Search results
Search for "fluorescence emission" in Full Text gives 111 result(s) in Beilstein Journal of Organic Chemistry.
Naphthalimide-phenothiazine dyads: effect of conformational flexibility and matching of the energy of the charge-transfer state and the localized triplet excited state on the thermally activated delayed fluorescence
Beilstein J. Org. Chem. 2022, 18, 1435–1453, doi:10.3762/bjoc.18.149

- . The synthesis of the dyads is based on the ordinary derivatization of the NI and PTZ chromophores [20]. The molecular structures were confirmed by 1H NMR, 13C NMR, and HRMS methods (Experimental section). UV–vis absorption and fluorescence emission spectra The UV–vis absorption spectra of the
- processes of these dyads upon photoexcitation. Experimental General methods All the chemicals used in synthesis are analytical pure and were used as received without purification. UV–vis absorption spectra were measured on a UV-2550 spectrophotometer (Shimadzu Ltd., Japan). Fluorescence emission spectra
Computational model predicts protein binding sites of a luminescent ligand equipped with guanidiniocarbonyl-pyrrole groups
Beilstein J. Org. Chem. 2022, 18, 1322–1331, doi:10.3762/bjoc.18.137
- prediction of two C2 related, dominating binding sites on 14-3-3ζ that may bind to two of the supramolecular ligand molecules. Keywords: AIE luminophores; fluorescence emission; guanidiniocarbonyl-pyrrole; ligand binding; 14-3-3 protein; Introduction The 14-3-3 protein family was one of the first
- overcome this issue is to use fluorescence emission as a read-out tool, such as an emission “on” or “off” behavior [15]. Selective and sensitive fluorescent ligands have been proven to be essential tools for the study of biological systems by biosensing and imaging [16]. There is an increasing demand for
- , Figures S22–S25). This compound 1 was tested in initial binding assays using fluorescence emission as well as native gel electrophoresis. We could indeed show that 1 binds to 14-3-3ζ as detected by native gel electrophoresis and fluorescence titration (Supporting Information File 1). Experiments show that
Synthesis, optical and electrochemical properties of (D–π)2-type and (D–π)2Ph-type fluorescent dyes
Beilstein J. Org. Chem. 2022, 18, 1047–1054, doi:10.3762/bjoc.18.106
- band, but also intense fluorescence emission both in solution and the solid state. Keywords: (D–π)2 structure; fluorescence; fluorescent dyes; photoabsorption; redox properties; Introduction The design and development of a new type of organic fluorescent dyes have been of considerable scientific and
- fluorescent dyes constructed of an electron-donating moiety (D) and an electron-withdrawing moiety (A), linked by a π-conjugated unit thanks to their intense photoabsorption and fluorescence emission characteristics originating from the intramolecular charge transfer (ICT) excitation from the D to the A
- photoabsorption band but also intense fluorescence emission both in solution and the solid state, compared to the (D–π)2Ph-type structure. The electrochemical properties of OTK-2 and OTT-2 (0.1 mM) were evaluated using CV in DMF containing 0.1 M tetrabutylammonium perchlorate (Bu4NClO4), in which the potentials
Morita–Baylis–Hillman reaction of 3-formyl-9H-pyrido[3,4-b]indoles and fluorescence studies of the products
Beilstein J. Org. Chem. 2022, 18, 926–934, doi:10.3762/bjoc.18.92
- . Fluorescence studies Fluorescence studies of these C-3-substituted pyrido[3,4-b]indole derivatives were examined and various parameters (contact time, concentration and solvent) were optimized for obtaining the best results using 7dA as a model substrate. Fluorescence emission spectra for optimizing the
- . Further, the fluorescence emission profile of 7dA was recorded in chloroform at different concentrations viz. 1 × 10−6 M, 2 × 10−6 M, 3 × 10−6 M, 4 × 10−6 M and 5 × 10−6 M which indicated that fluorescence intensity was found to increase with increase in concentration and fluorescence spectra above this
Post-synthesis from Lewis acid–base interaction: an alternative way to generate light and harvest triplet excitons
Beilstein J. Org. Chem. 2022, 18, 825–836, doi:10.3762/bjoc.18.83

- light, which confirmed that the emission was sensitive to BF3 concentration. Yang et al. used also TFA to shape the fluorescence emission based on the protonation effect between the dissociated H+ and the fluorescent material [32]. Lin et al. used the Lewis acids B(C6F5)3 and AlCl3 to regulate the
Synthesis of a novel aminobenzene-containing hemicucurbituril and its fluorescence spectral properties with ions
Beilstein J. Org. Chem. 2021, 17, 2840–2847, doi:10.3762/bjoc.17.195

