Search results
Search for "this compound" in Full Text gives 515 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
- Keishiro Tahara,
- Tetsufumi Nakakita,
- Alyona A. Starikova,
- Takashi Ikeda,
- Masaaki Abe and
- Jun-ichi Kikuchi
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- sides are almost coplanar with N···N distances between the NAr3 moieties of more than 13 Å (Figure S5 and Table S1 in Supporting Information File 1). Further investigations were not performed for 1c because the solubility of this compound in aprotic solvents including CH2Cl2 were extremely low. The

Graphical Abstract

Figure 1: Structures of target compounds 1 and reference compound Ph1b.

Figure 2: Cyclic voltammograms (left) and differential pulse voltammograms (right) of (top) 1a, (middle) Ph1b,...

Figure 3: Key frontier orbitals (isosurface values 0.02 au) (top), DFT-optimized structures with Mullliken ch...

Figure 4: UV–vis-NIR spectral changes of CH2Cl2/n-Bu4NPF6 (0.10 M) solutions containing (a) 1b (4.5 × 10−4 M)...

Figure 5: Vis-NIR spectra of 1b+ (green line) obtained by bulk electrolysis, with Gaussian deconvolutions (bl...
Aggregation-induced emission effect on turn-off fluorescent switching of a photochromic diarylethene
- Luna Kono,
- Yuma Nakagawa,
- Ayako Fujimoto,
- Ryo Nishimura,
- Yohei Hattori,
- Toshiki Mutai,
- Nobuhiro Yasuda,
- Kenichi Koizumi,
- Satoshi Yokojima,
- Shinichiro Nakamura and
- Kingo Uchida
Beilstein J. Org. Chem. 2019, 15, 2204–2212, doi:10.3762/bjoc.15.217

- is one of the examples which express the mechanisms of aggregation-induced emission (AIE). This compound emits orange fluorescence with a large Stokes shift derived from ESIPT in aprotic solvents such as THF or hexane, while it exhibits only a photochromic reaction in protic solvents such as methanol

Graphical Abstract

Figure 1: Synthetic procedure for a diarylethene (1o).

Figure 2: Photochromic reaction of diarylethene 1o having an ESIPT moiety.

Figure 3: Absorption spectral changes of diarylethene 1o having an ESIPT moiety in THF (c = 1.3×10-5 M). Blac...

Figure 4: Fluorescent spectra of 1o in several solvents (λex = 370 nm). Hexane (black line), chloroform (red ...

Figure 5: (a) The energy diagram of the ESIPT process of 1. (b) ESIPT fluorescence quenching upon UV light (λ...

Figure 6: (a) Fluorescence photographs of solutions/suspensions of 1o (1.2×10-4 M) in THF/water mixtures with...

Figure 7: (a) Crystals of 1o before UV light irradiation, (b) Green fluorescence of 1o observed under UV ligh...
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
- Arnault Heynderickx,
- Sébastien Nénon,
- Olivier Siri,
- Vladimir Lokshin and
- Vladimir Khodorkovsky
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- as the most convenient method of preparation (Scheme 1) [8]. This compound was described as yellow plates (from ethanol), which decomposed at about 300 °C giving a small amount of almost colorless crystalline sublimate identified as 1,4,5,8-tetraazaanthracene (5) [8]. The structure of 3 as the

Graphical Abstract

Figure 1: Quinoxaline derivatives 1–3.

Scheme 1: Synthesis of THTTA (3).

Scheme 2: Protonation, alkylation and acylation of 3.

Figure 2: The ORTEP view of 3. Torsion angles within the benzene ring are given in red.

Figure 3: The ORTEP view of 6a. Torsion angles within the benzene ring are given in red.

Figure 4: The ORTEP view of 7a (the iodine counter anion has been omitted for clarity). Torsion angles within...

Scheme 3: Charge transfer and delocalization within 3 and its diprotonated (6a) and monomethylated (7a) deriv...

Figure 5: Normalized absorption spectra of 3 in: toluene (black), acetone (blue), ethanol (green), water (red...

Figure 6: Protonation of 3 in ethanol. Two isosbestic points (IBP) are indicated by arrows.

Figure 7: Absorption spectra of 7a (blue) and 8 (red) in ethanol.

Figure 8: Absorption spectra of 10a (black), 10b (red). Solid curves: in toluene, dashed curves: in acetone.

Figure 9: View of the supramolecular array generated by 3 in the solid state. Color coding: nitrogen, blue; c...

Figure 10: View of the supramolecular array generated by 6a in the solid state. Color coding: nitrogen, blue; ...

Figure 11: View of the supramolecular array generated by 7a in the solid state. Color coding: nitrogen, blue; ...
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
- Annika Stein,
- Dave Compera,
- Bianka Karge,
- Mark Brönstrup and
- Jakob Franke
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- Withanolides are steroidal lactones widespread in Nightshade plants with often potent antiproliferative activities. Additionally, the structural diversity of this compound class holds much potential for the discovery of novel biological activity. Here, we report two newly characterised withanolides, named

Graphical Abstract

Figure 1: Withanolides from Physalis peruviana. A) Structures of the newly characterised truncated withanolid...

Figure 2: Key NMR correlations. (A) COSY and HMBC correlations for irinan A (2). (B) COSY and HMBC correlatio...

Figure 3: Structures and biosynthesis of androstanes. (A) Androstane backbone and androsterone (7) as a typic...

Figure 4: Intrinsic reactivity of 4ß-hydroxywithanolide E (1) under acidic/basic and oxidative conditions, re...
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
- Iwona E. Głowacka,
- Aleksandra Trocha,
- Andrzej E. Wróblewski and
- Dorota G. Piotrowska
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- syntheses of this compound the general approach which employs the aziridine ester (2R,1'R)-5b as a starting material allows to also obtain its 5'-epimer and norfuranomycin (2S,2'R)-133 [87]. To construct the 2,5-dihydrofurane ring the aziridine alcohol (2R,1'R,1''R)-134 (Scheme 35) [88] was converted to the

Graphical Abstract

Figure 1: Examples of three-carbon chirons.

Figure 2: Structures of derivatives of N-(1-phenylethyl)aziridine-2-carboxylic acid 5–8.

Figure 3: Synthetic equivalency of aziridine aldehydes 6.

Scheme 1: Synthesis of N-(1-phenylethyl)aziridine-2-carboxylates 5. Reagents and conditions: a) TEA, toluene,...

Scheme 2: Absolute configuration at C2 in (2S,1'S)-5a. Reagents and conditions: a) 20% HClO4, 80 °C, 30 h the...

Scheme 3: Major synthetic strategies for a 2-ketoaziridine scaffold [R* = (R)- or (S)-1-phenylethyl; R′ = Alk...

Scheme 4: Synthesis of cyanide (2S,1'S)-13. Reagents and conditions: a) NH3, EtOH/H2O, rt, 72 h; b) Ph3P, CCl4...

Scheme 5: Synthesis of key intermediates (R)-16 and (R)-17 for (R,R)-formoterol (14) and (R)-tamsulosin (15)....

Scheme 6: Synthesis of mitotic kinesin inhibitors (2R/S,1'R)-23. Reagents and conditions: a) H2, Pd(OH)2, EtO...

Scheme 7: Synthesis of (R)-mexiletine ((R)-24). Reagents and conditions: a) TsCl, TEA, DMAP, CH2Cl2, rt, 1 h;...

