Search results
Search for "carbonylation" in Full Text gives 48 result(s) in Beilstein Journal of Organic Chemistry.
Enantioselective desymmetrization strategy of prochiral 1,3-diols in natural product synthesis
- Lihua Wei,
- Rui Yang,
- Zhifeng Shi and
- Zhiqiang Ma
Beilstein J. Org. Chem. 2025, 21, 1932–1963, doi:10.3762/bjoc.21.151
- acetate gave monoacetate 111 in 98% yield and >98% ee. Subsequently, monoacetate 111 was converted into compound 112 with a 1,3-dioxan-2-one moiety in three steps, which underwent Pd-catalyzed decarboxylation/carbonylation to form the lactone 113. The N-methylimidazole ring was installed through a three

Graphical Abstract

Scheme 1: General mechanism of a lipase-catalyzed esterification.

Scheme 2: Shishido’s synthesis of (−)-xanthorrhizol (4) and (+)-heliannuol D (8).

Scheme 3: Shishido’s synthesis of a) (−)-heliannuol A (15) and b) heliannuol G (20) and heliannuol H (21).

Scheme 4: Deska’s synthesis of hyperione A (30) and ent-hyperione B (31).

Scheme 5: Huang’s synthesis of (+)-brazilin (37).

Scheme 6: Shishido’s synthesis of (−)-heliannuol D (42) and (+)-heliannuol A (43).

Scheme 7: Chênevert’s synthesis of (S)-α-tocotrienol (49).

Scheme 8: Kita’s synthesis of monoester 53.

Scheme 9: Kita’s synthesis of fredericamycin A (60).

Scheme 10: Takabe’s synthesis of (E)-3,7-dimethyl-2-octene-1,8-diol (64).

Scheme 11: Takabe’s synthesis of (18S)-variabilin (70).

Scheme 12: Kawasaki’s synthesis of (S)-Rosaphen (74) and (R)-Rosaphen (75).

Scheme 13: Tokuyama’s synthesis of a) (−)-petrosin (84) and b) (+)-petrosin (86).

Scheme 14: Fukuyama’s synthesis of leustroducsin B (96).

Scheme 15: Nanda’s synthesis of a) fragment 100, b) fragment 106 and c) (−)-rasfonin (109).

Scheme 16: Davies’ synthesis of (+)-pilocarpine (115) and (+)-isopilocarpine (116).

Scheme 17: Ōmura’s synthesis of salinosporamide A (125).

Scheme 18: Kang’s synthesis of ʟ-cladinose (124) and its derivative.

Scheme 19: Kang’s preparation of fragment 139.

Scheme 20: Kang’s synthesis of azithromycin (149).

Scheme 21: Kang’s synthesis of (−)-dysiherbaine (156).

Scheme 22: Kang’s synthesis of (−)-kaitocephalin (166).

Scheme 23: Kang’s synthesis of laidlomycin (180).

Scheme 24: Snyder’s synthesis of arboridinine (190).

Scheme 25: Ma’s synthesis of (+)-alstrostine G (203).

Scheme 26: Trost’s synthesis of (−)-18-epi-peloruside A (215).

Scheme 27: Lindel’s synthesis of (–)-dihydroraputindole (223).

Scheme 28: Iwata’s synthesis of a) (−)-talaromycin B (232) and b) (+)-talaromycin A (235).

Scheme 29: Cook’s synthesis of a) (−)-vincamajinine (240) and b) (−)-11-methoxy-17-epivincamajine (245).

Scheme 30: Cook’s synthesis of (+)-dehydrovoachalotine (249) and voachalotine (250).

Scheme 31: Cook’s synthesis of a) (−)-12-methoxy-Nb-methylvoachalotine (257) and b) (+)-polyneuridine, macusin...

Scheme 32: Trauner’s synthesis of stephadiamine (273).

Scheme 33: Garg’s synthesis of (–)-ψ-akuammigine (285).

Scheme 34: Ding’s synthesis of (+)-18-benzoyldavisinol (293) and (+)-davisinol (294).
Photoswitches beyond azobenzene: a beginner’s guide
- Michela Marcon,
- Christoph Haag and
- Burkhard König
Beilstein J. Org. Chem. 2025, 21, 1808–1853, doi:10.3762/bjoc.21.143


Graphical Abstract

Figure 1: Energy diagram of a two-state photoswitch. Figure 1 was redrawn from [2].

Figure 2: Example of the absorption spectra of the isomers of a photoswitch with most efficient irradiation w...

Scheme 1: Photoswitch classes described in this review.

Figure 3: Azoheteroarenes.

Scheme 2: E–Z Isomerisation (top) and mechanisms of thermal Z–E isomerisation (bottom).

Scheme 3: Rotation mechanism favoured by the electron displacement in push–pull systems. Selected examples of...

