Search results
Search for "hydrogen bonding" in Full Text gives 455 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Heterogeneous photocatalysis in flow chemical reactors
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125

- via physisorption. However, functionalised molecules and ions can strongly bind through chemisorption by covalent bonds, hydrogen bonding, or electrostatic attraction and have been shown to influence the photophysical properties of TiO2, generally causing a bathochromic shift of the absorption
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- hydrogen bonding, which was found for these and the other cycloaddends between a proton of the benzene ring of the diene and an oxygen atom of the dienophile (Table 7), also stabilize the endo TS. Therefore, this theoretical study indicated that the non-covalent π···π and C–H···π interactions control the
Synthesis, antiinflammatory activity, and molecular docking studies of bisphosphonic esters as potential MMP-8 and MMP-9 inhibitors
Beilstein J. Org. Chem. 2020, 16, 1277–1287, doi:10.3762/bjoc.16.108
- ligand to the zinc ion, with the same applying to 5. This could explain why no predicted hydrogen bonding was present in 6 and just very weak ones in 5. For MMP-9, the hydrophobic zones were formed by Ile159, Leu160, and Ala161, as was the case for Gly158 and Val194. As stated before, π–π interactions
Aldehydes as powerful initiators for photochemical transformations
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- then kept constant or slightly decreased. The possible initiation of the polymerization is presented in Scheme 11, where hydrogen bonding of 36 with water is crucial. In 2013, Wang and co-workers also reported the use of aliphatic ketones and aldehydes as photoinitiators for the photopolymerization of
- when they were alone present in an aqueous solution, they tended to form hydrates 45 with water via hydrogen bonding. The hydrogen bonding increased the energy levels of the excited states of 45, permitting the formation of radicals after UV light absorption [48]. These radicals either dissociated via
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- evidenced the hydrogen-bonding network of amphidinolide N (6a, Scheme 10). Furthermore, a rigorous evaluation of the 13C NMR chemical shift differences suggested that amphidinolide N and its analog, carbenolide I, are identical chemical compounds [72]. In the course of their comprehensive studies on the
Exploring the scope of DBU-promoted amidations of 7-methoxycarbonylpterin
Beilstein J. Org. Chem. 2020, 16, 509–514, doi:10.3762/bjoc.16.46
- attractive scaffold in the field of supramolecular chemistry, due to their well-defined donor/acceptor hydrogen-bonding arrays [10][11]. One limitation often encountered with the synthesis of structurally diverse pterins is the notorious insolubility in most solvents. This can be dealt with by preemptive
- (product 5). For β-hydroxyamines it is expected the hydroxy group expedites the amidation via hydrogen bonding to the 7-CMP carbonyl group, to assist in bringing the amine nucleophile into place (Figure 2). Quite unsurprisingly, secondary amines were less reactive, with N-methylbenzylamine (for 16) being
- insolubility of pterins in most solvents. We have shown that this reaction also benefits in its ease and often rapid reaction times (typically 5–10 min). While this amidation reaction can be hindered by typical steric effects, we have shown these issues are largely overcome in amines with additional hydrogen
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- cm−1, characteristic of the amide moiety. This low carbonyl absorption probably indicates an intramolecular hydrogen bonding between NH2 and the oxime oxygen, which further verifies the Z-conformation of the amidoxime derivatives. The hydroxylimino structure is verified in 1H NMR spectra from the
Synthesis of 4-(2-fluorophenyl)-7-methoxycoumarin: experimental and computational evidence for intramolecular and intermolecular C–F···H–C bonds
Beilstein J. Org. Chem. 2020, 16, 190–199, doi:10.3762/bjoc.16.22
- amongst the weakest of hydrogen bonding phenomena because a carbon acid (C–H) is weak, therefore is a weak donor, and the acceptor is non-polarizable, therefore is a poor acceptor [34][35][36]. Wang and co-workers reported the existence of the C–F···H–C intramolecular hydrogen bond in the structure of