- +, and Mn2+ (nitrate salts were used as cation sources) in DMF resulted in different degrees of quenching of the fluorescence emission of host 4. The results are collected in Figure 3 as the corresponding fluorescence quenching efficiency which was quantified using the equation ΔI = (I0 − I), where I is
- halide ions, especially the heavier iodide ion, to the macrocyclic sensor, slightly enhanced the fluorescence emission in CH2Cl2/CH3OH 4:1 (v/v) at 298 K, instead of quenching the fluorescence as predicted by the classic heavy-atom effect. The corresponding fluorescence enhancement efficiency of selected
- −1. In another perspective, the presence of the coordination of selected anions to the macrocyclic sensor enhanced the fluorescence emission in various degree, extremely contrary to the classic heavy-atom effect caused by a heavy atom. In general, this macrocyclic sensor showed high fluorescence
Photophysical, photostability, and ROS generation properties of new trifluoromethylated quinoline-phenol Schiff bases
Beilstein J. Org. Chem. 2021, 17, 2799–2811, doi:10.3762/bjoc.17.191
- coefficients of the derivatives studied here. All absorption spectra are listed in Supporting Information File 1 (Figures S2–S7). The steady-state fluorescence emission spectra of the Schiff base compounds from their absorption in the UV–vis region was carried out. Derivatives 3aa–fa and 3bb–be were analyzed
- fluorescence yields (Φf) were calculated in order to prove the quantum efficiency of these derivatives in terms of fluorescence emission and thus discuss the influence of the different solvents and substituents. Firstly, by comparing the selected solvents, compound 3aa (R = Ph, R1 = H) presented an emission in
- showing coplanarity of the same system. Normalized absorption spectra in the UV–vis region of compounds (a) 3ea and (b) 3be in CHCl3, MeOH or DMSO solution, respectively ([ ] = 1.50 × 10−5 M). Normalized steady-state fluorescence emission spectra of compound 3aa (R = Ph, R1 = H) in CHCl3 (black solid line
Exfoliated black phosphorous-mediated CuAAC chemistry for organic and macromolecular synthesis under white LED and near-IR irradiation
Beilstein J. Org. Chem. 2021, 17, 2477–2487, doi:10.3762/bjoc.17.164

- acetylene proton around 4.42 ppm. a) 1H NMR spectrum of chain end modified PCL-Anth; b) UV–vis spectra of (azidomethyl)anthracene (black) and PCL-Anth (red); c) fluorescence emission spectrum of PCL-Anth. a) GPC traces of PS-Az, PCL-Alk and block copolymer (Ps-b-PCL) b) 1H NMR spectrum of the block
Chemical syntheses and salient features of azulene-containing homo- and copolymers
Beilstein J. Org. Chem. 2021, 17, 2164–2185, doi:10.3762/bjoc.17.139
- displayed fluorescence emission at 385 nm in the protonated state. The electrochemical bandgap of these polymers was in the impressive range of 1.57–1.62 eV. In 2014, Hawker, Robb, and co-workers [35] also reported the synthesis of azulene-fluorene copolymers 117–121, containing varying ratios of 1,3-and
Synthesis of 1-indolyl-3,5,8-substituted γ-carbolines: one-pot solvent-free protocol and biological evaluation
Beilstein J. Org. Chem. 2021, 17, 1453–1463, doi:10.3762/bjoc.17.101
- were obtained from a plot of the semi-logarithmic [conc] vs the intensity of the fluorescence emission, and the IC50 (concentration at which 50% of the enzymatic activity is inhibited) was calculated for the carboline derivatives or doxorubicin using GraphPad Prism, version 7.02 for Windows (GraphPad
Double-headed nucleosides: Synthesis and applications
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98
Selected peptide-based fluorescent probes for biological applications
Beilstein J. Org. Chem. 2020, 16, 2971–2982, doi:10.3762/bjoc.16.247

- with nucleic acids. Reprinted with permission from [34]. Copyright (2012) American Chemical Society. A) Molecular structure of peptidic probe 1, Inset: HeLa cells incubated with peptide 1 (50 μM), showing an ATP responsive green fluorescence; B) fluorescence emission spectra of probe 1 (20.0 µM) (λex
- = 410 nm) with increasing concentration (0–10.0 µM) of ATP in 10 mM HEPES buffer, pH 7.4. Reproduced from [39] with permission from The Royal Society of Chemistry. A) Molecular structure of probe 2; B) fluorescence emission spectra for the titration of a 10 μM solution of 2 with p(dA·dT)2 in aqueous
- buffer (pH 7.4) (with base pair/2 molar ratios ranging from 0 to 4.0), inset: nuclei of HeLa cells stained with 2. Reprinted with permission from [34]. Copyright (2012) American Chemical Society. A) Molecular structure of 3; B) fluorescence emission spectra for the titration of a 10 μM solution of 3 with
Incorporation of a metal-mediated base pair into an ATP aptamer – using silver(I) ions to modulate aptamer function
Beilstein J. Org. Chem. 2020, 16, 2870–2879, doi:10.3762/bjoc.16.236