Figure 4: A) T-shaped and twisted Z-isomers determine the thermal stability and the Z–E-PSS (selected example...

Figure 5: Effect of di-ortho-substitution on thermal half-life and PSS.

Figure 6: Selected thermal lifetimes of azoindoles in different solvents and concentrations. aConcentration o...

Figure 7: Aryliminopyrazoles: N-pyrazoles (top) and N-phenyl (bottom).

Scheme 4: Synthesis of symmetrical heteroarenes through oxidation (A), reduction (B), and the Bayer–Mills rea...

Scheme 5: Synthesis of diazonium salt (A); different strategies of azo-coupling: with a nucleophilic ring (B)...

Scheme 6: Synthesis of arylazothiazoles 25 (A) and heteroaryltriazoles 28 (B).

Scheme 7: Synthesis of heteroarylimines 31a,b [36-38].

Figure 8: Push–pull non-ionic azo dye developed by Velasco and co-workers [45].

Scheme 8: Azopyridine reported by Herges and co-workers [46].

Scheme 9: Photoinduced phase transitioning azobispyrazoles [47].

Figure 9: Diazocines.

Scheme 10: Isomers, conformers and enantiomers of diazocine.

Scheme 11: Partial overlap of the ππ* band with electron-donating substituents and effect on the PSS. Scheme 11 was ada...

Figure 10: Main properties of diazocines with different bridges. aMeasured in n-hexane [56]. bMeasured in THF. cMe...

Scheme 12: Synthesis of symmetric diazocines.

Scheme 13: Synthesis of asymmetric diazocines.

Scheme 14: Synthesis of O- and S-heterodiazocines.

Scheme 15: Synthesis of N-heterodiazocines.

Scheme 16: Puromycin diazocine photoswitch [60].

Figure 11: Indigoids.

Figure 12: The main representatives of the indigoid photoswitch class.

Scheme 17: Deactivation process that prevents Z-isomerisation of indigo.

Figure 13: Stable Z-indigo derivative synthesised by Wyman and Zenhäusern [67].

Figure 14: Selected examples of indigos with aliphatic and aromatic substituents [68]. Dashed box: proposed π–π in...

Scheme 18: Resonance structures of indigo and thioindigo involving the phenyl ring.

Scheme 19: Possible deactivation mechanism for 4,4'-dihydroxythioindigo [76].

Scheme 20: Effect of different heteroaryl rings on the stability and the photophysical properties of hemiindig...

Figure 15: Thermal half-lives of red-shifted hemithioindigos in toluene [79]. aMeasured in toluene-d8.

Scheme 21: Structures of pyrrole [81] and imidazole hemithioindigo [64].

Figure 16: Examples of fully substituted double bond hemithioindigo (left), oxidised hemithioindigos (centre),...

Scheme 22: Structure of iminothioindoxyl 72 (top) and acylated phenyliminoindolinone photoswitch 73 (bottom). ...

Scheme 23: (top) Transition states of iminothioindoxyl 72. The planar transition state is associated with a lo...

Scheme 24: Baeyer–Drewsen synthesis of indigo (top) and N-functionalisation strategies (bottom).

Scheme 25: Synthesis of hemiindigo.

Scheme 26: Synthesis of hemithioindigo and iminothioindoxyl.

Scheme 27: Synthesis of double-bond-substituted hemithioindigos.

Scheme 28: Synthesis of phenyliminoindolinone.

Scheme 29: Hemithioindigo molecular motor [85].

Figure 17: Arylhydrazones.

Scheme 30: Switching of arylhydrazones. Note: The definitions of stator and rotor are arbitrary.

Scheme 31: Photo- and acidochromism of pyridine-based phenylhydrazones.

Scheme 32: A) E–Z thermal inversion of a thermally stable push–pull hydrazone [109]. B) Rotation mechanism favoured...

Scheme 33: Effect of planarisation on the half-life.

Scheme 34: The longest thermally stable hydrazone switches reported so far (left). Modulation of thermal half-...

Figure 18: Dependency of t1/2 on concentration and hypothesised aggregation-induced isomerisation.

Figure 19: Structure–property relationship of acylhydrazones.

Scheme 35: Synthesis of arylhydrazones.

Scheme 36: Synthesis of acylhydrazones.

Scheme 37: Photoswitchable fluorophore by Aprahamian et al. [115].

Scheme 38: The four-state photoswitch synthesised by the Cigáň group [116].

Figure 20: Diarylethenes.

Scheme 39: Isomerisation and oxidation pathway of E-stilbene to phenanthrene.

Scheme 40: Strategies adapted to avoid E–Z isomerisation and oxidation.

Scheme 41: Molecular orbitals and mechanism of electrocyclisation for a 6π system.

