Search results
Search for "macrocycle" in Full Text gives 174 result(s) in Beilstein Journal of Organic Chemistry.
Synthesis of a novel aminobenzene-containing hemicucurbituril and its fluorescence spectral properties with ions
Beilstein J. Org. Chem. 2021, 17, 2840–2847, doi:10.3762/bjoc.17.195

- hemicucurbituril-based macrocycle, alternately consisting of amidobenzene and 2-imidazolidione moieties was designed and synthesized. Based on the fragment coupling strategy, nitrobenzene-containing hemicucurbituril was firstly prepared facilely under alkaline environment, and reduction of the nitro groups
- produced the desired amidobenzene-containing hemicucurbituril. As an original fluorescent chemosensor, it exhibited strong interactions with Fe3+ over other metal cations. The experimental evidence of fluorescence spectra suggested that a 1:1 complex was formed between this macrocycle and Fe3+ with an
- association constant up to (2.1 ± 0.3) × 104 M−1. Meanwhile, this macrocycle showed no obvious or only slight enhancement of the fluorescence intensity with selected anions. Keywords: amidobenzene-containing macrocycle; hemicucurbituril; host–guest interaction; macrocycle; modification; Introduction
Halides as versatile anions in asymmetric anion-binding organocatalysis
Beilstein J. Org. Chem. 2021, 17, 2270–2286, doi:10.3762/bjoc.17.145
- macrocycle 81 (Scheme 17a) [78]. Compared to bis-thiourea 80, the higher rigidity in the macrocycle 81 not only enforces halide abstraction significantly, but also allowed for a better control of the stereoselectivity in the glycosylation of glycosyl halides 78 with a variety of coupling partners. In this
A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries
Beilstein J. Org. Chem. 2021, 17, 1181–1312, doi:10.3762/bjoc.17.90
Structural effects of meso-halogenation on porphyrins
Beilstein J. Org. Chem. 2021, 17, 1149–1170, doi:10.3762/bjoc.17.88

- -contained structural discussion [24]. However, with all the advances made in the crystal engineering of planar porphyrins, investigation through exploration into the effect of meso-halogenation on the macrocycle architecture have, to date, not been carried out. Herein we present a comparative analysis of
- porphyrin conformation are observed due to the metal center insertion. The first of these is the stacking between the porphyrin rings (at 3.581(3) Å), which now features the bromine atoms pointing in opposite directions with a ruffled conformation of the porphyrin macrocycle (Figure 3A). Moreover, a similar
- motif of bromine atoms pointing towards the phenyl rings is visible in this structure and seems to be a staple of this series (Figure 3B). Secondly, the edge-on interaction in this structure has changed, where the pyrrole moiety is pointing towards the face of the porphyrin macrocycle as seen in Figure
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- -dependent selective arrangement of one or two substrates within the cavity for photocatalysis. Therefore, this review will focus on: i) the role of the supramolecular system in mediating the photocatalytic selectivity, yield, and the rate of the photocatalytic products and ii) macrocycle-assisted hybrid
- can easily gain information on host–guest interactions in supramolecular systems as well as the photocatalytic reaction efficiency. Review Macrocycle-promoted photocatalysis Photocatalysis based on crown ethers and crown ether derivatives CEs are a class of macrocycles that consist of a ring with a
- on pillararene is a relatively new field, and only a few examples have been reported [53][54]. Wen and co-workers reported the selective photocatalytic oxidation of sulfides in the presence of conjugated macrocycle polymers (COP) with pillar[5]arene struts (Figure 17) [55]. The host–guest
Chiral anion recognition using calix[4]arene-based ureido receptors in a 1,3-alternate conformation
Beilstein J. Org. Chem. 2020, 16, 2999–3007, doi:10.3762/bjoc.16.249
- synthetic intermediate as the presence of the allyl groups immobilizes the required conformation and at the same time enables the potential incorporation of the macrocycle into a polymeric matrix. Consequently, the subsequent reduction step was carried out in two alternative ways: (i) exclusive reduction of
