Search results
Search for "lactone" in Full Text gives 288 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
A green, economical synthesis of β-ketonitriles and trifunctionalized building blocks from esters and lactones
Beilstein J. Org. Chem. 2019, 15, 2930–2935, doi:10.3762/bjoc.15.287
- solubility of both the KOt-Bu base and the acetonitrile derived KCH2CN salt. This effectively accelerates the generation of the latter and as such, its reaction with the lactone (or ester) carbonyl carbon. By increasing the solubility of the conjugate base salt, the pKa of acetonitrile in the ethereal
- alkoxide anions. This reduces the occurrence of undesirable side-reactions, e.g., intermolecular aldol reaction, lactone/ketonitrile product dimerization and ring-opening polymerization. The application of this method to produce the analogous hemiketal 6 from γ-butyrolactone was not as efficient. Although
- from lactone to 20% (Scheme 2). Due to the interest in the potential preparation of pyrimidines from β-ketonitriles and guanidine [18], we wanted to test the general applicability of this method for access to a variety of alkyl and aryl-substituted β-ketonitriles (as summarized in Table 1). When we
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- File 1) yielded ten drimane metabolites shown in Figure 1: one trihydroxylated drimane sesquiterpenoid lactone, nanangenine A (1), four drimane lactones bearing C6/C8 acyl chains, nanangenines B, C, D and E (2, 4, 5 and 6), two acylated drimanes bearing isomeric lactones, isonanangenines B and D (3 and
- ), three methyl groups (C-13, C-14, C-15) and three hydroxy groups (1-OH, 6-OH, 9-OH). Detailed analysis of the 2D NMR data for 1 (Table S3 in Supporting Information File 1) confirmed the presence of a drimane sesquiterpenoid lactone scaffold. A search of the literature revealed 1 to be almost identical to
- revealed the only difference to be the position of the lactone carbonyl group, which was determined to be at C-12 instead of C-11 based on key HBMC correlations from H-7 to C-12 and 9-OH to C-11. Therefore, the structure of 3 was assigned as shown in Figure 1. Compound 3 was previously reported in 2013 as
Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase
Beilstein J. Org. Chem. 2019, 15, 2590–2602, doi:10.3762/bjoc.15.252
- ]. Oxidation, iodocarboxylation and elimination yielded the lactone 43. A series of functional group manipulations provided enone 44, which underwent a cuprate-mediated Michael addition and liberation of the aldehyde 46 upon ozonolysis. After intramolecular aldol condensation the resulting enone 47 was
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- was based on the conservation of the 16-membered macrocyclic scaffold and the apolar tripeptidyl moiety found in the solonamides. Both features are important to guarantee the interference with S. aureus QS [12][13][14][15]. The ester linkage of the lactone core was substituted by the sulfide group
- File 1). Three main absorption bands could be readily observed around 3280, 1650 and 1520 cm−1. The first one was assigned to the stretch for N–H bonds of the peptide linkage. The stretch for the lactam and lactone C=O bonds gives rise to the broad absorption close to 1650 cm−1. The lowering on the
- wavenumber values for the lactone C=O stretch was also observed for bands assigned to the C=C bonds as consequence of their conjugation. Evaluation of the growth inhibition and hemolytic activity of S. aureus for the solonamide analogues Initially, the antibacterial activity of all analogues 9 was tested by
Sugar-derived oxazolone pseudotetrapeptide as γ-turn inducer and anion-selective transporter
Beilstein J. Org. Chem. 2019, 15, 2419–2427, doi:10.3762/bjoc.15.234

- showed a broad band at 3444–3421 cm−1 indicating the presence of -NHs of amine/amide functionalities. The bands at 1740 and 1688 cm−1 were assigned to the lactone carbonyl and amide (as well as imine) groups, respectively. In the 1H NMR spectrum, the downfield signals at δ 9.03 and 8.52 ppm were assigned