Scheme 8: Synthesis of (−)-cathinone ((S)-27). Reagents and conditions: a) PhMgBr, ether, 0 °C; b) H2, 10% Pd...

Scheme 9: Synthesis of N-Boc-norpseudoephedrine ((1S,2S)-(+)-29) and N-Boc-norephedrine ((1R,2S)-29). Reagent...

Scheme 10: Synthesis of (−)-ephedrine ((1R,2S)-31). Reagents and conditions: a) TfOMe, MeCN then NaBH3CN, rt; ...

Scheme 11: Synthesis of xestoaminol C ((2S,3R)-35), 3-epi-xestoaminol C ((2S,3S)-35) and N-Boc-spisulosine ((2S...

Scheme 12: Synthesis of ʟ-tryptophanol ((S)-41). Reagents and conditions: a) CDI, MeCN, rt, 1 h then TMSI, MeC...

Scheme 13: Synthesis of ʟ-homophenylalaninol ((S)-42). Reagents and conditions: a) NaH, THF, 0 °C to −78 °C, 1...

Scheme 14: Synthesis of ᴅ-homo(4-octylphenyl)alaninol ((R)-47) and a sphingolipid analogue (R)-48. Reagents an...

Scheme 15: Synthesis of florfenicol ((1R,2S)-49). Reagents and conditions: a) (S)-1-phenylethylamine, TEA, MeO...

Scheme 16: Synthesis of natural tyroscherin ((2S,3R,6E,8R,10R)-55). Reagents and conditions: a) I(CH2)3OTIPS, t...

Scheme 17: Syntheses of (−)-hygrine (S)-61, (−)-hygroline (2S,2'S)-62 and (−)-pseudohygroline (2S,2'R)-62. Rea...

Scheme 18: Synthesis of pyrrolidine (3S,3'R)-68, a fragment of the fluoroquinolone antibiotic PF-00951966. Rea...

Scheme 19: Synthesis of sphingolipid analogues (R)-76. Reagents and conditions: a) BnBr, Mg, THF, reflux, 6 h;...

Scheme 20: Synthesis of ᴅ-threo-PDMP (1R,2R)-81. Reagents and conditions: a) TMSCl, NaI, MeCN, rt, 1 h 50 min,...

Scheme 21: Synthesis of the sphingolipid analogue SG-14 (2S,3S)-84. Reagents and conditions: a) LiAlH4, THF, 0...

Scheme 22: Synthesis of the sphingolipid analogue SG-12 (2S,3R)-88. Reagents and conditions: a) 1-(bromomethyl...

Scheme 23: Synthesis of sphingosine-1-phosphate analogues DS-SG-44 and DS-SG-45 (2S,3R)-89a and (2S,3R)-89a. R...

Scheme 24: Synthesis of N-Boc-safingol ((2S,3S)-95) and N-Boc-ᴅ-erythro-sphinganine ((2S,3R)-95). Reagents and...

Scheme 25: Synthesis of ceramide analogues (2S,3R)-96. Reagents and conditions: a) NaBH4, ZnCl2, MeOH, −78 °C,...

Scheme 26: Synthesis of orthogonally protected serinols, (S)-101 and (R)-102. Reagents and conditions: a) BnBr...

Scheme 27: Synthesis of N-acetyl-3-phenylserinol ((1R,2R)-105). Reagents and conditions: a) AcOH, CH2Cl2, refl...

Scheme 28: Synthesis of (S)-linezolid (S)-107. Reagents and conditions: a) LiAlH4, THF, 0 °C to reflux; b) Boc2...

Scheme 29: Synthesis of (2S,3S,4R)-2-aminooctadecane-1,3,4-triol (ᴅ-ribo-phytosphingosine) (2S,3S,4R)-110. Rea...

Scheme 30: Syntheses of ᴅ-phenylalanine (R)-116. Reagents and conditions: a) AcOH, CH2Cl2, reflux, 4 h; b) MsC...

Scheme 31: Synthesis of N-Boc-ᴅ-3,3-diphenylalanine ((R)-122). Reagents and conditions: a) PhMgBr, THF, −78 °C...

Scheme 32: Synthesis of ethyl N,N’-di-Boc-ʟ-2,3-diaminopropanoate ((S)-125). Reagents and conditions: a) NaN3,...

Scheme 33: Synthesis of the bicyclic amino acid (S)-(+)-127. Reagents and conditions: a) BF3·OEt2, THF, 60 °C,...

Scheme 34: Synthesis of lacosamide, (R)-2-acetamido-N-benzyl-3-methoxypropanamide (R)-130. Reagents and condit...

Scheme 35: Synthesis of N-Boc-norfuranomycin ((2S,2'R)-133). Reagents and conditions: a) H2C=CHCH2I, NaH, THF,...

Scheme 36: Synthesis of MeBmt (2S,3R,4R,6E)-139. Reagents and conditions: a) diisopropyl (S,S)-tartrate (E)-cr...

Scheme 37: Synthesis of (+)-polyoxamic acid (2S,3S,4S)-144. Reagents and conditions: a) AD-mix-α, MeSO2NH2, t-...

Scheme 38: Synthesis of the protected 3-hydroxy-ʟ-glutamic acid (2S,3R)-148. Reagents and conditions: a) LiHMD...

Scheme 39: Synthesis of (+)-isoserine (R)-152. Reagents and conditions: a) AcCl, MeCN, rt, 0.5 h then Na2CO3, ...

Scheme 40: Synthesis of (3R,4S)-N3-Boc-3,4-diaminopentanoic acid (3R,4S)-155. Reagents and conditions: a) Ph3P...

Scheme 41: Synthesis of methyl (2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoate (2S,3S,4S)-159. ...

Scheme 42: Syntheses of methyl (3S,4S) 4,5-di-N-Boc-amino-3-hydroxypentanoate ((3S,4S)-164), methyl (3S,4S)-4-N...

Scheme 43: Syntheses of (3R,5S)-5-(aminomethyl)-3-(4-methoxyphenyl)dihydrofuran-2(3H)-one ((3R,5S)-168). Reage...

Scheme 44: Syntheses of a series of imidazolin-2-one dipeptides 175–177 (for R' and R'' see text). Reagents an...

Scheme 45: Syntheses of (2S,3S)-N-Boc-3-hydroxy-2-hydroxymethylpyrrolidine ((2S,3S)-179). Reagents and conditi...

Scheme 46: Syntheses of enantiomers of 1,4-dideoxy-1,4-imino-ʟ- and -ᴅ-lyxitols (2S,3R,4S)-182 and (2R,3S,4R)-...

Scheme 47: Synthesis of 1,4-dideoxy-1,4-imino-ʟ-ribitol (2S,3S,4R)-182. Reagents and conditions: a) AcOH, CH2Cl...

Scheme 48: Syntheses of 1,4-dideoxy-1,4-imino-ᴅ-arabinitol (2R,3R,4R)-182 and 1,4-dideoxy-1,4-imino-ᴅ-xylitol ...

Scheme 49: Syntheses of natural 2,5-imino-2,5,6-trideoxy-ʟ-gulo-heptitol ((2S,3R,4R,5R)-184) and its C4 epimer...

Scheme 50: Syntheses of (−)-dihydropinidine ((2S,6R)-187a) (R = C3H7) and (2S,6R)-isosolenopsins (2S,6R)-187b ...

Scheme 51: Syntheses of (+)-deoxocassine ((2S,3S,6R)-190a, R = C12H25) and (+)-spectaline ((2S,3S,6R)-190b, R ...