- chain of calixarene molecules joined together by intermolecular hydrogen bonds between the ureido groups (the C=O···H–N distances were 2.293 and 2.048 Å). One of the ureido functions in the macrocycle also held acetone via a C=O···H–N hydrogen bond (2.085 Å) and via a C=O···H–C bond (2.670 Å) from the
- immobilised in the 1,3-alternate conformation resulted in a macrocycle with a preorganised ureido cavity bearing chiral alkyl substituents in the near proximity. As shown by 1H NMR titration experiments, these compounds function as receptors for chiral anions in DMSO-d6. The chiral recognition ability can
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- corresponding free macrocycle. The detailed understanding of the thermodynamic and electrochemical properties of the tailor-made crown ethers lays the foundation for the construction of new types of molecular redox switches with emergent properties. Keywords: crown ether; isothermal titration calorimetry
- -functionalized crown ether as for the TTF crown ethers, where the NDI unit is in a position more remote from the crown ether binding site. Yet, keeping the formal C2-symmetry of the macrocycle is important to avoid mixtures of isomers upon the threading of directional axles, such as A1·PF6 (Figure 1c) [40
- Information File 1). Slow diffusion of CH3CN into a concentrated solution of NDIC7 in CH2Cl2 yielded single crystals suitable for X-ray diffraction (Figure 2b). The macrocycle displays a folded conformation in the solid state due to the flexible linker, featuring an intramolecular NDI/naphthalene stacking
NMR Spectroscopy of supramolecular chemistry on protein surfaces
Beilstein J. Org. Chem. 2020, 16, 2505–2522, doi:10.3762/bjoc.16.203

- adds more hydrophobic interactions with the aromatic side walls of the bowl-shaped calixarene core. A similar, but even more pronounced trend, is observed for the pumpkin-shaped macrocycle cucurbit[7]uril, favoring the trimethylated over unmethylated lysine by a factor of 3500 [49]. Methylated lysines
Design and synthesis of a bis-macrocyclic host and guests as building blocks for small molecular knots
Beilstein J. Org. Chem. 2020, 16, 2314–2321, doi:10.3762/bjoc.16.192
- multiple guests in order to systematically explore the lower size limits of trefoil knots. For example, if TLC were successful with host 1 and guest 2, then a 73 backbone-atom trefoil (and the corresponding unknotted macrocycle) would be formed (Figure 3 and Scheme S1 in Supporting Information File 1
- ), whereas host 1 and guest 3 would lead to a 75 backbone-atom trefoil (and unknotted macrocycle). Results and Discussion The synthesis of bis-macrocyclic host 1 began by breaking the symmetry of naphthalene-1,5-diol (4) by alkylation of one of the alcohols with 2-azidoethyl mesylate to yield azide 5 in 27
- spectrum showed the loss of the ethyl ester peaks (Scheme 4). Bis-macrocyclization of 15 under high-dilution using Shiina’s mixed-anhydride method [29] afforded host 1 in 28% yield. As with the analogous first-generation knot-precursor bis-macrocycle [15], host 1 was formed as a mixture of meta- and ortho
Design, synthesis and application of carbazole macrocycles in anion sensors
Beilstein J. Org. Chem. 2020, 16, 1901–1914, doi:10.3762/bjoc.16.157

- example, a carbazole-urea macrocycle was reported previously [10], however, the binding of anions occurred outside the receptor due to modest dimensions of the macrocyclic cavity. Using click-chemistry, a carbazole-triazole macrocycle, “tricarb”, was prepared that showed the ability to form non-covalent
- superstructures [11]. The rings should be of remarkable size for accommodating even small anions, such as acetate. In many cases the receptor is formally a macrocycle, but the anion is bound on its “surface”, not inside the ring [10][12]. A well-known example is the calix[4]pyrrole family, which emerged as an
- “cyanostar” macrocycle [15][16]. Although not a macrocycle, a successful binding motif that is noteworthy for its ability to bind carboxylates in polar environments, is the guanidiniocarbonylpyrrole (GCP) moiety designed by Carsten Schmuck and Michael Schwegmann [17]. The transition from complexation studies
Five-component, one-pot synthesis of an electroactive rotaxane comprising a bisferrocene macrocycle
Beilstein J. Org. Chem. 2020, 16, 1564–1571, doi:10.3762/bjoc.16.128