- the fluorescence (Figure 8). The addition of oligonucleotide 1d, which forms one mismatched base pair with 2f close to the fluorescein moiety, leads to hardly any reduction in the fluorescence emission, indicating that 2f binds less effectively (if at all). The oligonucleotide 2q quenches the
- present in the duplex. Except for the system involving the aptamer 1a, none of the systems shows a significant response to AMP in the absence of Ag(I). Unexpectedly, even after the addition of 1 equiv of Ag(I), the fluorescence emission does not significantly increase either. Adding an excess of Ag(I
Encrypting messages with artificial bacterial receptors
Beilstein J. Org. Chem. 2020, 16, 2749–2756, doi:10.3762/bjoc.16.225

- three dyes (Figure 3B), confirmed their presence on the membrane of individual bacteria, as expected from our design (Figure 3, step 1). The images also showed that, in the course of 24 hours, the fluorescence emission generated from the bacterial cells decreased (Figure 3B), as a result of bacterial
Styryl-based new organic chromophores bearing free amino and azomethine groups: synthesis, photophysical, NLO, and thermal properties
Beilstein J. Org. Chem. 2020, 16, 2282–2296, doi:10.3762/bjoc.16.189

- ][12][13][14]. Among dyestuffs classes, the push-pull fluorescent dyes are renowned to own such special behaviors. The push-pull dyes generate higher charge delocalization upon excitation, thus enhance both polarizability and fluorescence emission [12][13][14][18]. The charge delocalization upon
The B & B approach: Ball-milling conjugation of dextran with phenylboronic acid (PBA)-functionalized BODIPY
Beilstein J. Org. Chem. 2020, 16, 2272–2281, doi:10.3762/bjoc.16.188

- with roughly 1 equiv of 1), Dex-1c (ball milling with roughly 0.5 equiv of 1), and Dex-1d (ball milling with roughly 0.1 equiv of 1) expressed as % w/w on the modified dextrans. A) UV–vis absorption and B) fluorescence emission spectra (λexc = 380 nm) of the BODIPY-dextran conjugate Dex-1b solution in
- water. A) Hydrodynamic diameter of (nm) conjugate Dex-1b (at 1 mg/mL in H2O, black curve) and PBS (red curve), respectively. B) TEM image of conjugate Dex-1b in H2O. Fluorescence emission spectra of pyrene (4.4 × 10−8 M) in water and in a water solution in the presence of conjugate Dex-1b. Schematic
Naphthalene diimide bis-guanidinio-carbonyl-pyrrole as a pH-switchable threading DNA intercalator
Beilstein J. Org. Chem. 2020, 16, 2201–2211, doi:10.3762/bjoc.16.185