Figure 21: Aromatic stabilisation energy correlated with the thermal stability of the diarylethenes [127,129].

Figure 22: Half-lives of diarylethenes with increasing electron-withdrawing groups [128,129].

Scheme 42: Photochemical degradation pathway promoted by electron-donating groups [130].

Figure 23: The diarylethenes studied by Hanazawa et al. [134]. Increased rigidity leads to bathochromic shift.

Scheme 43: The dithienylethene synthesised by Nakatani's group [135].

Scheme 44: Synthesis of perfluoroalkylated diarylethenes.

Scheme 45: Synthesis of 139 and 142 via McMurry coupling.

Scheme 46: Synthesis of symmetrical derivatives 145 via Suzuki–Miyaura coupling.

Scheme 47: Synthesis of acyclic 148, malonic anhydride 149, and maleimide derivatives 154.

Figure 24: Gramicidin S (top left) and two of the modified diarylethene derivatives: first generation (bottom ...

Scheme 48: Pyridoxal 5'-phosphate and its reaction with an amino acid (top). The analogous dithienylethene der...

Figure 25: Fulgides.

Scheme 49: The three isomers of fulgides.

Scheme 50: Thermal and photochemical side products of unsubstituted fulgide [150].

Figure 26: Maximum absorption λc of the closed isomer compared with the nature of the aromatic ring and the su...

Scheme 51: Possible rearrangement of the excited state of 5-dimethylaminoindolylfulgide [153].

Figure 27: Quantum yields of ring closure (ΦE→C) and E–Z isomerisation (ΦE→Z) correlated with the increasing s...

Scheme 52: Active (Eα) and inactive (Eβ) conformers (left) and the bicyclic sterically blocked fulgide 169 (ri...

Scheme 53: Quantum yield of ring-opening (ΦC→E) and E–Z isomerisation (ΦE→Z) for different substitution patter...

Scheme 54: Stobbe condensation pathway for the synthesis of fulgides 179, fulgimides 181 and fulgenates 178.

Scheme 55: Alternative synthesis of fulgides through Pd-catalysed carbonylation.

Scheme 56: Optimised synthesis of fulgimides [166].

Scheme 57: Photoswitchable FRET with a fulgimide photoswitch [167].

Scheme 58: Three-state fulgimide strategy by Slanina's group.

Figure 28: Spiropyrans.

Scheme 59: Photochemical (left) and thermal (right) ring-opening mechanisms for an exemplary spiropyran with a...

Figure 29: Eight possible isomers of the open merocyanine according to the E/Z configurations of the bonds hig...

Scheme 60: pH-Controlled photoisomerisation between the closed spiropyran 191-SP and the open E-merocyanine 19...

Scheme 61: Behaviour of spiropyran in water buffer according to Andréasson and co-workers [180]. 192-SP in an aqueo...

Scheme 62: (left box) Proposed mechanism of basic hydrolysis of MC [184]. (right box) Introduction of electron-dona...

Scheme 63: Photochemical interconversion of naphthopyran 194 (top) and spirooxazine 195 (bottom) photoswitches...

Scheme 64: Synthesis of spiropyrans and spirooxazines 198 and the dicondensation by-product 199.

Scheme 65: Alternative synthesis of spiropyrans and spirooxazines with indolenylium salt 200.

Scheme 66: Synthesis of 4’-substituted spiropyrans 203 by condensation of an acylated methylene indoline 201 w...

Scheme 67: Synthesis of spironaphthopyrans 210 by acid-catalysed condensation of naphthols and diarylpropargyl...

Scheme 68: Photoswitchable surface wettability [194].

Figure 30: Some guiding principles for the choice of the most suitable photoswitch. Note that this guide is ve...
Recent advances in oxidative radical difunctionalization of N-arylacrylamides enabled by carbon radical reagents
- Jiangfei Chen,
- Yi-Lin Qu,
- Ming Yuan,
- Xiang-Mei Wu,
- Heng-Pei Jiang,
- Ying Fu and
- Shengrong Guo
Beilstein J. Org. Chem. 2025, 21, 1207–1271, doi:10.3762/bjoc.21.98

Graphical Abstract

Scheme 1: DTBP-mediated oxidative alkylarylation of activated alkenes.

Scheme 2: Iron-catalyzed oxidative 1,2-alkylarylation.

Scheme 3: Possible mechanism for the iron-catalyzed oxidative 1,2-alkylation of activated alkenes.

Scheme 4: A metal-free strategy for synthesizing 3,3-disubstituted oxindoles.

Scheme 5: Iminoxyl radical-promoted cascade oxyalkylation/alkylarylation of alkenes.

Scheme 6: Proposed mechanism for the iminoxyl radical-promoted cascade oxyalkylation/alkylarylation of alkene...

Scheme 7: Bicyclization of 1,n-enynes with alkyl nitriles.