- of Bordeaux, Pessac, France 10.3762/bjoc.16.128 Abstract The templated clipping of a ferrocene-grafted isophthalic acid derivative to encircle a hydrogen-bonding axle through the reaction with 1,4-bis(aminomethyl)benzene is described. The constituent electroactive macrocycle of the resultant [2
- crystal X-ray structure of a doubly ferrocene-decorated [2]rotaxane are further reported. Keywords: ferrocene; macrocycle; rotaxane; single crystal X-ray structure; template; Introduction The development of interlocked molecules with tailored properties allowed the preparation of molecular machines able
- act as stimuli-responsive molecular “shuttles” with potential applications to prepare nanoscale devices for computing and biomimetic engineering [2][3]. The highly efficient rotaxane formation developed by Leigh allowed the generation of a tetraamide macrocycle on a fumaramide or succinamide thread in
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- molecule. In this structure, it should be noted that there is a high degree of π-stacking between the porphyrin macrocycle. This is highlighted in Figure 4; however, there is a clear preference for the homo-metallic selective interactions between the porphyrin rings. As such, in the crystal packing the Ni
- minor disorder are omitted for clarity (thermal displacement 50%). Porphyrin macrocycle mean-planes are indicated by maroon shading. UV–vis of symmetric and unsymmetric triptycene porphyrin dimers 9 and 16, and the triptycene porphyrin monomer 14 in CHCl3. Emission spectrum of symmetric and unsymmetric
Light-controllable dithienylethene-modified cyclic peptides: photoswitching the in vivo toxicity in zebrafish embryos
Beilstein J. Org. Chem. 2020, 16, 39–49, doi:10.3762/bjoc.16.6

- side-chain hydroxylation modifications (series iii), with macrocycle ring-size variations (series iv), and with macrocycle homodimerization (series v). We systematically screened the ring-open and ring-closed photoforms of all 29 compounds in vitro, using a range of cellular toxicity assays (against
Photoreversible stretching of a BAPTA chelator marshalling Ca2+-binding in aqueous media
Beilstein J. Org. Chem. 2019, 15, 2801–2811, doi:10.3762/bjoc.15.273

- cyclization reaction yielding the corresponding azobenzene 1f was performed using a Cu(I) catalyst generated in situ [36]. Finally, the esters were hydrolyzed under mild conditions resulting in the azobenzene-BAPTA macrocycle 1. To gain some insight into the possible structures of the 1E and 1Z chelators and
- +, giving rise to a highly fluorescent species upon excitation at 485 nm. The fluorescence is greatly diminished after the addition of 10 equivalents of 1E, as the azobenzene macrocycle abstracts the Ca2+ ion from fluorophore 3. Upon irradiation of the ensemble at 365 nm at these low concentrations, a small
Fluorinated maleimide-substituted porphyrins and chlorins: synthesis and characterization
Beilstein J. Org. Chem. 2019, 15, 2704–2709, doi:10.3762/bjoc.15.263
- ability to selectively accumulate in the target tissue, the absence of toxicity, toxic byproducts and mutagenic effects, and an opportunity for medical administration. An additional advantage of porphyrins is the possibility of functionalization of the macrocycle periphery with various substituents and
- widely used to generate singlet oxygen for PDT applications [9][23]. We intend here to combine within one molecule the structural specificity of a meso-fuorinated porphyrin/chlorin macrocycle and maleimide units to develop novel multifunctional compounds with improved properties for various applications
- zinc from the coordination sphere of the porphyrin macrocycle and gave the free base maleimide porphyrin 6 in a quantitative yield. A successful formation of the fluorinated porphyrin azides 3a and 3b allowed their utility as intermediates in the further porphyrin core functionalisation especially with
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- ), as expected for the C-terminal carboxamide, we observed three ions derived from the breaking of two amide bonds, starting from the opening of the macrocycle by the loss of one, two or three amino acid residues as neutral fragments. This fragmentation pattern agrees with the one expected for cyclic
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
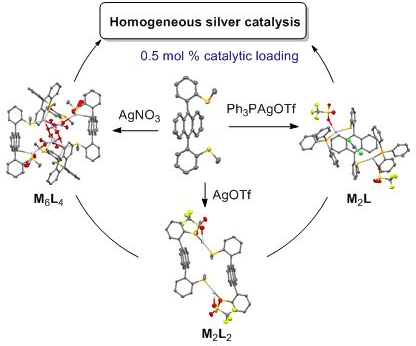