Scheme 52: Synthesis of (−)-microgrewiapine A ((2S,3R,6S)-194a) and (+)-microcosamine A ((2S,3R,6S)-194b). Rea...

Scheme 53: Syntheses of ʟ-1-deoxynojirimycin ((2S,3S,4S,5R)-200), ʟ-1-deoxymannojirimycin ((2S,3S,4S,5S)-200) ...

Scheme 54: Syntheses of 1-deoxy-ᴅ-galacto-homonojirimycin (2R,3S,4R,5S)-211. Reagents and conditions: a) MeONH...

Scheme 55: Syntheses of 7a-epi-hyacinthacine A1 (1S,2R,3R,7aS)-220. Reagents and conditions: a) TfOTBDMS, 2,6-...

Scheme 56: Syntheses of 8-deoxyhyacinthacine A1 ((1S,2R,3R,7aR)-221). Reagents and conditions: a) H2, Pd/C, PT...

Scheme 57: Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, L...

Scheme 58: Syntheses of 8-epi-swainsonine (1S,2R,8S,8aR)-231. Reagents and conditions: a) Ph3P=CHCOOMe, MeOH, ...

Scheme 59: Synthesis of a protected vinylpiperidine (2S,3R)-237, a key intermediate in the synthesis of (−)-sw...

Scheme 60: Synthesis of a modified carbapenem 245. Reagents and conditions: a) AcOEt, LiHMDS, THF, −78 °C, 1.5...
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
- Xiaodong Zhang,
- Wei Wu,
- Zhu Tao and
- Xin-Long Ni
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
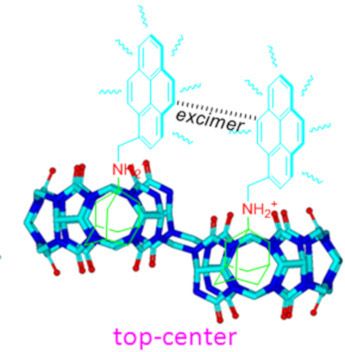
- designed and prepared the ADA-benzyl-based ammonium guest molecules G1. We synthesized this compound because ADA can form a highly stable complex with Q[7] (the highest reported K value for the ADA·Q[7] complex is 1012 M−1) [29]; while benzylammonium ions can be included in both cavities of Q[7] and Q[6

Graphical Abstract

Scheme 1: Chemical structures of host-1, host-2, G1, and G2.

Figure 1: 1H NMR spectra of G1 (1.0 mmol, D2O, pD = 2.0) in the presence of host-1 at different concentration...

Figure 2: 1H NMR spectra of G1 (1.0 mmol, D2O, pD = 2.0) in the presence of host-2 at different concentration...

Scheme 2: Plausible diastereomers showing the fluorescence response of G2 with host-1.

Figure 3: Fluorescence spectral changes of G2 (10.0 μM) in the presence of host-1 (a) and host-2 (b) at diffe...

Figure 4: 1H NMR spectra of G2 (1.0 mmol, D2O, pD = 2.0) in the presence of different concentrations of host-1...
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165


Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.
Different reactivity of phosphorylallenes under the action of Brønsted or Lewis acids: a crucial role of involvement of the P=O group in intra- or intermolecular interactions at the formation of cationic intermediates
- Stanislav V. Lozovskiy,
- Alexander Yu. Ivanov and
- Aleksander V. Vasilyev
Beilstein J. Org. Chem. 2019, 15, 1491–1504, doi:10.3762/bjoc.15.151
- 2). This compound in reaction with AlCl3 without benzene afforded a mixture of allyl alcohol Z-9 and diene E-10a after aqueous work-up (Table 2, entries 1 and 2). The amount of 2.1 equivalents of AlCl3 is sufficient for activation of this transformation (compared to the amount of AlCl3 in entries 1

Graphical Abstract

Figure 1: Allenes 1a–j used in this study.

Scheme 1: Transformations of allene 1g in TfOH leading to the formation of cations E1, E2 and E4 including se...

Figure 2: 31P NMR monitoring of the progress of transformation of E1 into E2 and E4 in TfOH at room temperatu...

Scheme 2: Results of the hydrolysis of cations A–H.

Scheme 3: Preparation of amides 6a,b from cations A, B, and F–H.

Scheme 4: Large-scale one-pot solvent-free synthesis of amides 6a,b from the corresponding propargylic alcoho...

Scheme 5: AlCl3-promoted hydroarylation of allene 1b by benzene leading to alkene Z-11n.

Scheme 6: Reaction of allene 1a with benzene under the action of AlCl3 followed by quenching of the reaction ...

Scheme 7: Multigram-scale one-pot synthesis of indane 12d from 2-methylbut-3-yn-2-ol.

Figure 3: NMR spectra of starting allene 1a (black) and its complex with 1 equivalent of AlCl3 13 (red) in CD2...

Scheme 8: 1H, 13C, and 31P NMR monitoring of AlCl3-promoted reactions of allene 1a leading to compounds E-14 ...

Scheme 9: Plausible reaction mechanism A for the formation of compounds 9, 10, 11, 12 from aillene 1a involvi...

Scheme 10: Plausible reaction mechanism B of formation of compounds 11, 12 from allene 1a involving HCl–AlCl3 ...

Figure 4: Visualization of LUMO, only positive values are shown, isosurface value 0.043: (a) species 16, (b) ...
Synthesis of pyrrolo[1,2-a]quinolines by formal 1,3-dipolar cycloaddition reactions of quinolinium salts
- Anthony Choi,
- Rebecca M. Morley and
- Iain Coldham
Beilstein J. Org. Chem. 2019, 15, 1480–1484, doi:10.3762/bjoc.15.149
- reaction of quinolinium salts bearing electron-withdrawing groups other than ketones, we prepared ester 4 [55] and amide 5 by alkylation of quinoline. Arylidenemalononitriles such as 6a are known to undergo related chemistry [41], so we heated this compound with the quinolinium salts in the presence of

Graphical Abstract

Scheme 1: Reaction of ketone 1 with electron-deficient alkenes 2.

Scheme 2: Reactions of ester 4 and amide 5 with electron-deficient alkenes 6.

Figure 1: Single crystal X-ray structure for 7c.

Scheme 3: Reactions of ester 4 and amide 5 with N-methylmaleimide.

Figure 2: Single crystal X-ray structure for 9.

Scheme 4: Reduction and oxidation of adducts 9 and 10.

Scheme 5: Formation of amides 15a and 15b and Suzuki–Miyaura coupling to yield 16.
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
- Sven Götz,
- Andreas Schneider and
- Arne Lützen
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- elaborated derivatives [58][59] and the ease of a recently established large-scale synthesis reported by Rousseau and co-workers [60]. Hence, we decided to revisit this compound in order to improve the separation in terms of both better resolution of the enantiomeric bowl-shaped conformers and isolation of
- details). Analysis of the data gave a racemization barrier of 114.3 kJ mol−1 which is in good agreement with the value of 114.0 kJ mol−1 reported by Collet for his experiments in dioxane [62]. Perhaps equally interesting for anyone planning to work with this compound this translates into the following

Graphical Abstract

Scheme 1: CTV and chiral CTV derivatives.

Scheme 2: The two enantiomeric crown isomers of chiral CTV 1 and its saddle isomer 1-S.

Scheme 3: Synthesis of CTV 1.

