of pentose sugars, 2′-deoxyribose or ribose [1][2]. Nucleotides are constituted by addition of a phosphate group at the 5′-position of the nucleosides and these
Synthesis of multiply fluorinated N-acetyl-D-glucosamine and D-galactosamine analogs via the corresponding deoxyfluorinated glucosazide and galactosazide phenyl thioglycosides
Beilstein J. Org. Chem. 2021, 17, 1086–1095, doi:10.3762/bjoc.17.85
- . Keywords: amino sugars; deoxyfluorination; fluorinated carbohydrates; hexosamine hemiacetals; thioglycosides; Introduction Fluorinated carbohydrates are versatile carbohydrate mimetics used to probe or manipulate the recognition of carbohydrates by carbohydrate-binding proteins or carbohydrate-processing
- molecules [12]. The fluorination of sugars is also a promising strategy to improve unfavorable pharmacokinetic properties of natural carbohydrates such as low lipophilicity [13][14][15][16] and fast metabolic degradation [17][18][19]. Over the last few years, considerable effort has been expended on the
- -catalyzed hydrogenolysis in ethanol/acetic anhydride appeared to be a logical deprotection step [26], the desired fluoro sugars were contaminated with varying quantities of unidentified byproducts. However, clean debenzylation was achieved by first converting the azide to an acetamide on reaction with
Beyond ribose and phosphate: Selected nucleic acid modifications for structure–function investigations and therapeutic applications
Beilstein J. Org. Chem. 2021, 17, 908–931, doi:10.3762/bjoc.17.76

- group between the sugars in DNA and RNA is at the origin of the vastly expanded fold [29][30][31][32] and functional spaces of RNA [33][34][35][36][37][38][39]. Perhaps less known is the fact that the sugar moiety in the backbone of a nucleic acid determines the base pairing priorities. For example, in
- adenosine [114]. This duplex adopted an overall A-form, with the sugars in the C3'-endo orientation and the two, well solvated methoxy groups, pointing into the relatively wide minor groove of the duplex. It was shown that as the number of carbons in the 2'-O-alkyl chain increased, so too did the
Simulating the enzymes of ganglioside biosynthesis with Glycologue
Beilstein J. Org. Chem. 2021, 17, 739–748, doi:10.3762/bjoc.17.64
- glycolipid or some other oligosaccharide, the nucleotide A is the product of the donor and xB is the acceptor product. The strings can be seen as a compression of the familiar condensed linear IUPAC notation, using a single-letter notation to represent sugars, with upper-case denoting ᴅ, and lowercase, the ʟ
- , sugars, and are read from right to left starting with the base (reducing-end) sugar. The letters a and b are reserved for α and β-anomers, respectively, while brackets are used to delimit branches, and the letter T is used to denote the connection point to ceramide, or to another conjugate depending on
- before sugars units, and multiple modifiers on the same monosaccharide are again ordered by linkage position, from lowest to highest, reading right to left. Nomenclature of gangliosides Gangliosides are commonly labelled according to the abbreviated Svennerholm [20] nomenclature, or else by the expanded
Direct synthesis of anomeric tetrazolyl iminosugars from sugar-derived lactams
Beilstein J. Org. Chem. 2021, 17, 115–123, doi:10.3762/bjoc.17.12
- Michal M. Wieclaw Bartlomiej Furman Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224, Warsaw, Poland 10.3762/bjoc.17.12 Abstract Herein we present the direct asymmetric synthesis of tetrazole-functionalized 1-deoxynojirimycin derivatives from simple sugars via a
Selected peptide-based fluorescent probes for biological applications
Beilstein J. Org. Chem. 2020, 16, 2971–2982, doi:10.3762/bjoc.16.247

- of the PDA liposomes to about 48% of the initial value (Figure 10B). The calculated binding constant from Stern–Volmer analysis of fluorescence titration is 1.5 × 106 M−1. This system is very selective towards LPS as other analytes, including nucleotides, anionic sugars, and ctDNA did not increase
Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- cytosine (C) [2]. Similarly, proteins, like microtubules and actin filaments, are self-sorted on the molecular level in living cells [3]. Furthermore, the smaller molecules of life such as sugars [4], peptides, and fatty acids [5] undergo self-sorting in the construction of a cell [6][7]. The above
A consensus-based and readable extension of Linear Code for Reaction Rules (LiCoRR)
Beilstein J. Org. Chem. 2020, 16, 2645–2662, doi:10.3762/bjoc.16.215

- interoperability; Linear Code monosaccharides may not be sufficient for every project while IUPAC-extended nomenclature [18] is actively maintained to ensure complete coverage of known sugars. If a user wants to specify that they are using LiCoRR with IUPAC monosaccharides, they can specify it as “LiCoRRICE” the
Computational tools for drawing, building and displaying carbohydrates: a visual guide
Beilstein J. Org. Chem. 2020, 16, 2448–2468, doi:10.3762/bjoc.16.199