- of the two L2·(AgOTf)2 stereoisomers highlighted their different geometry. The catalytic activity of all silver(I) complexes was effective under homogeneous conditions in two tandem addition/cycloisomerization of alkynes using 0.5–1 mol % of catalytic loading. Keywords: coordination macrocycle
- ) cation was coordinated to two sulfur atoms with (R)- and (S)-configuration, respectively (head-to-tail ligand coordination mode). These diastereoisomeric crystals presented a slightly different spatial arrangement. The head-to head macrocycle 1a had a parallelepiped shape (Figure 1a): the interplanar
- distance between two anthracenes was ca. 6.31 Å and the dihedral angle between the anthracene core and its 9,10-aryl substituents was 89° and 104°, respectively. The head-to-tail macrocycle 1a adopted a V-shape (Figure 1b): the angle between the planes of the two anthracenes was 73.2° and the dihedral
Effect of ring size on photoisomerization properties of stiff stilbene macrocycles
Beilstein J. Org. Chem. 2019, 15, 2408–2418, doi:10.3762/bjoc.15.233

- have been studied to investigate the possible impact of the macrocycle ring size on their photodynamic properties. The results show that reducing the ring size counteracts the photoisomerization ability of the macrocycles. However, even the smallest macrocycle studied (stiff stilbene subunits linked by
- largest macrocycle (linked by a twelve carbon chain) is significantly higher than that of the stiff stilbene unit itself. In general, it is indicated that addition of even a flexible chain to the stiff stilbene unit may significantly affect its photochemical properties and increase the photostability of
- the resulting macrocycle. Keywords: DFT; molecular mechanics; photostability; photo-switch; ring-strain; stiff stilbene; Introduction The stiff stilbene (SS) molecule has drawn a lot of interest due to its photodynamic properties [1]. Stiff stilbenes typically undergo light triggered isomerization
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- bulky anion. The observed selectivity trend of 1c was H2PO4− > Cl− > F− > Br− > I− > CH3COO− with the dissociation constants 1.9 × 103, 3.9 × 102, 3.6 × 102, 2.0 × 102, 1.0 × 102 and 3.0 × 101 M−1, respectively [36]. Conversely, another bile acid-based macrocycle 2 which was synthesized by the same
- association constant of macrocycle 3 in the more competitive 15% water/acetone medium, still demonstrating a high binding affinity for sulfate anion (Ka = 2.5 × 105 M−1) while the cage 3 did not show any binding toward the other tested anions in the more aqueous solvent mixture. 2.3. Redox-active 1,2,3
- -triazolium receptors A ferrocene-containing dicationic bis-triazolium macrocycle 4 (see Figure 4) has been designed and synthesized by utilising the intramolecular Eglinton cyclisation of an acyclic bis(triazolylalkyne)ferrocene precursor followed by alkylation. The anion sensing ability was investigated by
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- of a high energy barrier for the rotation of diethyl terephthalate units through the corona[6]arene macrocyclic annulus [23][24]. To circumvent the formation of structural isomers arising from the restricted rotation of aromatic fragments though the macrocycle annulus, we selected in the current
- tetrazine and phenolic oxygen and the high stability of corona[6]arene macrocycle under the reaction conditions. The spectroscopic data supported the macrocyclic structure of all products. To determine the structure beyond any doubt, and also to shed light on the conformation of phthalimide-containing O6
- -corona[6]arenes in the solid state, high quality single crystals of 3a were grown at room temperature from diffusion of diethyl ether vapor into the solution of 3a in acetonitrile. X-ray diffraction analysis revealed that the macrocycle 3a adopted an interesting conformation. As depicted in Figure 1, it
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- disintegrative for these complexes. This result is quite non-intuitive and worth attention in the context of formation of supramolecular complexes in polar environment, for which DMSO is most often a first-choice solvent. Keywords: cavitands; chirality; macrocycle; resorcin[4]arene; self-assembly
Nanopatterns of arylene–alkynylene squares on graphite: self-sorting and intercalation
Beilstein J. Org. Chem. 2019, 15, 1848–1855, doi:10.3762/bjoc.15.180