Figure 1: a) Chromatogram of an analytical separation of (rac)-1 on a CHIRALPAK IB column as the stationary p...

Figure 2: a) Chromatogram of the analytical separation of (rac)-1 (CHIRALPAK IB, 100% MeOH, 293 K, flow rate:...

Figure 3: a) 1H NMR spectrum of the neat crown isomers of (rac)-1 in CD3OD (400 MHz, 298 K); b) 1H NMR spectr...

Figure 4: Chromatograms of the analytical separations (CHIRALPAK IB, acetonitrile/water 40:60, 293 K, flow ra...

Figure 5: The mole fractions obtained in the racemization experiment plotted against the time, with black tri...
Synthesis of dipolar molecular rotors as linkers for metal-organic frameworks
- Sebastian Hamer,
- Fynn Röhricht,
- Marius Jakoby,
- Ian A. Howard,
- Xianghui Zhang,
- Christian Näther and
- Rainer Herges
Beilstein J. Org. Chem. 2019, 15, 1331–1338, doi:10.3762/bjoc.15.132
- coupling in a low yield of 10% alongside with phthalocyanine byproducts. The triisopropylsilyl-protected derivative 12b could be obtained in a slightly higher yield of 21%, presumably due to the bulkier TIPS-groups. A crystal structure of this compound was obtained (see Supporting Information File 2). To

Graphical Abstract

Figure 1: Interactions of a pair of dipolar rotors in different orientations. The axes of the rotors are para...

Figure 2: Structures of molecular dipolar rotors/linker molecules 1–5.

Scheme 1: General synthetic strategy to prepare the dipolar rotors 1–5.

Scheme 2: Synthesis of 3,3'-(2,3-difluoro-1,4-phenylene)dipropiolic acid (1) starting with diiodination of 1,...

Scheme 3: Synthesis of 3,3'-(5,6-Difluoro-2,1,3-benzothiadiazol-4,7-diyl)dipropiolic acid (2) and 3,3'-(5,6-D...

Scheme 4: Synthesis of 3,3'-(5,6-dicyano-1,3-benzodioxole-4,7-diyl)dipropiolic acid (4) and 3,3'-(6,7-dicyano...
Genomics-inspired discovery of massiliachelin, an agrochelin epimer from Massilia sp. NR 4-1
- Jan Diettrich,
- Hirokazu Kage and
- Markus Nett
Beilstein J. Org. Chem. 2019, 15, 1298–1303, doi:10.3762/bjoc.15.128
- -producing bacterium Massilia sp. NR 4-1 and predicted to direct the biosynthesis of a molecule that is structurally related to the thiazoline-containing siderophore micacocidin. In order to track this compound, we analyzed the metabolic profiles of Massilia cultures grown under different iron concentrations

Graphical Abstract

Figure 1: A) Organization of the micacocidin-type gene cluster from Massilia sp. NR 4-1 (top) and of the mic ...

Figure 2: Structures of massiliachelin (1), agrochelin (2), micacocidin (3), pyochelin I (4), pyochelin II (5...
Steroid diversification by multicomponent reactions
- Leslie Reguera,
- Cecilia I. Attorresi,
- Javier A. Ramírez and
- Daniel G. Rivera
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- library of androstanic derivatives 21 (Figure 2B) and their reduced analogues bearing the 3β-hydroxy group were biologically tested showing antifungal activity against Fusarium virguliforme and Fusarium solani. The generation of this compound library suffered from the formation of the Passerini reaction

Graphical Abstract

Figure 1: Structures of natural steroids of A) animal and B) plant origin.

Scheme 1: Synthesis of a steroidal β-lactam by Ugi reaction of a cholanic aldehyde [14].

Scheme 2: Synthetic route to steroidal 2,5-diketopiperazines based on a diastereoselective Ugi-4CR with an an...

Scheme 3: Multicomponent synthesis of a heterocycle–steroid hybrid using a ketosteroid as carbonyl component [18]....

Scheme 4: Synthesis of peptidomimetic–steroid hybrids using the Ugi-4CR with spirostanic amines and carboxyli...

Scheme 5: Synthesis of azasteroids using the Ugi-4CR with androstanic and pregnanic carboxylic acids [22].

Figure 2: Ugi-4CR-derived library of androstanic azasteroids with diverse substitution patterns at the phenyl...

Scheme 6: Synthesis of 4-azacholestanes by an intramolecular Ugi-4C-3R [26].

Scheme 7: Synthesis of amino acid–steroid hybrid by multiple Ugi-4CR using steroidal isocyanides [29].

Scheme 8: Synthesis of ecdysteroid derivatives by Ugi-4CR using a steroidal isocyanide [30].

Scheme 9: Stereoselective multicomponent synthesis of a steroid–tetrahydropyridine hybrid using a chiral bifu...

Scheme 10: Pd(II)-catalyzed three-component reaction with an alkynyl seco-cholestane [34].

Scheme 11: Multicomponent synthesis of steroid–thiazole hybrids from a steroidal ketone [36].

Scheme 12: Synthesis of cholanic pseudo-peptide derivatives by novel MCRs based on the reactivity of ynamide [37,38].

Scheme 13: Synthesis of steroid-fused pyrimidines and pyrimidones using the Biginelli-3CR [39,42,43].

Scheme 14: Synthesis of steroidal pyridopyrimidines by a reaction sequence comprising a 4CR followed by a post...

Scheme 15: Synthesis of steroid-fused pyrimidines by MCR of 2-hydroxymethylene-3-ketosteroids [46].

Scheme 16: Synthesis of steroid-fused naphthoquinolines by the Kozlov–Wang MCR using ketosteroids [50,51].

Scheme 17: Conjugation of steroids to carbohydrates and peptides by the Ugi-4CR [62,63].

Scheme 18: Solid-phase multicomponent conjugation of peptides to steroids by the Ugi-4CR [64].

Scheme 19: Solid-phase multicomponent conjugation of peptides to steroids by the Petasis-3CR [68].

Scheme 20: Synthesis of steroidal macrobicycles (cages) by multiple multicomponent macrocyclizations based on ...

Scheme 21: One-pot synthesis of steroidal cages by double Ugi-4CR-based macrocyclizations [76].
Novel (2-amino-4-arylimidazolyl)propanoic acids and pyrrolo[1,2-c]imidazoles via the domino reactions of 2-amino-4-arylimidazoles with carbonyl and methylene active compounds
- Victoria V. Lipson,
- Tetiana L. Pavlovska,
- Nataliya V. Svetlichnaya,
- Anna A. Poryvai,
- Nikolay Yu. Gorobets,
- Erik V. Van der Eycken,
- Irina S. Konovalova,
- Svetlana V. Shiskina,
- Alexander V. Borisov,
- Vladimir I. Musatov and
- Alexander V. Mazepa
Beilstein J. Org. Chem. 2019, 15, 1032–1045, doi:10.3762/bjoc.15.101
- (CYP11B2) and 11β-hydroxylase (CYP11B1), which is responsible for cortisol production [35]. This compound is under development for the treatment of Cushing's syndrome and pituitary ACTH hypersecretion [36]. From this point of view the approach to pyrrolo[1,2-c]imidazole moiety by using acyclic methylene

Graphical Abstract

Figure 1: 2-Aminoimidazole alkaloids from marine sponges.

Figure 2: The Knoevenagel–Michael adduct [24] and expected products.

Scheme 1: The three component condensation of imidazo[1,2-a]pyridine, aldehydes and Meldrum’s acid described ...