- a continuous development and evolution of the description of monosaccharides [2]. Glycans are puzzles to many chemists, and biologists as well as bioinformaticians. This complexity occurs at different levels (which makes it incremental). Amongst the most recognisable “sugars”, glucose is merely one
Azo-dimethylaminopyridine-functionalized Ni(II)-porphyrin as a photoswitchable nucleophilic catalyst
Beilstein J. Org. Chem. 2020, 16, 2119–2126, doi:10.3762/bjoc.16.179

- of sugars [6], enzyme mimics [7], and the utilization of intermolecular cooperative effects [8] are further applications of photoswitchable catalysis. Particularly interesting and close to our approach is an early work by Inoue et al. who achieved control of the transformation of CO2 and 1,2
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
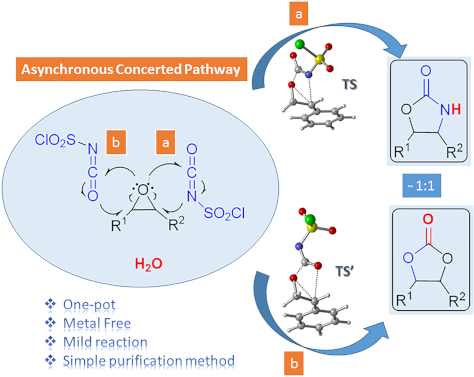
- natural products ranging from small molecules, such as sugars, lipids and amino acids to huge molecules [56]. Computational results A detailed mechanistic investigation of the synthesis of oxazolidinone and five-membered cyclic carbonate derivatives by the reaction between epoxide 7f and CSI has been
One-pot synthesis of isosorbide from cellulose or lignocellulosic biomass: a challenge?
Beilstein J. Org. Chem. 2020, 16, 1713–1721, doi:10.3762/bjoc.16.143

- of isosorbide. Another important factor is the nature of the feedstocks used. Hence, most of the isosorbide syntheses are from pure cellulose and sugars as substrates, such as microcrystalline cellulose and glucose, while studies describing an isosorbide synthesis from lignin-containing cellulosic
Synthesis of new asparagine-based glycopeptides for future scanning tunneling microscopy investigations
Beilstein J. Org. Chem. 2020, 16, 888–894, doi:10.3762/bjoc.16.80

- mechanisms of the interaction of glycopeptides with the cell surface [16]. Our focus lay on the preparation of glycopeptide libraries containing ʟ-asparagine since many saccharides on the cell surface are N-glycosidically linked to this amino acid [8]. The most abundant sugars found in these asparagine
- -linked structures are N-acetylglycosylamines, glucose, galactose, mannose, cellobiose, lactose, and maltose [19]. Therefore, we intended to prepare glycopeptide structures containing these sugars in a stepwise way, up to tripeptides. An orthogonal Fmoc/t-Bu protecting group strategy was chosen along with
Convenient synthesis of the pentasaccharide repeating unit corresponding to the cell wall O-antigen of Escherichia albertii O4
Beilstein J. Org. Chem. 2020, 16, 106–110, doi:10.3762/bjoc.16.12
- of pentasaccharide 1 was achieved using a convergent as well as a block synthetic strategy. For this purpose, a series of suitably functionalized monosaccharide intermediates 2 [20], 3 [21], 4 [22], 5 [23], 6 [24] and 7 [25] were prepared from the commercially available reducing sugars utilizing the
Synthesis of C-glycosyl phosphonate derivatives of 4-amino-4-deoxy-α-ʟ-arabinose
Beilstein J. Org. Chem. 2020, 16, 9–14, doi:10.3762/bjoc.16.2
- ; lipopolysaccharide; Introduction Glycosyltransferases are important enzymes that accomplish the transfer of activated sugar phosphates onto their respective acceptor molecules [1]. In most cases, nucleotide diphosphate sugars serve as the reactive species, but lipid-linked diphosphate derivatives are equally
- ). Conversion of benzylated reducing sugars, such as glucose [15], N-acetyl-ᴅ-glucosamine [16], and galactose [17], into the corresponding C-glycosyl phosponates using a Wittig reaction with methylenetriphenylphosphorane furnished the respective glycoenitols, which were then subjected to mercuriocyclization
SnCl4-catalyzed solvent-free acetolysis of 2,7-anhydrosialic acid derivatives
Beilstein J. Org. Chem. 2019, 15, 2990–2999, doi:10.3762/bjoc.15.295
- have reported one-pot multienzyme synthetic protocols for 2,7-anhydro-Neu5Ac [17][18][19]. Our group has developed one-pot syntheses of several anhydro sugars via microwave (MW)-assisted intramolecular anomeric protection (iMAP) of silylated sugars as well as ring-opening protocols for their 1,6
- Ac2O afforded glycal 13 (Table 1, entries 1–4). None of these conditions were successful for the acetolysis of these 2,7-anhydro derivatives, although they worked for the 1,6-anhydro sugars [29][32][33]. Adding 10 equiv of acetic acid to suppress the 2,3-elimination reaction failed to give the desired
- in the synthesis of inhibitors for N-acetylneuraminidases from different sources [42]. The ring opening of 5 was not trivial since the substrate had a sterically hindered quaternary anomeric center, unlike the tertiary anomeric center C-1 of 1,6-anhydro sugars [29][30][31][32][33][43]. Furthermore
Regioselectivity of glycosylation reactions of galactose acceptors: an experimental and theoretical study
Beilstein J. Org. Chem. 2019, 15, 2982–2989, doi:10.3762/bjoc.15.294