- to the amide NH(I) and NH(II), respectively. The signal at δ 1.80 ppm, integrating for two protons, was assigned to the presence of an NH2 functionality. In the 13C NMR spectrum, the appearance of signals at δ 170.8, 170.6 and 166.7 ppm were assigned to the lactone/amide carbonyl functionalities. The
- δ 2.0 ppm, integrating for three protons, was assigned to the NHCOCH3. In the 13C NMR spectrum, the appearance of five signals in the downfield region (at δ 171.6, 170.9, 167.5, 165.0, and 164.0 ppm) indicated the presence of three amides, lactone carbonyl and imine carbon (-C=N) suggesting the
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- (3b’ and 6b’) and anti-aromatic (4a’ and 5a’) ring currents. Reaction of 6,6-dimethylpentafulvene with singlet state oxygen to form an enol lactone via the multistep rearrangement proposed by Harada et al. (supporting information was not provided) [51]. Photosensitized oxygenation of 8
Characterization of two new degradation products of atorvastatin calcium formed upon treatment with strong acids
Beilstein J. Org. Chem. 2019, 15, 2085–2091, doi:10.3762/bjoc.15.206

- /dehydration process in the side chain), treatment with conc. aqueous hydrochloric acid gave a complex, bridged molecule under C–C-bond formation of the lactone moiety with the pyrrole, migration of the isopropyl group and loss of the carboxanilide residue. The novel degradation products were characterized by
- process (stereoisomers, products resulting from impure starting materials or side reactions), and only one of these impurities, lactone 2, is most likely a degradation product, resulting from acid-mediated lactonization of the 3,5-dihydroxyheptanoate side chain. A couple of previous publications deal with
- -dihydroxyheptanoate side chain under moderately acidic conditions (0.1 M HCl) [11][12][13][14]. Shah et al. [15] identified six additional decomposition products upon treatment with 0.1 M HCl at 80 °C for 24 h, among which the dehydrated lactone 3 was dominating, accompanied by minor amounts of products arising from
Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
Beilstein J. Org. Chem. 2019, 15, 2020–2028, doi:10.3762/bjoc.15.198
- chromatographed repeatedly with silica gel and RP-HPLC to afford four new sativene-type sesquiterpenoids, bipolenins K–N (1–4), along with eight previously reported compounds (5–12), which were identified as sativene-type sesquiterpenoids prehelminthosporol lactone (5) [1], helminthosporic acid (6) [1
- , 1.88, 2.65 and 3.77), which are typical resonances for sativene-type sesquiterpenoids. The NMR data for 1 were very similar to those for prehelminthosporol lactone (5) except for the replacement of a methine group (δC 32.1; δH 1.42) at C-9 in 5 with a hydroxylated quaternary carbon (δC 73.1) in 1. This
- with reduction of the lactone ring to the dialcohol and dihydroxylation of the Δ2,12 double bond. Detailed analysis of the 2D NMR data for 4 (Figure 2) confirmed the seco-sativene-type scaffold. The relative configurations at C-1, C-3, C-6, C-7 and C-13 were determined to be the same as those of 1–3
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- well as the H-22 oxymethine proton of the lactone moiety. Surprisingly, both compounds showed only two putative methyl signals, and no signal which might correspond to H-22. Thus, we reasoned that both unknown compounds might be truncated withanolide-like compounds. HRESIMS suggested a sum formula of
- ). Irinans represent highly unusual withanolide derivatives, as they lack the side-chain lactone ring that is a common structural feature of virtually all known withanolides [3], but possess an androstane backbone instead. While androstanes such as androsterone (7) are well-known human sex hormones (Figure
- without the lactone side chain. We therefore propose that the side-chain cleavage enzyme in withanolide biosynthesis acts at a late stage, using common pathway end products such as 4ß-hydroxywithanolide E (1) as its substrates. Two mechanisms are conceivable for this transformation (Figure 3C): A non