Scheme 8: Possible reaction mechanism for the bicyclization of 1,n-enynes with alkyl nitriles.

Scheme 9: Radical cyclization of N-arylacrylamides with isocyanides.

Scheme 10: Plausible mechanism for the radical cyclization of N-arylacrylamides with isocyanides.

Scheme 11: Electrochemical dehydrogenative cyclization of 1,3-dicarbonyl compounds.

Scheme 12: Plausible mechanism for the dehydrogenative cyclization of 1,3-dicarbonyl compounds.

Scheme 13: Photocatalyzed cyclization of N-arylacrylamide and N,N-dimethylaniline.

Scheme 14: Proposed mechanism for the photocatalyzed cyclization of N-arylacrylamides and N,N-dimethylanilines....

Scheme 15: Electrochemical monofluoroalkylation cyclization of N-arylacrylamides with dimethyl 2-fluoromalonat...

Scheme 16: Proposed mechanism for the electrochemical radical cyclization of N-arylacrylamides with dimethyl 2...

Scheme 17: Photoelectrocatalytic carbocyclization of unactivated alkenes using simple malonates.

Scheme 18: Plausible mechanism for the photoelectrocatalytic carbocyclization of unactivated alkenes with simp...

Scheme 19: Bromide-catalyzed electrochemical trifluoromethylation/cyclization of N-arylacrylamides.

Scheme 20: Proposed mechanism for the electrochemical trifluoromethylation/cyclization of N-arylacrylamides.

Scheme 21: Visible light-mediated trifluoromethylarylation of N-arylacrylamides.

Scheme 22: Plausible reaction mechanism for the visible light-mediated trifluoromethylarylation of N-arylacryl...

Scheme 23: Electrochemical difluoroethylation cyclization of N-arylacrylamides with sodium difluoroethylsulfin...

Scheme 24: Electrochemical difluoroethylation cyclization of N-methyacryloyl-N-alkylbenzamides with sodium dif...

Scheme 25: Photoredox-catalyzed radical aryldifluoromethylation of N-arylacrylamides with S-(difluoromethyl)su...

Scheme 26: Proposed mechanism for the photoredox-catalyzed radical aryldifluoromethylation of N-arylacrylamide...

Scheme 27: Visible-light-induced domino difluoroalkylation/cyclization of N-cyanamide alkenes.

Scheme 28: Proposed mechanism of photoredox-catalyzed radical domino difluoroalkylation/cyclization of N-cyana...

Scheme 29: Palladium-catalyzed oxidative difunctionalization of alkenes.

Scheme 30: Two possible mechanisms of palladium-catalyzed oxidative difunctionalization.

Scheme 31: Silver-catalyzed oxidative 1,2-alkyletherification of unactivated alkenes with α-bromoalkylcarbonyl...

Scheme 32: Photochemical radical cascade cyclization of dienes.

Scheme 33: Proposed mechanism for the photochemical radical cascade 6-endo cyclization of dienes with α-carbon...

Scheme 34: Photocatalyzed radical coupling/cyclization of N-arylacrylamides and.

Scheme 35: Photocatalyzed radical-type couplings/cyclization of N-arylacrylamides with sulfoxonium ylides.

Scheme 36: Possible mechanism of visible-light-induced radical-type couplings/cyclization of N-arylacrylamides...

Scheme 37: Visible-light-promoted difluoroalkylated oxindoles systhesis via EDA complexes.

Scheme 38: Possible mechanism for the visible-light-promoted radical cyclization of N-arylacrylamides with bro...

Scheme 39: A dicumyl peroxide-initiated radical cascade reaction of N-arylacrylamide with DCM.

Scheme 40: Possible mechanism of radical cyclization of N-arylacrylamides with DCM.

Scheme 41: An AIBN-mediated radical cascade reaction of N-arylacrylamides with perfluoroalkyl iodides.

Scheme 42: Possible mechanism for the reaction with perfluoroalkyl iodides.

Scheme 43: Photoinduced palladium-catalyzed radical annulation of N-arylacrylamides with alkyl halides.

Scheme 44: Radical alkylation/cyclization of N-Alkyl-N-methacryloylbenzamides with alkyl halides.

Scheme 45: Possible mechanism for the alkylation/cyclization with unactivated alkyl chlorides.

Scheme 46: Visible-light-driven palladium-catalyzed radical cascade cyclization of N-arylacrylamides with unac...

Scheme 47: NHC-catalyzed radical cascade cyclization of N-arylacrylamides with alkyl bromides.

Scheme 48: Possible mechanism of NHC-catalyzed radical cascade cyclization.

Scheme 49: Electrochemically mediated radical cyclization reaction of N-arylacrylamides with freon-type methan...