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
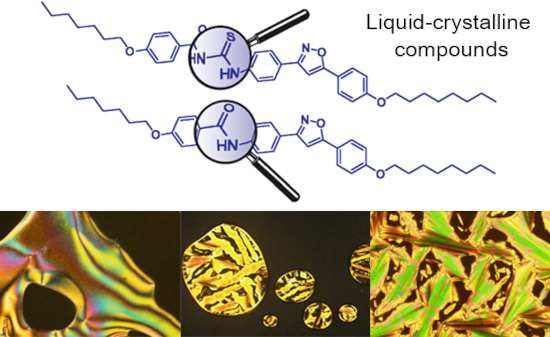
- thioureas is the reaction of carbon disulfide with either one or two different amines [9]. Due to their self-assembly and self-organization through intermolecular hydrogen bonding, thioureas display interesting technological applications to this group of molecules, one of which explores its application in
- crystals containing hydrogen bonds may exhibit a wide variety of phase polymorphism depending on the length of the chain, type of bonding and the functional groups involved [12][28][29][30]. Many liquid crystalline compounds have been developed exploring the ability of hydrogen bonding formation between
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
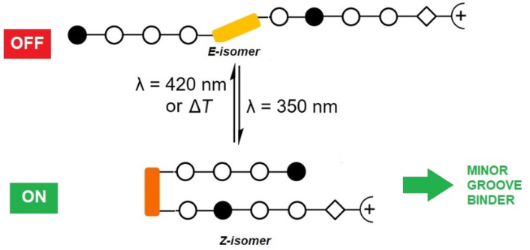
- 4,4'-substituted correlates, as the substituents are not conjugated to the N=N double bond [35][37][38][39]. Moreover, it was demonstrated that in the case of peptides, only the Z-isomer adopts the hairpin conformation. In our setup, this could influence the hydrogen bonding contacts between the
- required because this allowed for an alignment between hydrogen-bonding groups in long polyamides and in the minor groove of DNA [41]. The Fmoc-protected heterocyclic amino acids 2 were obtained from N-methylpyrrole and N-methylimidazole, respectively (Scheme 2A). The N-terminal N-methylpyrrole and N
SnCl4-catalyzed solvent-free acetolysis of 2,7-anhydrosialic acid derivatives
Beilstein J. Org. Chem. 2019, 15, 2990–2999, doi:10.3762/bjoc.15.295
- 2,7-anhydro backbone formation, which resulted in alleviation of hydrogen bonding, and thus enhancing the reactivity of the OH-4 group as an acceptor (Figure 1) [6]. Several chemical and enzymatic syntheses of 2,7-anhydro-Neu5Ac are currently being developed [6][25][26][27][28]. However, only few
- clearly showed that the NHAc group in position C-5 and the OH-4 group were oriented in a trans-diaxial configuration (Figure 2a). This enhanced the nucleophilicity of the OH-4 group due to the absence of hydrogen bonding between these moieties. After confirming the structure of 4 by single crystal X-ray
Regioselectivity of glycosylation reactions of galactose acceptors: an experimental and theoretical study
Beilstein J. Org. Chem. 2019, 15, 2982–2989, doi:10.3762/bjoc.15.294

- accurate prediction of the trends in selectivity could not be achieved. We have tried to explain the reduced regioselectivity of the β-anomers through hydrogen bonding interactions of the OH-3 and OH-4 groups of the model acceptors. Doutheau and co-workers proposed that such a reduced regioselectivity
Why do thioureas and squaramides slow down the Ireland–Claisen rearrangement?
Beilstein J. Org. Chem. 2019, 15, 2948–2957, doi:10.3762/bjoc.15.290
- states are only slightly more negative than in the starting acetal silyl ketenes. It means that transition states have only slightly dipolar character, which would be difficult to stabilize through hydrogen bonding and consequently reaction less prone to catalysis. Interestingly, the reaction comprising
- squaramide catalyst, these energies were similar (Table 6). These calculations further support the notion that hydrogen-bonding organocatalysts bind stronger to starting silyl ketene acetals than to the transition structure and thus are not efficiently catalyzing the Ireland–Claisen rearrangement. The reason
- for this difference, however, remains unclear. The explanation may be connected to a more compact transition state of sigmatropic rearrangements than their starting materials, which is thus less amenable to additional stabilization via hydrogen-bonding catalysts. To get further insight, we have
Influence of the cis/trans configuration on the supramolecular aggregation of aryltriazoles
Beilstein J. Org. Chem. 2019, 15, 2881–2888, doi:10.3762/bjoc.15.282
- stacking interactions, in addition to hydrogen bonding, with the aromatic rings adopting a high degree of parallelism, as seen in crystal packings and ECD data. Furthermore, π–bromine interactions between the bromine atom of the aryl substituents and the triazole units might also contribute to an overall
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein J. Org. Chem. 2019, 15, 2830–2839, doi:10.3762/bjoc.15.276