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- 88. Ester exchange of 88 with (+)-8-phenylmenthol gave the key cyclization precursor 16. Oxidative radical cyclization of 16 in the presence of Mn(OAc)3 and Yb(OTf)3·H2O afforded the major tricyclic diastereomer 89 (dr = 38:1). Then the construction of the unsaturated lactone was performed by
- conversion of 89 to vinyl triflate 90, followed by reduction and palladium-catalyzed carbonylation–lactone formation to give key intermediate 8 [75][76][77]. After that, the three successive epoxides were installed by known procedures with some modification. The highlights were the introduction of the second
- and its relatives from commercially available acid 32 (Figure 2, route J and Scheme 8) [46]. This synthesis highlights the utilization of an indium(III)-catalyzed cationic polycyclization of 17 and a palladium-catalyzed carbonylation–in situ lactone formation to construct the key intermediate 94
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
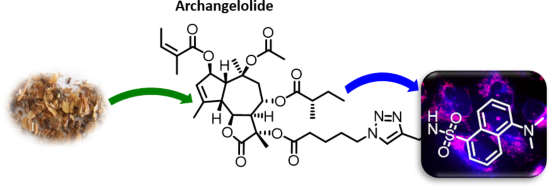
- biological effects. In plants, they are synthesized, among others, for pesticidal and antimicrobial effects. Two such compounds, archangelolide and trilobolide of the guaianolide type, are structurally similar to the well-known and clinically tested lactone thapsigargin. While trilobolide has already been
- fluorescent conjugate; sarco/endoplasmic reticulum calcium ATPase; sesquiterpene lactone; trilobolide analogue; Introduction Sesquiterpene lactones (SLs) have been attracting interest already for some time due to the plethora of biological effects they elicit. Various SLs show anticancer, antimicrobial
- takes place remarkably differs from that in compound 2 and thapsigargin. This is very likely due to stereochemistry on the lactone ring connection at C7 and the absence of a hydroxy group at the same position. The steric circumstances and the presence of the α-carbonyl group makes the tertiary C11
Application of chiral 2-isoxazoline for the synthesis of syn-1,3-diol analogs
Beilstein J. Org. Chem. 2019, 15, 1840–1847, doi:10.3762/bjoc.15.179
- broad spectrum of natural products [1][2]. (3R)-β-Hydroxy-δ-lactone or its open-ring equivalent (3R)-syn-3,5-dihydroxypentanoic acid, is a common structure in naturally occurring mevastatin (or compactin), lovastatin or closely related statins, and synthetic statins. Either the syn or anti-1,3-diol
- chiral β-hydroxy-δ-lactone moiety or its equivalents, pioneered by Wong [42], is equally competitive. Here, we report the preparations of tert-butyl (3S,5R)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate and related syn-1,3-diol analogs from a chiral 2-isoxazoline (Scheme 1b). This work is part of
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- transition state was involved. Acid-induced aziridine ring openings and subsequent conjugate additions to the α,β-unsaturated lactone led to the formation of cis-fused [5,5']bicyclic compounds 186a or 186b. Reduction of the lactone moiety in 186a and subsequent deprotection gave (2S,3R,4S,5R)-184. In order
- to synthesize the natural (2S,3R,4R,5R)-184 the lactone 186b was subjected to Mitsunobu reaction followed by the ester reduction and the hydrogenolytic cleavage of the 1-phenylethyl group. Piperidines: A cis-disubstituted piperidine scaffold was identified in several piperidine alkaloids of diverse
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- paraformaldehyde and benzyl isocyanide to form hybrid 11 in good yield. Similarly, the spirostanic lactone 12 was transformed into spirostanic acid 13 and amine 14, which were next ligated to alanine methyl ester and Boc-histidine leading to the amino acid–steroid conjugates 15 and 16, respectively. This approach
Mechanochemical synthesis of poly(trimethylene carbonate)s: an example of rate acceleration
Beilstein J. Org. Chem. 2019, 15, 963–970, doi:10.3762/bjoc.15.93
