Search results
Search for "supramolecular chemistry" in Full Text gives 202 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- ]tetrazines a useful macrocyclic platform for the study of supramolecular chemistry. Applications of the phthalimide-containing functionalized O6-corona[3]arene[3]tetrazines are being actively perused and results will be reported in due course. Experimental General procedure for the synthesis of 3a–e. To a
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- ; supramolecular chemistry; Introduction Despite of the great progress that has been made in supramolecular chemistry in polar and aqueous media, formation of discrete ordered supramolecular associates with precise patterns of noncovalent interactions still presents a challenge [1][2]. Especially self-assembly
- Bartosz Setner Agnieszka Szumna Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland 10.3762/bjoc.15.187 Abstract Directional self‐assembly of conformationally well-defined complexes in polar environment is still a major challenge in supramolecular
- chemistry. In the present study we demonstrate that resorcin[4]arene sulfonic acid (RSA) interacts with chiral amines (amino acid derivatives and aminocavitands) to form inclusion complexes and capsules based on electrostatic interactions. The complexes were characterized by circular dichroism and DOSY NMR
Novel macrocycles – and old ones doing new tricks
Beilstein J. Org. Chem. 2019, 15, 1838–1839, doi:10.3762/bjoc.15.178

- 10.3762/bjoc.15.178 Keywords: macrocycles; supramolecular chemistry; Macrocycles [1] are the workhorses in supramolecular chemistry. Many basic supramolecular concepts have been developed through studying crown ethers, cryptands, podands and spherands in the 1970s and 1980s. For these contributions
- , macrocycles played a central role for the fundamental science that established supramolecular chemistry as an independent field of chemical research as well as for its applications in contemporary research on functional supramolecules and materials. Nowadays, the use of macrocycles has significantly
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- groups. They have exhibited wide potential applications in supramolecular chemistry [31][32][33][34][35][36] for their unique structures and electron-rich cavities. In this paper, we report the complexation between 2,6-helic[6]arene and its four derivatives with 1,1′-dimethyl-4,4′-bipyridinium and
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
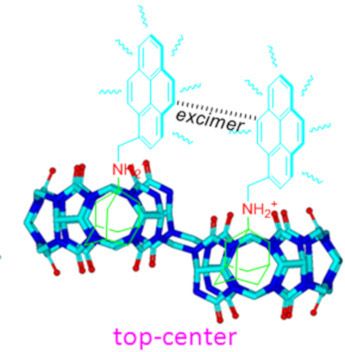
- Xiaodong Zhang Wei Wu Zhu Tao Xin-Long Ni Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.15.166 Abstract The unique monomer and excimer fluorescence emissions of pyrene were first exploited as distinctly
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- the high dispersion energy in the compact cis,cis-isomer. Keywords: azobenzene; macrocycles; molecular switch; Introduction In supramolecular chemistry rigid scaffolds are required to arrange different recognition units in predefined distances and spatial orientation to each other [1]. One example
- possible, should lead to containers which are due to their foldable feature promising candidates for applications in supramolecular chemistry. Experimental General remarks: All chemicals were reagent grade and were used as purchased. Reactions were monitored by TLC analysis with silica gel 60 F254 thin
- Abdulselam Adam Saber Mehrparvar Gebhard Haberhauer Institut für Organische Chemie, Universität Duisburg-Essen, Universitätsstr. 7, D-45117 Essen, Germany 10.3762/bjoc.15.156 Abstract The combination of photo-switchable units with macrocycles is a very interesting field in supramolecular
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- –guest chemistry; macrocycles; molecular recognition; precious metal chloride complexes; Introduction Macrocyclic receptors are the major workhorses in supramolecular chemistry. Design and synthesis of new macrocyclic receptors with new properties is always attractive but is also challenging [1
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- enantiomers of 3a is present, which assembled to a dimeric structure with the fifth chelation of a Zn2+ ion by the carbonyl group of the other molecule. The self-dimerization property of 3a may be utilized in supramolecular chemistry [50][51][52][53]. Experimental 1: Porphyrin phosphonium salt 1 was prepared
Reversible end-to-end assembly of selectively functionalized gold nanorods by light-responsive arylazopyrazole–cyclodextrin interaction
Beilstein J. Org. Chem. 2019, 15, 1407–1415, doi:10.3762/bjoc.15.140

- linker molecule (dAAP) led to light-responsive reversible self-assembly of AuNR in an end-to-end manner as depicted in Scheme 1 to open up a novel strategy for the design of hybrid nanomaterials by supramolecular chemistry. Results and Discussion The AuNR (length: 58 nm, width: 25 nm, aspect-ratio: 2.3
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- -consuming syntheses. Alternatively, supramolecular chemistry provides a non-covalent approach to achieve fluorescence sensing [2]. An elegant supramolecular strategy, named indicator displacement assay (IDA), was established and popularized by Anslyn and co-workers (Scheme 1a) [3][4]. The complexation of a
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- ; cyclotriveratrylenes; HPLC; macrocycles; racemization; saddle isomer; Introduction Cyclotriveratrylenes (CTVs) [1][2][3][4][5][6][7][8] are cyclic bowl-shaped molecules and belong to the most studied concave host molecules in supramolecular chemistry besides, e.g., calixarenes and resorcinarenes [9], cyclodextrins
Host–guest interactions between p-sulfonatocalix[4]arene and p-sulfonatothiacalix[4]arene and group IA, IIA and f-block metal cations: a DFT/SMD study
Beilstein J. Org. Chem. 2019, 15, 1321–1330, doi:10.3762/bjoc.15.131