Scheme 50: Proposed mechanistic pathway of electrochemically induced radical cyclization reaction.

Scheme 51: Redox-neutral photoinduced radical cascade cylization of N-arylacrylamides with unactivated alkyl c...

Scheme 52: Proposed mechanistic hypothesis of redox-neutral radical cascade cyclization.

Scheme 53: Thiol-mediated photochemical radical cascade cylization of N-arylacrylamides with aryl halides.

Scheme 54: Proposed possible mechanism of thiol-mediated photochemical radical cascade cyclization.

Scheme 55: Visible-light-induced radical cascade bromocyclization of N-arylacrylamides with NBS.

Scheme 56: Possible mechanism of visible-light-induced radical cascade cyclization.

Scheme 57: Decarboxylation/radical C–H functionalization by visible-light photoredox catalysis.

Scheme 58: Plausible mechanism of visible-light photoredox-catalyzed radical cascade cyclization.

Scheme 59: Visible-light-promoted tandem radical cyclization of N-arylacrylamides with N-(acyloxy)phthalimides....

Scheme 60: Plausible mechanism for the tandem radical cyclization reaction.

Scheme 61: Visible-light-induced aerobic radical cascade alkylation/cyclization of N-arylacrylamides with alde...

Scheme 62: Plausible mechanism for the aerobic radical alkylarylation of electron-deficient amides.

Scheme 63: Oxidative decarbonylative [3 + 2]/[5 + 2] annulation of N-arylacrylamide with vinyl acids.

Scheme 64: Plausible mechanism for the decarboxylative (3 + 2)/(5 + 2) annulation between N-arylacrylamides an...

Scheme 65: Rhenium-catalyzed alkylarylation of alkenes with PhI(O2CR)2.

Scheme 66: Plausible mechanism for the rhenium-catalyzed decarboxylative annulation of N-arylacrylamides with ...

Scheme 67: Visible-light-induced one-pot tandem reaction of N-arylacrylamides.

Scheme 68: Plausible mechanism for the visible-light-initiated tandem synthesis of difluoromethylated oxindole...

Scheme 69: Copper-catalyzed redox-neutral cyanoalkylarylation of activated alkenes with cyclobutanone oxime es...

Scheme 70: Plausible mechanism for the copper-catalyzed cyanoalkylarylation of activated alkenes.

Scheme 71: Photoinduced alkyl/aryl radical cascade for the synthesis of quaternary CF3-attached oxindoles.

Scheme 72: Plausible photoinduced electron-transfer (PET) mechanism.

Scheme 73: Photoinduced cerium-mediated decarboxylative alkylation cascade cyclization.

Scheme 74: Plausible reaction mechanism for the decarboxylative radical-cascade alkylation/cyclization.

Scheme 75: Metal-free oxidative tandem coupling of activated alkenes.

Scheme 76: Control experiments and possible mechanism for 1,2-carbonylarylation of alkenes with carbonyl C(sp2...

Scheme 77: Silver-catalyzed acyl-arylation of activated alkenes with α-oxocarboxylic acids.

Scheme 78: Proposed mechanism for the decarboxylative acylarylation of acrylamides.

Scheme 79: Visible-light-mediated tandem acylarylation of olefines with carboxylic acids.

Scheme 80: Proposed mechanism for the radical cascade cyclization with acyl radical via visible-light photored...

Scheme 81: Erythrosine B-catalyzed visible-light photoredox arylation-cyclization of N-arylacrylamides with ar...

Scheme 82: Electrochemical cobalt-catalyzed radical cyclization of N-arylacrylamides with arylhydrazines or po...

Scheme 83: Proposed mechanism of radical cascade cyclization via electrochemical cobalt catalysis.

Scheme 84: Copper-catalyzed oxidative tandem carbamoylation/cyclization of N-arylacrylamides with hydrazinecar...

Scheme 85: Proposed reaction mechanism for the radical cascade cyclization by copper catalysis.

Scheme 86: Visible-light-driven radical cascade cyclization reaction of N-arylacrylamides with α-keto acids.

Scheme 87: Proposed mechanism of visible-light-driven cascade cyclization reaction.

Scheme 88: Peroxide-induced radical carbonylation of N-(2-methylallyl)benzamides with methyl formate.

Scheme 89: Proposed cyclization mechanism of peroxide-induced radical carbonylation with N-(2-methylallyl)benz...

Scheme 90: Persulfate promoted carbamoylation of N-arylacrylamides and N-arylcinnamamides.

Scheme 91: Proposed mechanism for the persulfate promoted radical cascade cyclization reaction of N-arylacryla...

Scheme 92: Photocatalyzed carboacylation with N-arylpropiolamides/N-alkyl acrylamides.

Scheme 93: Plausible mechanism for the photoinduced carboacylation of N-arylpropiolamides/N-alkyl acrylamides.