- biomedical applications [24]. The amidine base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) is one of the best studied and most popular organocatalysts for ring-opening polymerizations of cyclic carbonates and lactones [25][26][27]. In contrast to the high activity of lactone polymerization, cyclic carbonate
Synthesis and biological investigation of (+)-3-hydroxymethylartemisinin
Beilstein J. Org. Chem. 2019, 15, 567–570, doi:10.3762/bjoc.15.51
- sesquiterpene lactone is still unknown [5]. Whereas artemisinins are almost non-toxic to normal cells, several studies have confirmed their potent antitumor activity [6][7]. In addition, they have been reported to possess immunosuppressive, anti-inflammatory, antiviral, antifungal and antiparasitic activities
- [8][9][10]. Recently, it was shown that artemisinin interacts with the mammalian protein gephyrin and by stabilizing it, it enhances GABAA receptor signaling resulting in in vivo conversion of pancreatic α-cells into functional β-like cells [11]. Therefore, this sesquiterpene lactone may also find an
- 24% and 16% yield, respectively. Protection of the free hydroxy group of 16 as a silyl ether and methylation of the obtained lactone in α-position (LDA/MeI/HMPA) afforded derivative 17. After removal of the TES protecting group (+)-3-hydroxymethyl-9-epi-artemisinin (18, Scheme 3) was obtained
A chemoenzymatic synthesis of ceramide trafficking inhibitor HPA-12
Beilstein J. Org. Chem. 2019, 15, 490–496, doi:10.3762/bjoc.15.42
- reaction [20], tandem approach from (S)-Wynberg lactone [21], chiral ruthenium-catalyzed N-demethylative rearrangement of 1,2-isoxazolidines [22], gold(I)-catalyzed cyclization of a propargylic N-hydroxylamine [23], from β-sulfinamido ketones derived from chiral sulfinimines [24], and a Kornblum–DeLaMare
Reusable and highly enantioselective water-soluble Ru(II)-Amm-Pheox catalyst for intramolecular cyclopropanation of diazo compounds
Beilstein J. Org. Chem. 2019, 15, 357–363, doi:10.3762/bjoc.15.31
- nature and have excellent biological activity, including strong antibiotic, antihelmetic, antifungal, antitumor, antiviral and anti-inflammatory, which make them interesting lead structures for new drugs [34]. That is why a great deal of attention has been paid to the synthesis of the lactone ring [35
- diazoacetate could be cyclopropanated affording the corresponding lactone with low yield and good enantioselectivity (Table 1, entry 1). In case of the diazo compound derived from cinnamyl diazoacetate the corresponding lactone was obtained in high yield with high enantioselectivity (Table 1, entry 2). A
Synthesis of nonracemic hydroxyglutamic acids
Beilstein J. Org. Chem. 2019, 15, 236–255, doi:10.3762/bjoc.15.22

- intramolecular lactonization to form 83 by implementation of the Mitsunobu reaction. After opening of the lactone ring with trichloroethanol and silylation of the hydroxy group oxidation at C5 was performed in the usual way to give a pyroglutamate 84. Benzyl or p-methoxybenzyl esters 85a or 85b were next
- decomposition of this stereoisomer including racemization at Cα. From pentose via 2,3-aziridino-γ-lactone In the so called “2,3-aziridino-γ-lactone methodology” [18][99][100] ribose (or lyxose) is used as a starting material [101][102] which is transformed into the lactone 95 in several steps [99]. Boron
- protected 3,4-dihydroxy-L-glutamic acid 96 and the respective γ-lactone 97 formed from 96 in the presence of acids. Benzyl alcohol was selected to refrain from decomposition of the final amino acids during the acid hydrolysis of, e.g., methyl esters [99] since for a mixture of 96 and 97 hydrogenolysis
Unexpected loss of stereoselectivity in glycosylation reactions during the synthesis of chondroitin sulfate oligosaccharides
Beilstein J. Org. Chem. 2019, 15, 137–144, doi:10.3762/bjoc.15.14
- )-protected disaccharides 1 and 2 would be prepared from known building blocks 3 [35] and 4 [36] using D-glucurono-6,3-lactone and D-galactosamine hydrochloride as starting materials, respectively. The N-TFA group [37][38][39][40][41] is an adequeate choice for 2-amino protection of GalNAc moieties because it