- involved in hydrogen bonding were His380, Asn277, Ser378, Ser381, Tyr382, Lys391, Ser387 and Ser386. In addition, the study also showed binding with the zinc ion and π-interactions, in particular, π–π T-shaped and amide–π shaped coupling with Ser377 and Tyr382. The molecular docking study of compound 5c
- exhibited π–π T-shaped interactions connecting the indole and furan rings with Tyr340. However, π–alkyl linkage was observed between the benzofuran moiety of compound 5c and amino acid Lys295. The fluorine atom of the 4-fluorophenyl group was involved in the binding with the zinc ion and Ser377. Hydrogen
- bonding was found between the oxygen of the benzofuran ring and the hydrogens of Lys291. When compound 6a was docked inside the active pocket of the homology model, it represented π–π stacked and π–alkyl attachment of the indole rings on both sides of the molecule with amino acids His380 and Lys295
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein J. Org. Chem. 2019, 15, 2664–2670, doi:10.3762/bjoc.15.259
- distorted half-chair. Presumably, this difference is due to the formation of an intramolecular hydrogen bond O6–H…O5 (H…O 1.95(2) Å, O–H…O 154(2)°) in molecule 6. In the crystal of 1 the amino group participates in intermolecular hydrogen bonding N1–H…O3 (H…O 2.39(2) Å, N–H…O 167(1)°) forming chains of
Anion-driven encapsulation of cationic guests inside pyridine[4]arene dimers
Beilstein J. Org. Chem. 2019, 15, 2486–2492, doi:10.3762/bjoc.15.241

- synthesis of pyridine[4]arenes dates back to 2001 [4], their host–guest chemistry is still under-explored. Both macrocycles are concave and are known to form capsular assemblies via intermolecular hydrogen bonding [5][6]. Pyridine is significantly less electron-rich than benzene. Consequently, pyridinearene
- simultaneous complexation of anionic and cationic guests, resulting in a partial rupture of the hydrogen-bonding seam and a ≈100 kJ/mol weaker interaction energy compared to [12 + Me4Nendo + Iexo] (Figure S7 and Table S2, Supporting Information File 1). To illustrate the unsuitability of the lower rim for
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- the competition between the intramolecular hydrogen bond between the N–H and C=O groups in the Z-form and hydrogen bonds formed by these functional groups with solvent molecules. The conformational distortion and the increased conformational disorder arising from hydrogen bonding with the solvent can
Sugar-derived oxazolone pseudotetrapeptide as γ-turn inducer and anion-selective transporter
Beilstein J. Org. Chem. 2019, 15, 2419–2427, doi:10.3762/bjoc.15.234

- the oxazolone pseudo-peptide showed intramolecular C=O···HN(II) hydrogen bonding in a seven-membered ring leading to a γ-turn conformation. This fact was supported by a solution-state NMR and molecular modeling studies. The oxazolone pseudotetrapeptide was found to be a better Cl−-selective
- (TSFAA)-derived homologated linear pentapeptide which showed a well defined intramolecular hydrogen-bonding-stabilized helical array [9][10][11]. Our group has reported a trans-vicinal ᴅ-glucofuranoroic-3,4-diacid with a TAA framework and incorporated it into the N-terminal tetrapeptide sequence (H-Phe
- , we obtained an oxazolone ring containing pseudo peptides 1 and 2a, respectively (Figure 1) The NMR studies of pseudotetrapeptide 2a indicated a γ-turn conformation stabilized by the intramolecular hydrogen bonding [(II)NH···O=C] in a seven-membered ring. The oxazolone pseudotetrapeptide 2a
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- substantially than did a bulky one. The effects contrary to those reported in conventional MV systems were explained by zwitterionization through hydrogen bonding between the urea bridge and the counteranions, increasing the electronic interactions between two triarylamino units. Furthermore, we clarified the
- intervalence charge transfer characteristics of the zwitterionic MV state. Keywords: anion binding; electrochemistry; hydrogen bonding; triarylamine; urea; zwitterionic mixed valency; Introduction Mixed-valence (MV) compounds have received increasing attention from the viewpoint of fundamental research on
- 2.75 Å), primarily reflecting the absence of packing in the former. In the optimized structure of 1a+–PF6−, the N–H···F hydrogen-bonding was comparable to that in 1b+–PF6− with regard to the atomic geometry and the N···F distances. The increased acidity of 1 upon one-electron-oxidation enhances the