- chiral properties of the DNA or RNA helical structures [12][13], could also take advantage of induced CD spectrum (ICD) in the visible spectrum range of small achiral dyes, which they show only upon binding to DNA/RNA [14]. Moreover, with recent advances in fluorescence emission-based polarisation
Et3N/DMSO-supported one-pot synthesis of highly fluorescent β-carboline-linked benzothiophenones via sulfur insertion and estimation of the photophysical properties
Beilstein J. Org. Chem. 2020, 16, 1740–1753, doi:10.3762/bjoc.16.146
- measurement of UV–vis absorption and fluorescence emission of the samples, stock solutions of 1.0 mM concentration were prepared using analytical grade CHCl3 as the solvent, and diluted to the final concentration of 4.0 μM. Next, we carefully measured the photophysical properties at room temperature including
One-pot synthesis of dicyclopenta-fused peropyrene via a fourfold alkyne annulation
Beilstein J. Org. Chem. 2020, 16, 791–797, doi:10.3762/bjoc.16.72
- 1.78 eV from the onset of its UV–vis absorption spectrum. Similar to the cyclopenta-fused pyrene derivatives [28][29], compound 1 does not show detectable fluorescence emission. Furthermore, the electrochemical properties of 1 was probed by cyclic voltammetry (CV) in DCM (Figure 3b). According to the
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- dimers and 8 nm (1.25 × 106 cm−1) for the monomer. The difference in properties of the unsymmetric zinc-nickel dimer 16 is observed significantly more in the corresponding fluorescence emission spectrum (Figure 7). The emission observed for the zinc monomer and dimer, 14 and 9, respectively, was
- nickel porphyrin is acting as the acceptor. An electron/energy transfer is occurring between the two porphyrins, therefore, when the molecule is excited at the wavelength of the zinc porphyrin, the fluorescence emission ordinarily observed for the zinc porphyrin does not occur as the energy has been
- fluorescence emission spectra of related dimers (Figure 8), connected via different linker groups, were taken (Figure 9 and Figure 10). Both the triptycene-linked zinc porphyrin dimer 9 and BODIPY dimer 7 were compared with a meso–meso-linked dimer 19 [50] and butadiyne-linked dimer 20 [51]. The UV–vis spectra
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- cause of the activity. Therefore, the interaction of selected compounds, i.e., 11 and 12, with CT DNA was monitored by UV–vis spectroscopy and viscosity measurements. Additionally, the EB−displacing ability of the compounds was evaluated by fluorescence emission spectroscopy. The UV–vis spectra of a CT
- intercalating ability. EB–DNA conjugate exhibits an intense fluorescence emission band at 592 nm, when its solution is excited at 540 nm. Compounds 11 and 12 have not presented any appreciable fluorescence emission either alone in solution or in the co-existence of CT DNA or EB under the same experimental
- conditions (λexcitation = 540 nm at room temperature). Thus, the quenching observed in an EB–DNA solution upon addition of the compounds 11 and 12 may reveal their competition to EB for the DNA-intercalation sites as monitored by fluorescence emission spectroscopy with λexcitation = 540 nm. A significant
Synthesis and circularly polarized luminescence properties of BINOL-derived bisbenzofuro[2,3-b:3’,2’-e]pyridines (BBZFPys)
Beilstein J. Org. Chem. 2020, 16, 325–336, doi:10.3762/bjoc.16.32
- fluorescence emission bands, whereas the (R)-isomers emitted right-handed CPL to produce the mirror images. The calculated luminescence dissymmetry factors [48] glum for the solutions were all within the range of 3.80 × 10−4 to 6.90 × 10−4. On the other hand, in the dispersed solid state in Fomblin® PFPE
Plasma membrane imaging with a fluorescent benzothiadiazole derivative
Beilstein J. Org. Chem. 2019, 15, 2644–2654, doi:10.3762/bjoc.15.257

- 2,1,3-benzothiadiazole (BTD) core and its derivatives that are successfully applied in bioimaging experiments. (Left) UV–vis, (center) fluorescence emission and (right) solvatochromic effect (Stokes shift in wavenumbers versus solvent polarity in ETN) of the synthesized BTD-4APTEG (10 μM for all
- microscopy. Arrows indicate the peripherical accumulation of the dyes in the plasma membranes. The letter N indicates the nuclei of the cells (scale bar of 10 μm). Synthesis of the plasma membrane BTD probe (BTD-4APTEG) and its structural features. UV–vis and fluorescence emission data (in different solvents
- probe operating at 400 MHz for 1H and at 100 MHz for 13C NMR. Chemical shifts were expressed in parts per million (ppm) and referenced by the signals of the residual hydrogen atoms of the deuterated solvent, as indicated in the legends. UV–vis absorption (Varian Cary 5000) spectroscopy and fluorescence
α,ß-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
Beilstein J. Org. Chem. 2019, 15, 2524–2533, doi:10.3762/bjoc.15.245
- potential dependent accumulation in mitochondria, indicated by a fluorescence emission shift from red to green. The exposure of 11b to the cells caused remarkable loss of mitochondrial membrane potential, hence the fluorescence gradually shifted from red to green as the membrane potential (Ψm) decreased
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
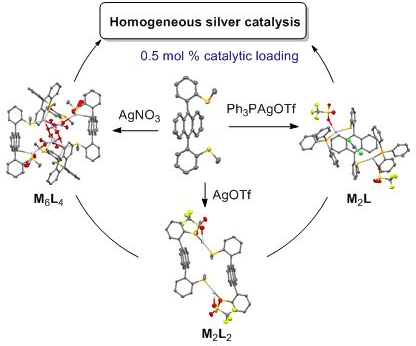
- ). Finally, the photophysical properties of ligand syn-1 (20 μM) and complexes 1a–d (30 μM) were evaluated in dichloromethane (Supporting Information File 1, Figures S4–S8). The UV–visible and fluorescence emission spectra (λexc = 345 nm) of ligand and complexes were similar and correspond to those of 9,10


































































































































































































































































































