Scheme 94: Electrochemical Fe-catalyzed radical cyclization with N-arylacrylamides.

Scheme 95: Plausible mechanism for the electrochemical Fe-catalysed radical cyclization of N-phenylacrylamide.

Scheme 96: Substrate scope of the selective functionalization of various α-ketoalkylsilyl peroxides with metha...

Scheme 97: Proposed reaction mechanism for the Fe-catalyzed reaction of alkylsilyl peroxides with methacrylami...

Scheme 98: EDA-complex mediated C(sp2)–C(sp3) cross-coupling of TTs and N-methyl-N-phenylmethacrylamides.

Scheme 99: Proposed mechanism for the synthesis of oxindoles via EDA complex.
Recent advances in synthetic approaches for bioactive cinnamic acid derivatives
- Betty A. Kustiana,
- Galuh Widiyarti and
- Teni Ernawati
Beilstein J. Org. Chem. 2025, 21, 1031–1086, doi:10.3762/bjoc.21.85

- scaled up to a gram scale. Uozumi and co-workers (2019) reported a carbonylation under aqueous flow conditions using alkenyl halide 240 to prepare cinnamic acid (7) catalyzed by an amphiphilic polystyrene-poly(ethylene glycol) resin-supported Pd-diphenylphosphine catalyst (cat 6) (Scheme 65A) [31]. Also
- product 245 or the corresponding Z-isomeric product 246, both in good yields. Alkenyl sulfides have also been used for the preparation of cinnamic acid esters. For example, Wu and co-workers (2020) studied the Pd-catalyzed carbonylation of alkenyl sulfides in the presence of NHC ligands via C–S cleavage

Graphical Abstract

Figure 1: Biologically active cinnamic acid derivatives.

Scheme 1: General synthetic strategies for cinnamic acid derivatizations.

Scheme 2: Cinnamic acid coupling via isobutyl anhydride formation.

Scheme 3: Amidation reaction via O/N-pivaloyl activation.

Scheme 4: Cinnamic acid amidation using TCCA/PPh3 reagent.

Scheme 5: Cinnamic acid amidation using triazine-based reagents.

Scheme 6: Cinnamic acid amidation using continuous flow mechanochemistry.

Scheme 7: Cinnamic acid amidation using COMU as coupling reagent.

Scheme 8: Cinnamic acid amidation using allenone coupling reagent.

Scheme 9: Cinnamic acid amidation using 4-acetamidophenyl triflimide as reagent.

Scheme 10: Cinnamic acid amidation using methyltrimethoxysilane (MTM).

Scheme 11: Cinnamic acid amidation utilizing amine–borane reagent.

Scheme 12: Cinnamic acid amidation using TCCA/PPh3 reagent.

Scheme 13: Cinnamic acid amidation using PPh3/I2 reagent.

Scheme 14: Cinnamic acid amidation using PCl3 reagent.

Scheme 15: Cinnamic acid amidation utilizing pentafluoropyridine (PFP) as reagent.

Scheme 16: Cinnamic acid amidation using hypervalent iodine(III).

Scheme 17: Mechanochemical amidation using 1,1,2,2-tetrafluoroethyl-N,N-dimethylamine (TFEDMA) reagent.

Scheme 18: Methyl ester preparation using tris(2,4,6-trimethoxyphenyl)phosphine (TMPP).

Scheme 19: N-Trifluoromethyl amide preparation using isothiocyanate and AgF.

Scheme 20: POCl3-mediated amide coupling of carboxylic acid and DMF.

Scheme 21: O-Alkylation of cinnamic acid using alkylating agents.

Scheme 22: Glycoside preparation via Mitsunobu reaction.

Scheme 23: O/N-Acylation via rearrangement reactions.

Scheme 24: Amidation reactions using sulfur-based alkylating agents.

Scheme 25: Amidation reaction catalyzed by Pd0 via C–N cleavage.

Scheme 26: Amidation reaction catalyzed by CuCl/PPh3.

Scheme 27: Cu(II) triflate-catalyzed N-difluoroethylimide synthesis.

Scheme 28: Cu/Selectfluor-catalyzed transamidation reaction.

Scheme 29: CuO–CaCO3-catalyzed amidation reaction.

Scheme 30: Ni-catalyzed reductive amidation.

Scheme 31: Lewis acidic transition-metal-catalyzed O/N-acylations.

Scheme 32: Visible-light-promoted amidation of cinnamic acid.

Scheme 33: Sunlight/LED-promoted amidation of cinnamic acid.

Scheme 34: Organophotocatalyst-promoted N–O cleavage of Weinreb amides to synthesize primary amides.

Scheme 35: Cinnamamide synthesis through [Ir] photocatalyst-promoted C–N-bond cleavage of tertiary amines.