Figure 3: Molecular structure of 1-([1,1'-biphenyl]-4-yl)-5-oxo-7-phenyl-6,7-dihydro-5H-pyrrolo[1,2-c]imidazo...

Scheme 2: Two forms of cation 9i.

Figure 4: Molecular structure of 3-(2-amino-4-phenyl-1H-imidazol-5-yl)-3-(p-tolyl)propanoic acid 11b accordin...

Scheme 3: Three forms of the compound 11b in the crystal phase.

Scheme 4: Synthesis of the mixture of compounds 13 and 14.

Figure 5: Molecular structure of aminoimidazo[1,2-c]pyrrole 16a according to X-ray diffraction data. Thermal ...

Scheme 5: Resonance structures of 16a.

Figure 6: 3,3’-Spirooxindole alkaloids.

Figure 7: Molecular structure of aminoimidazo[1,2-c]pyrrole 19a according to X-ray diffraction data. Thermal ...

Scheme 6: Resonance structures of 19a.
Mechanistic investigations on multiproduct β-himachalene synthase from Cryptosporangium arvum
- Jan Rinkel and
- Jeroen S. Dickschat
Beilstein J. Org. Chem. 2019, 15, 1008–1019, doi:10.3762/bjoc.15.99
- comparison with commercially available standards (Figures S6–S9, Supporting Information File 1). The non-enzymatic degradation of GPP as a background reaction to 15 resulted in a substantial loss of stereoinformation for this compound (7% ee). Also the cyclised products 10, 11 and 13 where not obtained in
- most products. However, in the side product 7 C-1 remains untouched after 1,11-cyclisation, which allows to investigate the stereochemical course of the first cyclisation step for this compound. First, the absolute configuration of 7 was assigned as shown in Figure 2 from the incubation experiments

Graphical Abstract

Figure 1: Total ion chromatograms of hexane extracts from the incubations of HcS with A) FPP, B) GPP and C) G...

Figure 2: Structures of HcS products arising A) from FPP together with related oxidation product 9, B) from G...

Scheme 1: Initial steps of the cyclisation of GPP towards monoterpene products [34]. Both pathways are likely co-...

Scheme 2: Late stage cyclisations of the himachalyl cation B to HcS products 1–6. Alternative mechanistic and...

Figure 3: EI mass spectrum of 1 arising from an incubation of (2-2H)GPP and IPP with FPPS and HcS showing a l...

Figure 4: Stereochemical course of the final deprotonation step towards 3, 5 and 6 investigated by GC–MS. EI ...

Scheme 3: Proposed cyclisation mechanism towards cation B via an initial 1,11-cyclisation (path A) and an hyp...

Figure 5: Total ion chromatogram of hexane extracts from HcS incubations with A) (R)-NPP, B) (S)-NPP and C) (...

Figure 6: The origin of the two diastereotopic methyl groups in 1. Partial 13C NMR spectrum of A) unlabelled 1...

Figure 7: Stereochemical course of the 1,11-cyclisation at C-1 for 7. Partial HSQC spectra of HcS incubation ...

Figure 8: Investigation of the 1,3-hydride shift in the cyclisation towards 1. Partial 13C NMR spectra of A) ...

Figure 9: Stereochemical course of the 1,3-hydride shift at C-10 in 1. Partial HSQC spectra of A) unlabelled 1...

Figure 10: Position specific mass shift analysis for selected EIMS ions of HcS products. Black dots represent ...
Diaminoterephthalate–α-lipoic acid conjugates with fluorinated residues
- Leon Buschbeck,
- Aleksandra Markovic,
- Gunther Wittstock and
- Jens Christoffers
Beilstein J. Org. Chem. 2019, 15, 981–991, doi:10.3762/bjoc.15.96
- of the wavelengths, the electron-withdrawing ALA-amide unit was electronically decoupled from the DAT chromophore by introducing a propylene spacer. Thus, diethyl DAT was first equipped with an N-Alloc-protected 3-aminopropyl side chain at one nitrogen function following a literature protocol. This
- compound, 5, was then submitted to reductive amination with para-(trifluoromethyl)benzaldehyde. After Alloc deprotection, the side chain was amidated with ALA following the same COMU–DIPEA protocol as described for 3. The second target compound 7 was obtained in 59% yield over three steps. Indeed, the

Graphical Abstract

Figure 1: Bifunctional DAT as a cross-linker for proteins. The compound is a turn-on-probe, i.e., the fluores...

Scheme 1: Preparation of DAT–ALA conjugate 3 with fluorinated benzyl residue.

Scheme 2: Preparation of conjugate 7 consisting of fluorinated DAT and ALA moieties with an additional propyl...

Figure 2: High-resolution XPS of SAM 3.

Figure 3: High-resolution XPS of SAM 7.
Towards the preparation of synthetic outer membrane vesicle models with micromolar affinity to wheat germ agglutinin using a dialkyl thioglycoside
- Dimitri Fayolle,
- Nathalie Berthet,
- Bastien Doumeche,
- Olivier Renaudet,
- Peter Strazewski and
- Michele Fiore
Beilstein J. Org. Chem. 2019, 15, 937–946, doi:10.3762/bjoc.15.90

- 8 showed the highest inhibitory effect of this study with an IC50 of 11 µM corresponding to a 3000-fold improvement compared to the monomer control. This result suggests a higher participation of sugar units in the lectin binding for this compound. Docking calculation for model glycolipids To

Graphical Abstract

Figure 1: Structure of the β-thiols 1a and 1b and of the commercial alkenes 2a and 2b.

Scheme 1: Synthesis of the n-alkyl thioglycosides 3–5, 7 and 8. Detailed reaction conditions are reported in ...

Scheme 2: Synthesis of the lipophilic scaffold 6; DMAP = N,N-dimethylaminopyridine.

Figure 2: Periodic monitoring by 1H NMR (300 MHz, DMF-d7) of the formation of product 8 from a mixture compou...

Figure 3: Micrographs of giant vesicles and lipid aggregates obtained from the gentle hydration (in PBS, pH 7...

Figure 4: A simplified (and not in scale) representation of the ELLA assay, to study the interaction between ...

Figure 5:
Inhibition curves for the binding of WGA-HRP to PAA-GlcNAc by D-GlcNAc The symbols (■), () and (○) ...

Figure 6: Main poses obtained from docking experiments. WGA (PDB 2UVO) surface is shown in white for monomer ...
A diastereoselective approach to axially chiral biaryls via electrochemically enabled cyclization cascade
- Hong Yan,
- Zhong-Yi Mao,
- Zhong-Wei Hou,
- Jinshuai Song and
- Hai-Chao Xu
Beilstein J. Org. Chem. 2019, 15, 795–800, doi:10.3762/bjoc.15.76
- stereoselectivity of the reaction was not controlled by relative thermodynamic stability of the diastereomers. The major isomer of 3c, which contained a sterically less demanding Ph group at the R1 position (cf. Table 1), did not isomerize at room temperature for 1 year. However, heating a solution of this compound

Graphical Abstract

Scheme 1: Reaction design.

Scheme 2: Scope of electrochemical synthesis of axially chiral biaryls. Reaction conditions: undivided cell, 2...

Scheme 3: Proposed reaction mechanism for the electrochemical synthesis of 3a.