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- receptors for molecular recognition of anionic species, pH sensors, mechanically interlocked molecules, molecular machines, and molecular reactors. Keywords: anion recognition; catenane; chalcogen bonding; click reaction; molecular reactor; hydrogen bonding; pH sensor; rotaxane; supramolecular; 1,2,3
- interactions such as hydrogen bonding, π–π stacking, electrostatic interactions, van der Waals forces, hydrophobic/solvatophobic effects and coordination bonds [2][3]. Advances in supramolecules from molecular to macroscopic size with pre-structured or functionalized receptors and multivalent binding positions
- results in the sensing of anions. In fact, strictly speaking this interaction is not a hydrogen bonding interaction seen as there is no X···H–Y unit (X, Y = O, N, F) but these interactions are often referred to as such in the literature. The anion binding can be enhanced by the alkylation of 1,2,3
Synthesis of 1-azaspiro[4.4]nonan-1-oxyls via intramolecular 1,3-dipolar cycloaddition
Beilstein J. Org. Chem. 2019, 15, 2036–2042, doi:10.3762/bjoc.15.200
- reduction among known nitroxides [25]. In contrast, structures 12a–c are asymmetric, and corresponding hydroxylamines could be additionally stabilised by hydrogen bonding between the nitrogen atom and the proton of the hydroxymethyl group. Conclusion In this study, we again demonstrated feasibility of the
Tautomerism as primary signaling mechanism in metal sensing: the case of amide group
Beilstein J. Org. Chem. 2019, 15, 1898–1906, doi:10.3762/bjoc.15.185

- been expected that the intramolecular hydrogen bonding (between the tautomeric hydroxy group and the nitrogen atom from the amide group) could stabilize the pure enol form in some solvents, the keto tautomer is also observed. This is a result from the formation of intramolecular associates in some
- intramolecular hydrogen bonding with the ionophore [7][8] is shown in Scheme 1. The complex formation ejects the tautomeric proton and stabilizes the keto tautomer. Several successful tautomeric ligands, based on 4-(phenyldiazenyl)naphthalen-1-ol (1) [8] (2 and 3, Scheme 2) as a tautomeric unit have been
- developed by us. We found that compounds 2 and 3 exist in the neutral state solely as enol tautomers due to intramolecular hydrogen bonding involving the tautomeric hydroxy group and that the complexation shifts the equilibrium to the K form. Although 3 exhibits a 3D structure and as a result, shows high
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- derivatives for the additional multiple hydrogen bonding interactions between the hosts and the guests, which were evidenced by 1H NMR titrations, X-ray crystal structures and DFT calculations. Moreover, it was also found that the association constants (Ka) of the complexes could be significantly enhanced
- by the additional multiple hydrogen-bonding interactions between the hosts and the guests, which were evidenced by 1H NMR titration, X-ray crystal structures and DFT calculations. Moreover, we also found that the Ka values of the complexes could be significantly enhanced with larger counteranions of
- upfield shifts, possibly due to hydrogen bonding between the hydrogen of the bipyridinium unit of G4 and the oxygen atoms of the host. Similarly, the complexation between other helic[6]arene derivatives (H2, H3, H5) with guests G3 and G4 could also be observed (Supporting Information File 1, Figures S15
Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding
Beilstein J. Org. Chem. 2019, 15, 1592–1600, doi:10.3762/bjoc.15.163

- an anchor for the subsequently formed hydrogen-bonded water clusters inside the host cavity. The attraction and crowding of water molecules at the “hot spot” narrow belt is not unexpected in view of the higher electron density concentrated in this pre-organized by hydrogen bonding location (Figure 4




































































































































































































































































































