- formation reactions. Keywords: complex formation; DFT; group IA; IIA and f-block metal cations; macrocycles; p-sulfonatocalix[4]arene; p-sulfonatothiacalix[4]arene; Introduction If macrocycles are pillars of the supramolecular chemistry, then calixarenes (“calix” = vase + “arene”) are the 3rd pillar after
Bambusuril analogs based on alternating glycoluril and xylylene units
Beilstein J. Org. Chem. 2019, 15, 1268–1274, doi:10.3762/bjoc.15.124

- Tomas Lizal Vladimir Sindelar Department of Chemistry and RECETOX, Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic 10.3762/bjoc.15.124 Abstract The glycoluril monomer is a popular building block in supramolecular chemistry as it is used for the synthesis of versatile host molecules
- ; supramolecular chemistry; Introduction Macrocycles consisting of urea building blocks play an important role in supramolecular chemistry [1]. Urea N–H motifs provide macrocycles with the ability to act as anion receptors due to the stabilizing effect of N–H···anion hydrogen bonding [2][3][4]. Furthermore, the
N-doped carbon dots covalently functionalized with pillar[5]arenes for Fe3+ sensing
Beilstein J. Org. Chem. 2019, 15, 1262–1267, doi:10.3762/bjoc.15.123

- units, the newly constructed fluorescent CCDs could recognize Fe3+ with high selectivity. Therefore, such CCDs can potentially serve as a promising chemical sensor for Fe3+ ions. Keywords: chemical sensor; CN-dots; fluorescence; ion recognition; supramolecular chemistry; Findings Carbon dots (C-dots
- macrocyclic compounds, especially new macrocyclic arenes, have become one of the research hotspots in supramolecular chemistry [11][12][13][14]. Among them pillarenes as a relatively new family of pillar-shaped members discovered a decade ago have played a key role due to their unique conformations
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- supramolecular chemistry applications. Keywords: conjugation; heterocycles; macrocycles; multicomponent reactions; steroids; Review 1 Introduction The utilization of multicomponent reactions (MCRs) [1] for the derivatization of biomolecules has continuously grown over the last years. These diversity-oriented
- in the pursuit of medicinal and supramolecular chemistry applications. Despite some review articles have described selected examples of MCRs used to modify and to macrocyclize steroidal compounds [12][13], to our knowledge there is no review exclusively dedicated to covering the applications of MCRs
Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium
Beilstein J. Org. Chem. 2019, 15, 1203–1209, doi:10.3762/bjoc.15.117

- -assemblies have been recognized as a type of exciting nanomaterials with tremendous potential for research and commercial applications [1][2][3][4][5][6]. Thanks to the development of supramolecular chemistry, the ordered structures could be obtained through programmed self-assembly coupled with covalent
- ][31][32]. Nowadays, as the research in supramolecular chemistry is expanding into the aqueous realm, a systematic understanding of the interaction relationship of supramolecular self-assemblies and aqueous medium is becoming increasingly important [33][34][35][36]. As the first generation of
Halogen bonding and host–guest chemistry between N-alkylammonium resorcinarene halides, diiodoperfluorobutane and neutral guests
Beilstein J. Org. Chem. 2019, 15, 947–954, doi:10.3762/bjoc.15.91

- supramolecular chemistry and materials science [1][2][3]. New systems both help us to better understand the nature and impetus behind the self-assembly of these fascinating systems, while also providing new materials that can provide the basis for a wide number of applications [4][5]. Halogen bonding (XB), as a
Mechanochemistry of supramolecules
Beilstein J. Org. Chem. 2019, 15, 881–900, doi:10.3762/bjoc.15.86
- . Contrastingly, achieving syntheses through mechanochemical methods are generally time-saving, environmentally friendly and more economical. This review is written to shed some light on supramolecular chemistry and the synthesis of various supramolecules through mechanochemistry. Keywords: ball milling
- conditions through covalent approaches. By using common synthetic methodologies chemists are able to proficiently synthesize a variety of both natural and unnatural molecular scaffolds [4][5][6]. The era of supramolecular chemistry began with the introduction of coordination theory by Alfred Werner in 1893
- supramolecular chemistry are dynamic combinatorial chemistry [11], subcomponent self-assembly approach [12][13][14], and systems chemistry [15][16][17][18], etc. There also has been growing interest towards exploration of nontraditional energy sources like visible light [19][20], microwave [21], mechanochemical
Homo- and hetero-difunctionalized β-cyclodextrins: Short direct synthesis in gram scale and analysis of regiochemistry
Beilstein J. Org. Chem. 2019, 15, 710–720, doi:10.3762/bjoc.15.66
- -difunctionalization; regioselectivity; Introduction Cyclodextrins (CDs) are cyclic oligomers of α-D-glucopyranose (Figure 1 illustrates the heptamer, β-CD) that have attracted worldwide interest in various fields of applied supramolecular chemistry due to their ability to form host–guest inclusion complexes [1]. A
LCST phase behavior of benzo-21-crown-7 with different alkyl chains
Beilstein J. Org. Chem. 2019, 15, 437–444, doi:10.3762/bjoc.15.38