- (OC6H13) side chains of opposing sides of each square are oriented towards the macrocycle interior, and are observed as darker image regions, and the remaining OC6H13 chains interact intermolecularly. The closer packing of 1b leads to the interaction of the butyloxy chains of the macrocycle corner units
- direction d1 with γ(a,d1) = (5 ± 1)°, whereas all (short) hexyloxy side chains point into the macrocycle interior – a result of the unhindered rotation of the p-phenylene units prior to the physisorption – or point towards the solution phase, leading to a close contact of the backbones along one direction
- with a slight offset (most probably due to the butyloxy (OC4H9) side chains of the macrocycle corners). Consequently, only seven TCB molecules can intercalate in the intramolecular nanopore of the (almost quadratic) backbones (α◊ = (87 ± 4)°) and are observed as bright dots (in rows of three, one, and
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- -conjugated polymers [29][30][31]. On the other hand, γ-CD is relatively less employed in the synthesis of mechanically interlocked molecules despite of its ability to form interesting 1:2 inclusion complexes, and there are only few examples of rotaxane and catenane featuring γ-CD as an interlocked macrocycle
- further interlocking at the γ-CD. Results and Discussion Building block design and rotaxane synthesis To encourage complex formation with γ-CD, axle 1 was designed with a biphenylene core to bind to the macrocycle through hydrophobic effect. The axle is terminated by 2-aminoethyl azide for CB[6]-mediated
- bond formation, interlocking of the macrocycle is ensured to result in a good efficiency of mechanical bond formation. The synthesis of 1 and 2 is depicted in Scheme 1. To synthesize hetero[n]rotaxanes containing both γ-CD and CB[6], a 1:1 mixture of the anthracene stopper 2 and CB[6] in 50 mM HCl was
Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding
Beilstein J. Org. Chem. 2019, 15, 1592–1600, doi:10.3762/bjoc.15.163

- ), there is no consensus regarding the number of bound water molecules and the location of their coordination. A number of intriguing questions remain: (1) Which localities of the host’s macrocycle are the strongest attractors for the guest water molecules? (2) What are the stabilizing factors for the
- leaving several outstanding questions unanswered: 1) Which localities of the host’s macrocycle are the strongest attractors for the incoming water molecules? (2) What are the major factors contributing to the stability of the water clusters entrapped in the β-CD interior and what type of interactions
- ). Thus only this mode of hydration was considered for β-CD. The cavity of the host’s macrocycle was probed for places/spots exhibiting enhanced affinity for the incoming water molecules. The first entrapped water molecule can bind to either rim (n = 1; Figure 2, structures a, b, d, e) or the cavity walls
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- are determined by the type and size of the macrocyclic platform, e.g., if all of the amino acid side chains are of the same configuration, they are presented on one face of the macrocycle in a convergent manner. The artificial Lissoclinum cyclopeptide platforms feature C2, C3 and C4 symmetry [3]. So
- isomerization’s resulting in a stepwise decrease of the distance of the two macrocycles. Accordingly, the distance between the recognition units at the upper and the lower macrocycle decreases stepwise as well. In the chiral container 5 two identical macrocycles are connected to each other. A further development
- , whereas platform 3a possesses two oxazole rings. Overall, both macrocycles feature four amino acid side chains (isopropyl groups), whereby all of them are of the same configuration (S). Therefore, they are presented on one face of the macrocycle in a convergent manner. Platform 3a was synthesized in a few






















































































































































































































































































































































































































