Scheme 4: Computation investigation on the vinyl radical cyclization. DFT (M06-2X/6-31G*) calculated energeti...
Synthesis of polydicyclopentadiene using the Cp2TiCl2/Et2AlCl catalytic system and thin-layer oxidation of the polymer in air
- Zhargolma B. Bazarova,
- Ludmila S. Soroka,
- Alex A. Lyapkov,
- Мekhman S. Yusubov and
- Francis Verpoort
Beilstein J. Org. Chem. 2019, 15, 733–745, doi:10.3762/bjoc.15.69

- . Indeed, as reported in previously published papers [15][16], the colored blue complex under these conditions is caused by a compound containing Ti(III) or Ti(IV). This compound corresponds to the final [Cp2TiEt]+·[AlEtCl3]− complex. The presence of an isosbestic point at 656 nm indicates the presence of

Graphical Abstract

Figure 1: Absorption spectra in the UV and visible spectral region: 1) bis(cyclopentadienyl)titan dichloride (...

Figure 2: Absorption spectra in the visible spectral region: 1) Cp2TiCl2·AlEt2Cl (toluene, 10 mmol/L, Ti/Al r...

Figure 3: 1Н NMR spectra of tricyclopentadiene (a) and the interaction product between Cp2TiCl2 and AlEt2Cl w...

Scheme 1: Mechanism of alkylation of Cp2TiCl2.

Figure 4: Visible spectra of a mixture of Cp2TiCl2 and AlEt2Cl as function of time.

Figure 5: Thermometric curve of DCPD polymerization using the catalyst system based on Cp2TiCl2 (a) and its s...

Scheme 2: The structures formed as a result of the cationic polymerization of dicyclopentadiene.

Scheme 3: The units resulting from ROMP of dicyclopentadiene.

Scheme 4: Mechanism of ROMP dicyclopentadiene.

Figure 6: FTIR spectrum of PDCPD obtained in toluene with the catalyst system based on Cp2TiCl2 and AlEt2Cl.

Figure 7: 1Н NMR spectrum of PDCPD obtained with the catalytic system based on Cp2TiCl2 and AlEt2Cl.

Figure 8: GPC traces for two samples of DCPD polymers obtained at a concentration of Cp2TiCl2/AlEt2Cl complex...

Figure 9: IR spectra of cationic polymerized dicyclopentadiene taken after certain periods of time exposed to...

Figure 10: Correlation of intensities of vibrational bands at 1620 and 700 cm−1 and layer exposure time in air...

Figure 11: DSC exotherm for PDCPD subjected to air oxidation for 700 hours.

Figure 12: DSC exotherm for PDCPD subjected to unexposed film: 1) in air atmosphere; 2) in argon.

Scheme 5: Possible radical formation in the reaction (1).

Scheme 6: The first step of the chain propagation.

Figure 13: Dependence of intensities of adsorption bands at 1410 and 700 cm−1 and dwell time of the layer in a...

Figure 14: Semi-logarithmic kinetic curve of PDCPD oxidation in air (thin layer on silicon) with respect to in...

Figure 15: The distribution of oxygen concentration in the polymer layer: 1 – a layer of oxidized cross-linked...

Figure 16: Dependence of the ratio of adsorption bands at 1700 and 700 cm−1 on the exposure time of the layer ...

Figure 17: Infrared spectra (a) of products of cationic polymerization of DCPD, stabilized with an antioxidant...
The LANCA three-component reaction to highly substituted β-ketoenamides – versatile intermediates for the synthesis of functionalized pyridine, pyrimidine, oxazole and quinoxaline derivatives
- Tilman Lechel,
- Roopender Kumar,
- Mrinal K. Bera,
- Reinhold Zimmer and
- Hans-Ulrich Reissig
Beilstein J. Org. Chem. 2019, 15, 655–678, doi:10.3762/bjoc.15.61
- give α-methoxy carbonyl compound 7. The isolation of this compound supports our mechanistic proposal as shown in Scheme 2 and it shows that sterically very hindered carboxylic acids are probably poor components in the route to β-ketoenamides. A systematic study of this possible limitation was not
- (see Scheme 10). This compound was converted into PM59 by the standard cyclocondensation reaction (Scheme 15) leading to a pyridin-4-ol moiety that was converted to the nonaflate. Compound PM60 bears a pyrimidyl and a pyridinyl substituent at the 2,2’-position of the biphenyl part [41]. Synthesis of
- approach to complex heterocycles. The aldehyde PM69 was further converted into the terminal alkyne PM73 by employing the Bestmann–Ohira protocol (Scheme 21). After its Sonogashira reaction with iodobenzene to the intermediate disubstituted alkyne PM74 this compound was converted into furopyrimidine

Graphical Abstract

Scheme 1: Discovery of the LANCA three-component reaction. The reaction of pivalonitrile (1) with lithiated m...

Scheme 2: Proposed mechanism of the LANCA three-component reaction to β-ketoenamides KE and pyridin-4-ol deri...

Scheme 3: One-pot preparation of pyridin-4-ols PY and their subsequent transformations to highly substituted ...

Scheme 4: Synthesis of β-ketoenamides KE by the LANCA three-component reaction of alkoxyallenes, nitriles and...

Scheme 5: β-Ketoenamides KE36–43 derived from enantiopure components.

Scheme 6: Bis-β-ketoenamides KE44–46 derived from aromatic dicarboxylic acids.

Scheme 7: Conversion of alkyl propargyl ethers E into aryl-substituted β-ketoenamides KEAr and selected produ...

Scheme 8: Condensation of LANCA-derived β-ketoenamides KE with ammonium salts to give 5-alkoxy-substituted py...

Scheme 9: Synthesis of PM31–35 from β-ketoenamides KE37, KE38, KE40, KE41 and KE78 obtained by method A (NH4O...

Scheme 10: Synthesis of bis-pyrimidine derivatives PM36, PM39 and PM40 from β-ketoenamides KE44–46 by method A...

Scheme 11: Functionalization of pyrimidine derivatives PM through selenium dioxide oxidations of PM5, PM9, PM15...

Scheme 12: Conversion of 2-vinyl-substituted pyrimidine PM7 into aldehyde PM50; (NMO = N-methylmorpholine N-ox...

Scheme 13: Deprotection of 5-alkoxy-substituted pyrimidines PM2, PM20 and PM29 and conversion into nonaflates ...

Scheme 14: Palladium-catalyzed coupling reactions of PM54 and PM12 giving rise to new pyrimidine derivatives P...

Scheme 15: Synthesis of pyrimidyl-substituted pyridyl nonaflate PM60.

Scheme 16: Condensation of LANCA-derived β-ketoenamides KE with hydroxylamine hydrochloride leading to pyrimid...

Scheme 17: Reactions of β-ketoenamides KE15 and KE7 with hydroxylamine hydrochloride leading to pyrimidine N-o...

Scheme 18: Structures of pyrimidine N-oxides PO30–33 derived from β-ketoenamides KE43, KE45, KE78 and KE80.

Scheme 19: Reduction of PO4 to PM5 and Boekelheide rearrangements of PO13, PO14, PO4 and PO30 to 4-acetoxymeth...

Scheme 20: Deprotection of 4-acetoxymethyl-substituted pyrimidine derivatives PM61 and PM63, oxidations to for...

Scheme 21: Synthesis of pyrimidinyl-substituted alkyne PM74 and conversion into furopyrimidine PM75 and Sonoga...

Scheme 22: Trifluoroacetic acid-promoted conversion of LANCA-derived β-ketoenamides KE into oxazoles OX and 1,...