Scheme 36: Blue LED-promoted FeCl3-catalyzed reductive transamidation.

Scheme 37: FPyr/TCT-catalyzed amidation of cinnamic acid derivative 121.

Scheme 38: Cs2CO3/DMAP-mediated esterification.

Scheme 39: HBTM organocatalyzed atroposelective N-acylation.

Scheme 40: BH3-catalyzed N-acylation reactions.

Scheme 41: Borane-catalyzed N-acylation reactions.

Scheme 42: Catalytic N-acylation reactions via H/F bonding activation.

Scheme 43: Brønsted base-catalyzed synthesis of cinnamic acid esters.

Scheme 44: DABCO/Fe3O4-catalyzed N-methyl amidation of cinnamic acid 122.

Scheme 45: Catalytic oxidation reactions of acylating agents.

Scheme 46: Preparation of cinnamamide-substituted benzocyclooctene using I(I)/I(III) catalysis.

Scheme 47: Pd-colloids-catalyzed oxidative esterification of cinnamyl alcohol.

Scheme 48: Graphene-supported Pd/Au alloy-catalyzed oxidative esterification via hemiacetal intermediate.

Scheme 49: Au-supported on A) carbon nanotubes (CNT) and B) on porous boron nitride (pBN) as catalyst for the ...

Scheme 50: Cr-based catalyzed oxidative esterification of cinnamyl alcohols with H2O2 as the oxidant.

Scheme 51: Co-based catalysts used for oxidative esterification of cinnamyl alcohol.

Scheme 52: Iron (A) and copper (B)-catalyzed oxidative esterification of cinnamaldehyde.

Scheme 53: NiHPMA-catalyzed oxidative esterification of cinnamaldehyde.

Scheme 54: Synthesis of cinammic acid esters through NHC-catalyzed oxidative esterification via intermolecular...

Scheme 55: Redox-active NHC-catalyzed esterification via intramolecular oxidation.

Scheme 56: Electrochemical conversion of cinnamaldehyde to methyl cinnamate.

Scheme 57: Bu4NI/TBHP-catalyzed synthesis of bisamides from cinnamalaldehyde N-tosylhydrazone.

Scheme 58: Zn/NC-950-catalyzed oxidative esterification of ketone 182.

Scheme 59: Ru-catalyzed oxidative carboxylation of terminal alkenes.

Scheme 60: Direct carboxylation of alkenes using CO2.

Scheme 61: Carboxylation of alkenylboronic acid/ester.

Scheme 62: Carboxylation of gem-difluoroalkenes with CO2.

Scheme 63: Photoredox-catalyzed carboxylation of difluoroalkenes.

Scheme 64: Ru-catalyzed carboxylation of alkenyl halide.

Scheme 65: Carboxylation of alkenyl halides under flow conditions.

Scheme 66: Cinnamic acid ester syntheses through carboxylation of alkenyl sulfides/sulfones.

Scheme 67: Cinnamic acid derivatives synthesis through a Ag-catalyzed decarboxylative cross-coupling proceedin...

Scheme 68: Pd-catalyzed alkyne hydrocarbonylation.

Scheme 69: Fe-catalyzed alkyne hydrocarbonylation.

Scheme 70: Alkyne hydrocarboxylation using CO2.

Scheme 71: Alkyne hydrocarboxylation using HCO2H as CO surrogate.

Scheme 72: Co/AlMe3-catalyzed alkyne hydrocarboxylation using DMF.

Scheme 73: Au-catalyzed oxidation of Au–allenylidenes.

Scheme 74: Pd-catalyzed C–C-bond activation of cyclopropenones to synthesize unsaturated esters and amides.

Scheme 75: Ag-catalyzed C–C-bond activation of diphenylcyclopropenone.

Scheme 76: Cu-catalyzed C–C bond activation of diphenylcyclopropenone.

Scheme 77: PPh3-catalyzed C–C-bond activation of diphenylcyclopropenone.

Scheme 78: Catalyst-free C–C-bond activation of diphenylcyclopropenone.

Scheme 79: Cu-catalyzed dioxolane cleavage.

Scheme 80: Multicomponent coupling reactions.

Scheme 81: Pd-catalyzed partial hydrogenation of electrophilic alkynes.

Scheme 82: Nickel and cobalt as earth-abundant transition metals used as catalysts for the partial hydrogenati...

Scheme 83: Metal-free-catalyzed partial hydrogenation of conjugated alkynes.

Scheme 84: Horner–Wadsworth–Emmons reaction between triethyl 2-fluoro-2-phosphonoacetate and aldehydes with ei...

Scheme 85: Preparation of E/Z-cinnamates using thiouronium ylides.

Scheme 86: Transition-metal-catalyzed ylide reactions.

Scheme 87: Redox-driven ylide reactions.

