Search results
Search for "Molecular recognition" in Full Text gives 168 result(s) in Beilstein Journal of Organic Chemistry.
Novel macrocycles – and old ones doing new tricks
Beilstein J. Org. Chem. 2019, 15, 1838–1839, doi:10.3762/bjoc.15.178

- diversified. They are not only tools for studying molecular recognition and molecular machines, but are also key components for sensing, supramolecular catalysis, (chiral) separation, drug delivery, or smart materials. Many new macrocycles have recently been reported, with pillararenes being one of the most
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- Jing Li Qiang Shi Ying Han Chuan-Feng Chen Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China University of Chinese Academy of Sciences, Beijing 100049, China
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
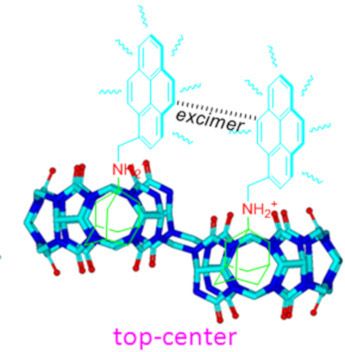
- photophysical signals to identify the possible diastereomers of guests within nor-seco-cucurbit[10]uril (NS-CB[10]) cavities. Further experiments revealed that balancing the hydrophilic and hydrophobic effects of the guest in aqueous solution can improve the molecular recognition and binding ability of NS-CB[10
- ]. Keywords: fluorescent; host–guest interaction; macrocycles; molecular recognition; nor-seco-cucurbit[10]uril; pyrene; Introduction Host–guest interactions that trigger molecular recognition are a current topic of interest. For example, understanding the protein–ligand molecular recognition is of paramount
- , the top–center isomer of G2 within both host-1 and host-2 could be characterized because of the novel monomer and excimer photophysical property of pyrene as fluorophore. As a result, we demonstrated here a novel guest-controlled molecular recognition and stereoisomerism for the first time. Results
Synthesis and conformational preferences of short analogues of antifreeze glycopeptides (AFGP)
Beilstein J. Org. Chem. 2019, 15, 1581–1591, doi:10.3762/bjoc.15.162

- better understanding of the correlation between structure and activity of AFGPs would not only improve the basic knowledge on molecular recognition, but could also help designing new tailored antifreeze agents for many applications, e.g., cooling systems, coating technology, ice-templating, food industry
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- ][22]. Beside the usage of the side chains of the amino acids and the azole rings for molecular recognition, the functional groups of the scaffolds of these cyclopeptides have also been applied as receptors for Y-shaped anions [23] and as ligands for copper(II) complexes [24][25]. Of special interest
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- –guest chemistry; macrocycles; molecular recognition; precious metal chloride complexes; Introduction Macrocyclic receptors are the major workhorses in supramolecular chemistry. Design and synthesis of new macrocyclic receptors with new properties is always attractive but is also challenging [1
- ]. Tetralactam macrocycles with four amide NH residues have been studied for ca. three decades and used in a wide range of fields including molecular recognition, optical sensing, molecular machine etc. [2][3][4][5][6][7][8][9][10][11][12][13]. The amide N−H groups directed into the cavity could form hydrogen
- bonds with guests, constituting the main driving forces for molecular recognition. This class of macrocycles, in particular the ones with single arene as sidewalls, have found wide applications in diverse fields: Leigh and co-workers reported many interlocked structures and molecular machines with
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- Yu-Ying Wang Yong Kong Zhe Zheng Wen-Chao Geng Zi-Yi Zhao Hongwei Sun Dong-Sheng Guo College of Chemistry, Key Laboratory of Functional Polymer Materials, State Key Laboratory of Elemento-Organic Chemistry, Tianjin Key Laboratory of Biosensing and Molecular Recognition, Nankai University, Tianjin
- supramolecular cavities [25]. Calixarenes are the third generation of macrocyclic compounds composed of phenolic units bridged with methylene groups at the o-positions of phenolic hydroxy groups [9]. We have focused on molecular recognition and self-assembly of water-soluble calixarene derivatives for a long
- luminescent materials, high-performance supramolecular dye lasers, and dye-sensitized solar cells. Although in our present study GC5A has served as a specific test case for molecular recognition of various dyes, such an investigation is inspirable for other artificial receptors, especially those of new
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- they bear a chiral cavity that can be used for molecular recognition [19]. Probably the most famous example for this behavior is the chiral discrimination and determination of the absolute configuration of the two enantiomers of CHFClBr using an enantioenriched cryptophane-C [20][21]. Other examples
Host–guest interactions between p-sulfonatocalix[4]arene and p-sulfonatothiacalix[4]arene and group IA, IIA and f-block metal cations: a DFT/SMD study
Beilstein J. Org. Chem. 2019, 15, 1321–1330, doi:10.3762/bjoc.15.131