Scheme 23: Conversion of β-ketoenamide KE79 into oxazole OX16 and transformation into 5-styryl-substituted oxa...

Scheme 24: Mechanisms of the formation of 1,2-diketones DK and of acetyl-substituted oxazole derivatives OX.

Scheme 25: Hydrogenolyses of benzyloxy-substituted β-ketoenamides KE52 and KE54 to 1,2-diketone DK14 and to di...

Scheme 26: Conversions of 2,4-dicyclopropyl-substituted oxazole OX7 into oxazole derivatives OX18–20 (PPA = po...

Scheme 27: Syntheses of vinyl and ethynyl-substituted oxazole derivatives OX21 and OX23 and their palladium-ca...

Scheme 28: Synthesis of C3-symmetric oxazole derivative OX28 and the STM current image of its 1-phenyloctane s...

Scheme 29: Condensation of 1,2-diketones DK with o-phenylenediamine to quinoxalines QU1–7 (CAN = cerium ammoni...

Scheme 30: The LANCA three-component reaction leading to β-ketoenamides KE and the structure of functionalized...
Cyclopropene derivatives of aminosugars for metabolic glycoengineering
- Jessica Hassenrück and
- Valentin Wittmann
Beilstein J. Org. Chem. 2019, 15, 584–601, doi:10.3762/bjoc.15.54
- high importance. Until now, the carbamate-linked methylcyclopropenes Ac4GlcNCyoc and Ac4GalNCyoc are the only cyclopropene derivatives that were examined in this context [25][26]. Ac4GlcNCyoc was used to visualize protein-specific glycosylation inside living cells [32]. However, this compound is

Graphical Abstract

Figure 1: Cyclopropene-modified mannosamine, glucosamine and galactosamine derivatives employed for MGE.

Figure 2: A) Reaction of ManNCyc and ManNCp, respectively, with Tz-PEG-OH to determine second-order rate cons...

Scheme 1: MGE with cyclopropene-modified mannosamines. Cells were grown with sugar for 48 hours and then incu...

Figure 3: HEK 293T cells were grown with 100 μM Ac4ManNCyc, Ac4ManNCp, Ac4ManNCyoc or DMSO only (negative con...

Scheme 2: Synthesis of Ac4ManNCp(H2) and Ac4ManNCyc(H2) and the corresponding DMB-labeled sialic acids. C/A =...

Scheme 3: Synthesis of Ac4ManNCyoc(H2) and the corresponding DMB-labeled sialic acid.

Scheme 4: Synthesis of Ac4GlcNCp and Ac4GalNCp.

Figure 4: HEK 293T cells were grown with 100 μM Ac4ManNCp, Ac4GlcNCp, Ac4GalNCp or DMSO only (negative contro...

Figure 5: HEK 293T cells were grown with 100 μM Ac4GlcNCp, Ac4GalNCp or DMSO only (negative control) for 48 h...

Figure 6: HEK 293T cells were grown with 50 μM (A) or 100 μM (B) Ac4GlcNCp, Ac4GlcNCyoc or DMSO only (negativ...

Figure 7: Western blot analysis of soluble glycoproteins. HEK 293T cells were grown for 48 h with 100 μM Ac4M...

Scheme 5: Synthesis of Ac4GlcNCp(H2) and Ac4GlcNCyoc(H2).
Synthesis and SAR of the antistaphylococcal natural product nematophin from Xenorhabdus nematophila
- Frank Wesche,
- Hélène Adihou,
- Thomas A. Wichelhaus and
- Helge B. Bode
Beilstein J. Org. Chem. 2019, 15, 535–541, doi:10.3762/bjoc.15.47
- that showed nanomolar activity against S. aureus [20]. To the detriment of this compound class, all derivatives lose their antibacterial activity in the presence of serum in vitro in serial broth and agar dilution method [16][20]. Even with the use of charged groups as modifiers, serum-protein binding

Graphical Abstract

Scheme 1: General procedure for the synthesis of nematophin and related derivatives. i) 1.5 equiv α-keto carb...

Scheme 2: Synthesis of azatryptamines (4-azatryptamine (4ATRA) and 1-methyl-4-azatryptamine (1M4ATRA)). i) 5....

Scheme 3: Synthesis of azatryptamines (7-azatryptamine (7ATRA) and 1-methyl-7-azatryptamine (1M7ATRA)). i) 5....
Selectivity in multiple multicomponent reactions: types and synthetic applications
- Ouldouz Ghashghaei,
- Francesca Seghetti and
- Rodolfo Lavilla
Beilstein J. Org. Chem. 2019, 15, 521–534, doi:10.3762/bjoc.15.46
- (Scheme 11B). This compound does not react under the initial reaction conditions, but can be forced to do so in a Joullié MCR with a new isocyanide/carboxylic acid pair [47]. Incidentally, this last process is also remarkably stereoselective, something very unusual in Ugi-type reactions. Finally, to

Graphical Abstract

Scheme 1: Selectivity levels found in multiple multicomponent reactions. I) Innate selectivity; II) sequentia...

Scheme 2: Indiscriminate double Ugi MCR upon pyridine-2,6-dicarboxylic acid.

Scheme 3: Representative examples of MCR-polymer synthesis. A) Biginelli HTS of polymers; B) Passerini;- C) U...

Scheme 4: Concept of multicomponent macrocyclization.

Scheme 5: Supramolecular structures out of MMCR macrocyclizations.

Scheme 6: Macrocyclization by MMCRs. A) Staudinger MCR; B) boronic-imine MCR.

Scheme 7: Selective Sequential MMCRs. A and B) MCRs involving terephthalaldehyde; C) Multiple GBB processes w...

Scheme 8: Biased substrates for selective MMCRs.

Scheme 9: The Union concept. A) Asinger–Ugi combination; B) Passerini–Ugi/azide from anthranilic acid; C) Pas...

Scheme 10: Relevant examples of consecutive MCRs exploiting the Union Concept. A) Petasis-Ugi combination; B) ...

Scheme 11: Selective MMCRs featuring FGs with distinct reactivity along the sequence. A) Synthesis of aminomet...

Scheme 12: High order MMCRs. A) Ugi/Ugi–Smiles 7C combination; B) imidazoline-N-cyanomethylamide-Ugi union lea...

Scheme 13: Consecutive Ugi 4CR-deprotection–Ugi 4CR strategy towards A) PNA oligomers and B) peptidic tetrazol...

Scheme 14: Sequential Ugi 4CR-deprotection access to cyclopeptoids.

Scheme 15: Stepwise access to 6-aminopenicillanic acid derivative through an Asinger, deprotection, Joullié ap...

Scheme 16: A triple MCR-deprotection approach affording anticancer peptidomimetics.
Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives
- Mikhail V. Makarov and
- Marie E. Migaud
Beilstein J. Org. Chem. 2019, 15, 401–430, doi:10.3762/bjoc.15.36


Graphical Abstract

Figure 1: Structural formulas of Nam, NA, NR+, NMN, and NAD+.

Figure 2: Main synthetic routes to nicotinamide riboside (NR+X−).

Scheme 1: Synthesis of NR+Cl− based on the reaction of peracylated chlorosugars with Nam.

Figure 3: Predominant formation of β-anomer over α-anomer of NR+X−.

Scheme 2: Synthesis of NR+Cl− by reacting 3,5-di-O-benzoyl-D-ribofuranosyl chloride (5) with Nam (1a).