Volatiles from the hypoxylaceous fungi Hypoxylon griseobrunneum and Hypoxylon macrocarpum
Beilstein J. Org. Chem. 2018, 14, 2974–2990, doi:10.3762/bjoc.14.277
- volatiles seem to be involved in the plant pathogenicity of fungi, as recently observed for 3,4-dimethylpentan-4-olide (4), a volatile from the ash pathogen Hymenoscyphus fraxineus that currently threatens the European ash population [10]. Both enantiomers of this lactone were found to inhibit ash seed
N-Acylated amino acid methyl esters from marine Roseobacter group bacteria
Beilstein J. Org. Chem. 2018, 14, 2964–2973, doi:10.3762/bjoc.14.276
- into the analytical window of the method. A wide variety of AHLs, e.g., widespread (Z)-N-(tetradec-7-enoyl)homoserine lactone (1, Z7-C-14:1-AHL, Figure 1), have been identified in roseobacters by these methods [19][20][21][22]. A related group of compounds occurring in Roseovarius only, are N
An overview on recent advances in the synthesis of sulfonated organic materials, sulfonated silica materials, and sulfonated carbon materials and their catalytic applications in chemical processes
Beilstein J. Org. Chem. 2018, 14, 2745–2770, doi:10.3762/bjoc.14.253
- and 37 from active carbonyl compounds (e.g., isatins 32, acenaphthoquinone (33), and aldehydes 38), a variety of C–H activated acids (cyclohexane-1,3-dione (20a), 5,5-dimethylcyclohexane-1,3-dione (20b), 2-naphthol (23), ethyl acetoacetate (34a), 4-hydroxycoumarin (34b), triacetic acid lactone (34c
Ring-opening metathesis of some strained bicyclic systems; stereocontrolled access to diolefinated saturated heterocycles with multiple stereogenic centers
Beilstein J. Org. Chem. 2018, 14, 2698–2707, doi:10.3762/bjoc.14.247
- . Starting from various cyclodienes, the corresponding derived bicyclic lactone, lactam, and isoxazoline derivatives were submitted to ROM under ethenolysis. These functionalized, strained bicyclic systems afforded novel highly-functionalized diolefinated heterocyclic scaffolds in ROM reactions with
- functionalized derivatives such as bicyclic lactones, γ-lactams or isoxazolines, derived from various cyclodienes and to evaluate their chemical behavior under Ru-catalyzed ring-opening conditions (Scheme 2). First, the ring opening of racemic bicyclic γ-lactone (±)-3 (derived from cyclodiene 1 via β-lactam
- -membered ring system thus possessing ring strain, did not afford any ROM products. Interestingly, lactone (±)-3 in the presence of second generation catalysts (G-2 and HG-2) provided the corresponding ring-opened compound (±)-5 albeit with modest yields (Scheme 3, Table 1). In the presence of G-2 and HG-2
Non-native autoinducer analogs capable of modulating the SdiA quorum sensing receptor in Salmonella enterica serovar Typhimurium
Beilstein J. Org. Chem. 2018, 14, 2651–2664, doi:10.3762/bjoc.14.243

- , host colonization, and biofilm formation at high population densities. This cell–cell signaling process is regulated by N-acyl L-homoserine lactone (AHL) signals, or autoinducers, and LuxR-type receptors in Gram-negative bacteria. SdiA is an orphan LuxR-type receptor found in Escherichia, Salmonella
- and antagonism. Keywords: N-acyl L-homoserine lactone; LuxR-type receptor; Quorum sensing; Salmonella enterica serovar Typhimurium; SdiA; Introduction In the fight against bacterial infections, microbes have a decisive advantage over the medical community: evolution [1]. Using bacteriocidal or
- to coordinate group beneficial behaviors such as virulence factor production, host colonization, and biofilm formation at high population densities [12]. Gram-negative bacteria typically use N-acyl L-homoserine lactone (AHL) signals for QS, which are produced by LuxI-type synthases and sensed by























































































































































































































































































































