- .15.131 Abstract The molecular recognition in aqueous solution is extremely important because most biological processes occur in aqueous solution. Water-soluble members of the calix[n]arene family (e.g., p-sulfonato substituted) can serve as model systems for studying the nature and manner of interactions
Enantioselective Diels–Alder reaction of anthracene by chiral tritylium catalysis
Beilstein J. Org. Chem. 2019, 15, 1304–1312, doi:10.3762/bjoc.15.129
- Qichao Zhang Jian Lv Sanzhong Luo Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, 100190, Beijing, China State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science
Robust perfluorophenylboronic acid-catalyzed stereoselective synthesis of 2,3-unsaturated O-, C-, N- and S-linked glycosides
Beilstein J. Org. Chem. 2019, 15, 1275–1280, doi:10.3762/bjoc.15.125
- molecular recognition, cell–cell interaction, immunological recognition and transmission of biological information [4][5][6]. They are easily transformed into important bioactive compounds such as oligosaccharides, glycopeptides, nucleosides, antibiotics, uronic acids and other natural products [1][2][3
Molecular recognition using tetralactam macrocycles with parallel aromatic sidewalls
Beilstein J. Org. Chem. 2019, 15, 1086–1095, doi:10.3762/bjoc.15.105
Fabrication, characterization and adsorption properties of cucurbit[7]uril-functionalized polycaprolactone electrospun nanofibrous membranes
Beilstein J. Org. Chem. 2019, 15, 992–999, doi:10.3762/bjoc.15.97

- ][23][24]. Through hydrophobic effects, ion–dipole interactions, and/or hydrogen bonding, CB[n]s show selective molecular recognition properties towards cationic and neutral guests [25][26]. In the last two decades, CB[n]s have been used for a variety of applications including supramolecular catalysis
Mechanochemistry of supramolecules
Beilstein J. Org. Chem. 2019, 15, 881–900, doi:10.3762/bjoc.15.86
- ]. Supramolecular catalysis The concept of supramolecular catalysis mainly is based on the use of supramolecular chemistry, molecular recognition, host–guest chemistry, etc. for catalysis [96]. The field originated with the understanding of enzymatic system which is conceptually different from traditional organic
Dispersion interactions
Beilstein J. Org. Chem. 2018, 14, 3076–3077, doi:10.3762/bjoc.14.286

- but it is certainly attenuated [8]. We are still in the process of understanding just by how much. LD is a driving force for molecular aggregation that plays a key role in the thermodynamic stability, molecular recognition, chemical selectivity through transition-state stabilization, protein folding
Microwave-assisted synthesis of biologically relevant steroidal 17-exo-pyrazol-5'-ones from a norpregnene precursor by a side-chain elongation/heterocyclization sequence
Beilstein J. Org. Chem. 2018, 14, 2589–2596, doi:10.3762/bjoc.14.236
- , keto–enol tautomerism can be challenging and is of special importance in biological systems, chemical reactivity, and molecular recognition [18]. The tautomeric equilibrium in solution is strongly influenced by the substitution pattern of the heterocyclic ring, the polarity and protic nature of the
Synthesis of a water-soluble 2,2′-biphen[4]arene and its efficient complexation and sensitive fluorescence enhancement towards palmatine and berberine
Beilstein J. Org. Chem. 2018, 14, 2236–2241, doi:10.3762/bjoc.14.198