Figure 4: Mechanism of the formation of the β-anomer of the glycosylated product in the case of the reaction ...

Scheme 3: Synthesis of NR+Br− by reacting bromosugars with Nam (1a).

Scheme 4: Synthesis of NR+OTf− based on the glycosylation of Nam (1a) with tetra-O-acetyl-β-D-ribofuranose (2a...

Scheme 5: Improved synthesis of NR+OTfˉ and NAR+OTfˉ based on the glycosylation of pre-silylated Nam or NA wi...

Scheme 6: Synthesis of triacetylated NAR+OTf− by glycosylation of nicotinic acid trimethylsilyl ester with te...

Scheme 7: Synthesis of NR+Cl− from NR+OTf− by means of ion exchange with sodium chloride solution.

Scheme 8: Synthesis of acylated NR+OTf− by means of ion exchange with sodium chloride.

Scheme 9: Synthesis of triacetylated derivatives of NAR+ by glycosylation of nicotinic acid esters with ribos...

Scheme 10: Synthesis of NR+OTf− from the triflate salt of ethyl nicotinate-2,3,5-triacetyl-β-D-riboside in met...

Scheme 11: Reaction of 2,3,5-tri-O-acetyl-β-phenyl nicotinate riboside triflate salt with secondary and tertia...

Scheme 12: Synthesis of NMN based on the Zincke reaction of N-(2,4-dinitrophenyl)-3-carbamoylpyridinium chlori...

Scheme 13: Synthesis of NMN based on the Zincke reaction of N-(2,4-dinitrophenyl)-3-carbamoylpyridinium chlori...

Scheme 14: Efficacious protection of 2′,3′-hydroxy groups of NR+X−.

Scheme 15: Protection of the 2′,3′-hydroxy groups of NR+Cl– with a mesitylmethylene acetal group.

Figure 5: Reduction of derivatives of NR+Xˉ into corresponding 1,2-; 1,4-; 1,6-NRH derivatives.

Figure 6: Mechanism of the reduction of the pyridinium core with dithionite as adapted from [67].

Scheme 16: Reduction of triacylated NR+OTf– derivatives by sodium dithionite followed by complete removal of a...

Figure 7: Structural formulas of iridium and rhodium catalysts (a)–(d) for regeneration of NAD(P)H from NAD(P)...

Figure 8: Two approaches to synthesis of 5′-derivatives of NR+.

Scheme 17: Synthesis of NMN starting from NR+ salt.

Scheme 18: Efficient synthesis of NMN by phosphorylation of 2′,3′-O-isopropylidene-NR+ triflate followed by re...

Scheme 19: Synthesis of a bisphosphonate analogue of β-NAD+ based on DCC-induced conjugation of 2′,3′-O-isopro...

Scheme 20: Synthesis of 5′-acyl and 2′,3′,5′-triacyl derivatives of NR+.

Figure 9: Structural formulas of NMN analogues 39–41.

Scheme 21: Synthesis of 5′-phosphorylated derivatives of NR+ using a “reduction–modification–oxidation” approa...

Scheme 22: Synthesis of 5′-phosphorylated derivatives of NR+ using a “reduction–modification–reoxidation” appr...

Figure 10: Structural formulas of 5′-phosphorylated derivatives of NR+.

Scheme 23: Synthesis of 5′-phosphorylated derivatives of NR+ using a direct NR+ phosphorylation approach.

Figure 11: Structural formulas of amino acid NR+ conjugates.

Scheme 24: Synthesis of amino acid NR+ conjugates using NRH and protected amino acid under CDI-coupling condit...

Figure 12: Chemical structures of known isotopically labelled NR+ analogues and derivatives.

Scheme 25: Synthesis of [2′-3H]-NR+ and [2′-3H]-NMN.

Scheme 26: Synthesis of α- and β-anomers of [1′-2H]-NMN.
Sigmatropic rearrangements of cyclopropenylcarbinol derivatives. Access to diversely substituted alkylidenecyclopropanes
- Guillaume Ernouf,
- Jean-Louis Brayer,
- Christophe Meyer and
- Janine Cossy
Beilstein J. Org. Chem. 2019, 15, 333–350, doi:10.3762/bjoc.15.29
- (TFDA) [68] to a solution of propargyl glycolates 64a–n containing NaF in diglyme at 120 °C. Difluorocyclopropene 65a could be purified by flash chromatography on silica gel and was isolated in 86% yield but this compound rapidly underwent decomposition upon storage. The instability of glycolates 65a–n

Graphical Abstract

Scheme 1: Representative strategies for the formation of alkylidenecyclopropanes from cyclopropenes and scope...

Scheme 2: [2,3]-Sigmatropic rearrangement of phosphinites 2a–h.

Scheme 3: [2,3]-Sigmatropic rearrangement of a phosphinite derived from enantioenriched cyclopropenylcarbinol...

Scheme 4: Selective reduction of phosphine oxide (E)-3f.

Scheme 5: Attempted thermal [2,3]-sigmatropic rearrangement of phosphinite 6a.

Scheme 6: Computed activation barriers and free enthalpies.

Scheme 7: [2,3]-Sigmatropic rearrangement of phosphinites 6a–j.

Scheme 8: Proposed mechanism for the Lewis base-catalyzed rearrangement of phosphinites 6.

Scheme 9: [3,3]-Sigmatropic rearrangement of tertiary cyclopropenylcarbinyl acetates 10a–c.

Scheme 10: [3,3]-Sigmatropic rearrangement of secondary cyclopropenylcarbinyl esters 10d–h.

Scheme 11: [3,3]-Sigmatropic rearrangement of trichoroacetimidates 12a–i.

Scheme 12: Reaction of trichloroacetamide 13f with pyrrolidine.

Scheme 13: Catalytic hydrogenation of (arylmethylene)cyclopropropane 13f.

Scheme 14: Instability of trichloroacetimidates 21a–c derived from cyclopropenylcarbinols 20a–c.

Scheme 15: [3,3]-Sigmatropic rearrangement of cyanate 27 generated from cyclopropenylcarbinyl carbamate 26.

Scheme 16: Synthesis of alkylidene(aminocyclopropane) derivatives 30–37 from carbamate 26.

Scheme 17: Scope of the dehydration–[3,3]-sigmatropic rearrangement sequence of cyclopropenylcarbinyl carbamat...

Scheme 18: Formation of trifluoroacetamide 50 from carbamate 49.

Scheme 19: Formation of alkylidene[(N-trifluoroacetylamino)cyclopropanes] 51–54.

Scheme 20: Diastereoselective hydrogenation of alkylidenecyclopropane 51.

Scheme 21: Ireland–Claisen rearrangement of cyclopropenylcarbinyl glycolates 56a–l.

Scheme 22: Synthesis and Ireland–Claisen rearrangement of glycolate 61 possessing gem-diester substitution at ...

Scheme 23: Synthesis of alkylidene(gem-difluorocyclopropanes) 66a–h, and 66k–n from propargyl glycolates 64a–n....

Scheme 24: Ireland–Claisen rearrangement of N,N-diBoc glycinates 67a and 67b.

Scheme 25: Diastereoselective hydrogenation of alkylidenecyclopropanes 58a and 74.

Scheme 26: Synthesis of functionalized gem-difluorocyclopropanes 76 and 77 from alkylidenecyclopropane 66a.

Scheme 27: Access to oxa- and azabicyclic compounds 78–80.