- -crown-7, B21C7s) can effectively control the thermo-responsive properties. Keywords: crown ethers; hydrophobic units; lower critical solution temperature; LCST; thermo-responsiveness; supramolecular chemistry; Introduction The introduction of stimuli-responsiveness into artificial materials is vital
- behavior, is a type of thermo-responsiveness [14][15][16], which has gained much attention in supramolecular chemistry, because of its profound impact on the development of stimuli-sensitive supramolecular materials with controllable and/or programmable phase separation properties [17][18][19]. Though LCST
First synthesis of cryptands with sucrose scaffold
Beilstein J. Org. Chem. 2019, 15, 210–217, doi:10.3762/bjoc.15.20
- ’,4,4’-penta-O-benzylsucrose and 1’,2,3,3’,4,4’-hexa-O-benzylsucrose. Keywords: cryptands; macrocyclization; sucrose; Introduction The design and synthesis of macrocyclic receptors is one of the main challenges of supramolecular chemistry [1][2]. These artificial systems exhibit interesting properties
Calixazulenes: azulene-based calixarene analogues – an overview and recent supramolecular complexation studies
Beilstein J. Org. Chem. 2018, 14, 2488–2494, doi:10.3762/bjoc.14.225

- investigated. Keywords: azulene; calixarenes; calixazulenes; supramolecular chemistry; tetraalkylammonium salts; Introduction Among the great variety of synthetic macrocyclic molecular receptors which have been reported, those that are referred to by their generic name “calixarene” loom large [1][2][3]. The
- ]azulene 3. To shed light on possible explanations for these findings, our attention was again directed to computational results derived from DFT calculations which are increasingly being commonly used in supramolecular chemistry. The ωB97xD functional [25] which combines the long range functional ωB97x
- , in light of recent developments in the facile syntheses of other functionalzed azulenes as reported by Narita et al. [34] the potential for further syntheses of hetero-functionalized calixazulenes and their supramolecular chemistry may be realized. Further studies by us on these intriguing
Coordination-driven self-assembly of discrete Ru6–Pt6 prismatic cages
Beilstein J. Org. Chem. 2018, 14, 2242–2249, doi:10.3762/bjoc.14.199
- supramolecular chemistry leading to the formation of a single major product. Keywords: arene–ruthenium(II); heterometallic cages; platinum metalloligand; self-assembly; supramolecular architectures; Introduction Coordination-driven self-assembly of discrete architectures has evolved as a unique protocol to
- –ruthenium(II) acceptor clips/building blocks have been extensively utilized in supramolecular chemistry because of their rigid directionality toward electron-rich donors due to their restricted coordination sites as a result of the fixed position of the p-cymene moiety [24][93][98][99][100][101][102][103
Synthesis of a water-soluble 2,2′-biphen[4]arene and its efficient complexation and sensitive fluorescence enhancement towards palmatine and berberine
Beilstein J. Org. Chem. 2018, 14, 2236–2241, doi:10.3762/bjoc.14.198

- Xiayang Huang Xinghua Zhang Tianxin Qian Junwei Ma Lei Cui Chunju Li School of Chemical and Environmental Engineering, Shanghai Institute of Technology, 100 Hai-Quan Road, Shanghai 201418, P. R. China Department of Chemistry, Center for Supramolecular Chemistry and Catalysis, Shanghai University
- , medicine, and environment. Cyclodextrins [1][2][3][4], cucurbiturils [5][6][7][8][9][10][11], and calixarenes [12][13][14][15][16][17][18][19][20] have been widely used in aqueous supramolecular chemistry. In the past ten years, the chemistry of pillar[n]arenes has developed very quickly because of their
Dynamic light scattering studies of the effects of salts on the diffusivity of cationic and anionic cavitands
Beilstein J. Org. Chem. 2018, 14, 2212–2219, doi:10.3762/bjoc.14.195

- light scattering; Hofmeister effect; ion–ion interactions; water; Introduction Although all life on planet Earth depends on aqueous solutions, our understanding of aqueous supramolecular chemistry is limited. As a result, as Smith has eloquently pointed out [1], the effects of buffers and salts on



















































































































































































