- = (2.29 ± 0.27) × 106 M−1) is 3.9 times larger than that of P (Ka = (5.87 ± 0.24) × 105 M−1). Keywords: berberine; biphenarenes; host–guest complexes; molecular recognition; palmatine; Introduction Host–guest chemistry in water is significantly important due to its extensive applications in biology
Synthesis and post-functionalization of alternate-linked-meta-para-[2n.1n]thiacyclophanes
Beilstein J. Org. Chem. 2018, 14, 2190–2197, doi:10.3762/bjoc.14.192
- molecular recognition of organic compounds as well as metals. Herein, we report the selective and high-yielding synthesis of novel alternate-linked-meta-para-thiacyclophanes. These novel thiacyclophanes are selectively synthesized in high-yielding procedures. Furthermore, post-functionalization of the
- cyclophanes is formed by thiacyclophanes, in which the thioether linkages impose less conformational strain and which have an increased cavity size compared to other (oxa/aza)cyclophanes. Thiacalix[n]arenes are among the most widely known thiacyclophanes with significant ability for molecular recognition [5
- was further analysed by X-ray diffraction. Results and Discussion Macrocyclization Macrocyclization and post-functionalization of cyclophanes is of high interest for the development of various applications in molecular recognition. Based on our previous one-pot procedure towards homothiacalixarenes
Calix[6]arene-based atropoisomeric pseudo[2]rotaxanes
Beilstein J. Org. Chem. 2018, 14, 2112–2124, doi:10.3762/bjoc.14.186

- bis(benzylalkylammonium) axle [19], where the stereoselective formation of the pseudo[3]rotaxane with endo-alkyl orientation VIII was observed [19]. Calixarene macrocycles [22] have found numerous applications in several areas of supramolecular chemistry, such as (bio)molecular recognition [23] and
Synthesis and supramolecular self-assembly of glutamic acid-based squaramides
Beilstein J. Org. Chem. 2018, 14, 2065–2073, doi:10.3762/bjoc.14.180

- asymmetric catalysis and molecular recognition [5][6]. Besides, squaramides present a dual ability to recognize anions and cations through hydrogen bonding interactions, acting as ion sensors and transmembrane anion transporters [7]. This property has been crucial for the development of new drugs [8][9
Synthesis of new p-tert-butylcalix[4]arene-based polyammonium triazolyl amphiphiles and their binding with nucleoside phosphates
Beilstein J. Org. Chem. 2018, 14, 1980–1993, doi:10.3762/bjoc.14.173

- was elaborated for the naked-eye detection of ADP with a detection limit of 0.5 mM. Keywords: ADP; amphiphile; ATP; calix[4]arene; CuAAC; eosin Y probe; molecular recognition; polydiacetylene; self-assembly; triazole; Introduction During the last two decades many researcher groups have paid much
Diazirine-functionalized mannosides for photoaffinity labeling: trouble with FimH
Beilstein J. Org. Chem. 2018, 14, 1890–1900, doi:10.3762/bjoc.14.163
- X-ray crystallography, studies in solution add valuable information in molecular recognition studies as they take molecular dynamics as well as solvent effects into consideration. In the latter respect, photoaffinity labeling has evolved as a useful tool for studies under physiological conditions [1
Strong binding and fluorescence sensing of bisphosphonates by guanidinium-modified calix[5]arene
Beilstein J. Org. Chem. 2018, 14, 1840–1845, doi:10.3762/bjoc.14.157

- to their facial modification, Prof. Böhmer demonstrated calixarenes as having “(almost) unlimited possibilities” [17]. We have focused on molecular recognition and self-assembly of water-soluble calixarene derivatives for a long time [18][19][20][21][22][23][24], directed by exploring biomedical
Host–guest complexes of conformationally flexible C-hexyl-2-bromoresorcinarene and aromatic N-oxides: solid-state, solution and computational studies
Beilstein J. Org. Chem. 2018, 14, 1723–1733, doi:10.3762/bjoc.14.146

- properties by tuning the structure of the interacting partners. Over the past decade, the N-oxide family has attracted the attention of the H–G community in molecular recognition processes [33][34][35]. In order to tune the resorcinarene-PyNO H–G recognition events at the molecular level, a better
The phenyl vinyl ether–methanol complex: a model system for quantum chemistry benchmarking
Beilstein J. Org. Chem. 2018, 14, 1642–1654, doi:10.3762/bjoc.14.140

- hydrogen bonds; Introduction The balance of different noncovalent interactions is crucial for chemical and biochemical processes as it controls molecular recognition and aggregation [1][2][3][4][5][6]. In order to gain a deeper understanding of these processes, knowledge on exact structural arrangements











































































































































































