- supported by theoretical studies [10][11]. ᴅ-Galactose (ᴅ-Gal) is one of the most abundant sugars in nature and a component of oligosaccharides and glycoconjugates with relevant functions [12]. Following a methodology previously applied to ᴅ-glucosamine acceptors [8] with some modifications, in the present
A green, economical synthesis of β-ketonitriles and trifunctionalized building blocks from esters and lactones
Beilstein J. Org. Chem. 2019, 15, 2930–2935, doi:10.3762/bjoc.15.287
- example, in sugars, hemiacetal pyranoses (due to reduced bond angle strain) are energetically much more favored in solution compared to the corresponding 5-membered furanoses [17]. Significantly, 1H (500 MHz) and 13C NMR (126 MHz) of the purified product 6 (pure by TLC) indicated traces of what appears to
Bacterial terpene biosynthesis: challenges and opportunities for pathway engineering
Beilstein J. Org. Chem. 2019, 15, 2889–2906, doi:10.3762/bjoc.15.283

- rearrangements, creating the basic hydrocarbon skeleton of a terpene [10][11]. This basic hydrocarbon skeleton is then modified to generate a large number of terpenoid structures, which can be further modified by addition of other building blocks, like sugars, amino acids, or fatty acids [12]. Terpenes are named
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein J. Org. Chem. 2019, 15, 2830–2839, doi:10.3762/bjoc.15.276
- aryl substituent located on the nitrogen atom. Nucleotide pyrophosphatase activity Nucleotide pyrophosphatases belong to the family of ecto-nucleotidases [34][35]. They can hydrolyze nucleotides, dinucleotides, and nucleotide sugars, e.g., ATP, ADP, NAD+, ADP-ribose and diadenosine polyphosphates [36
Chemical synthesis of the pentasaccharide repeating unit of the O-specific polysaccharide from Escherichia coli O132 in the form of its 2-aminoethyl glycoside
Beilstein J. Org. Chem. 2019, 15, 2563–2568, doi:10.3762/bjoc.15.249
- + 2] strategy where the required monosaccharide building blocks are prepared from commercially available sugars through rational protecting group manipulation. The NIS-mediated activation of thioglycosides was used extensively for the glycosylation reactions throughout. Keywords: 2-aminoethyl
Indium-mediated C-allylation of melibiose
Beilstein J. Org. Chem. 2019, 15, 2458–2464, doi:10.3762/bjoc.15.238
- reported for the first time a tin-mediated allylation of unprotected carbohydrates followed by ozonolysis allowing for easy accessibility of the corresponding elongated sugars [2]. In the same year, Chan and Li introduced indium for the allylation of aldehydes and furthermore demonstrated the applicability
Robust perfluorophenylboronic acid-catalyzed stereoselective synthesis of 2,3-unsaturated O-, C-, N- and S-linked glycosides
Beilstein J. Org. Chem. 2019, 15, 1275–1280, doi:10.3762/bjoc.15.125
- δ 6.01 (H2, H3) [19][21]. Using the optimized conditions in Table 1, entry 4, we then examined the substrate scope. Therefore, glucal 1a was reacted with various O-nucleophiles (using primary, secondary, tertiary, allyl, propargyl alcohols and sugars), C-nucleophiles (using trimethylsilyl cyanide
- results obtained using boron trifluoride diethyl etherate [32]. We then applied the perfluorophenylboronic acid catalyst to promote the reaction between 2,3,4,6-tetra-O-acetyl-D-glucal (4a) and O- and S-nucleophiles (Figure 2). The Ferrier-catalyzed rearrangements of 2-substituted sugars such as 2,3,4,6
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- (e.g., biotin) [64]. Importantly, the effective implementation of on-resin Ugi-4CRs paved the way for the subsequent development of multicomponent macrocyclizations permitting the introduction of PEGs, sugars and fluorescent labels at resin-linked peptides [66][67]. More recently, Wessjohann and Rivera
Towards the preparation of synthetic outer membrane vesicle models with micromolar affinity to wheat germ agglutinin using a dialkyl thioglycoside
Beilstein J. Org. Chem. 2019, 15, 937–946, doi:10.3762/bjoc.15.90

- addition to its high efficiency and selectivity, the TEC reaction does not require any metal, a key feature for the preparation of potential vaccines. Results and Discussion Preparation of alkyl glycosides from unprotected sugars and lipophilic scaffolds Thioglycolipids are not native in OMV [1], but



















































































































































































































































































