Scheme 88: Noble transition-metal-catalyzed olefination via carbenoid species.

Scheme 89: TrBF4-catalyzed olefination via carbene species.

Scheme 90: Grubbs catalyst (cat 7)/photocatalyst-mediated metathesis reactions.

Scheme 91: Elemental I2-catalyzed carbonyl-olefin metathesis.

Scheme 92: Cu-photocatalyzed E-to-Z isomerization of cinnamic acid derivatives.

Scheme 93: Ni-catalyzed E-to-Z isomerization.

Scheme 94: Dehydration of β-hydroxy esters via an E1cB mechanism to access (E)-cinnamic acid esters.

Scheme 95: Domino ring-opening reaction induced by a base.

Scheme 96: Dehydroamination of α-aminoester derivatives.

Scheme 97: Accessing methyl cinnamate (44) via metal-free deamination or decarboxylation.

Scheme 98: The core–shell magnetic nanosupport-catalyzed condensation reaction.

Scheme 99: Accessing cinnamic acid derivatives from acetic acid esters/amides through α-olefination.

Scheme 100: Accessing cinnamic acid derivatives via acceptorless α,β-dehydrogenation.

Scheme 101: Cu-catalyzed formal [3 + 2] cycloaddition.

Scheme 102: Pd-catalyzed C–C bond formation via 1,4-Pd-shift.

Scheme 103: NHC-catalyzed Rauhut–Currier reactions.

Scheme 104: Heck-type reaction for Cα arylation.

Scheme 105: Cu-catalyzed trifluoromethylation of cinnamamide.

Scheme 106: Ru-catalyzed alkenylation of arenes using directing groups.

Scheme 107: Earth-abundant transition-metal-catalyzed hydroarylation of α,β-alkynyl ester 374.

Scheme 108: Precious transition-metal-catalyzed β-arylation of cinnamic acid amide/ester.

Scheme 109: Pd-catalyzed β-amination of cinnamamide.

Scheme 110: S8-mediated β-amination of methyl cinnamate (44).

Scheme 111: Pd-catalyzed cross-coupling reaction of alkynyl esters with phenylsilanes.

Scheme 112: Pd-catalyzed β-cyanation of alkynyl amide/ester.

Scheme 113: Au-catalyzed β-amination of alkynyl ester 374.

Scheme 114: Metal-free-catalyzed Cβ-functionalizations of alkynyl esters.

Scheme 115: Heck-type reactions.

Scheme 116: Mizoroki–Heck coupling reactions using unconventional functionalized arenes.

Scheme 117: Functional group-directed Mizoroki–Heck coupling reactions.

Scheme 118: Pd nanoparticles-catalyzed Mizoroki–Heck coupling reactions.

Scheme 119: Catellani-type reactions to access methyl cinnamate with multifunctionalized arene.

Scheme 120: Multicomponent coupling reactions.

Scheme 121: Single atom Pt-catalyzed Heck coupling reaction.

Scheme 122: Earth-abundant transition metal-catalyzed Heck coupling reactions.

Scheme 123: Polymer-coated earth-abundant transition metals-catalyzed Heck coupling reactions.

Scheme 124: Earth-abundant transition-metal-based nanoparticles as catalysts for Heck coupling reactions.

Scheme 125: CN- and Si-based directing groups to access o-selective cinnamic acid derivatives.

Scheme 126: Amide-based directing group to access o-selective cinnamic acid derivatives.

Scheme 127: Carbonyl-based directing group to access o-selective cinnamic acid derivatives.

Scheme 128: Stereoselective preparation of atropisomers via o-selective C(sp2)–H functionalization.

Scheme 129: meta-Selective C(sp2)–H functionalization using directing group-tethered arenes.

Scheme 130: para-Selective C(sp2)–H functionalization using directing group-tethered arenes.

Scheme 131: Non-directed C(sp2)–H functionalization via electrooxidative Fujiwara–Moritani reaction.

Scheme 132: Interconversion of functional groups attached to cinnamic acid.

Scheme 133: meta-Selective C(sp2)–H functionalization of cinnamate ester.

Scheme 134: C(sp2)–F arylation using Grignard reagents.

Scheme 135: Truce–Smiles rearrangement of N-aryl metacrylamides.

Scheme 136: Phosphine-catalyzed cyclization of γ-vinyl allenoate with enamino esters.
Recent advances in controllable/divergent synthesis
- Jilei Cao,
- Leiyang Bai and
- Xuefeng Jiang
Beilstein J. Org. Chem. 2025, 21, 890–914, doi:10.3762/bjoc.21.73

- tactics in chemical synthesis. Ligand-controlled regiodivergent C1 insertion into arynes [19]. Ligand effect in homogenous gold catalysis enabling regiodivergent π-bond-activated cyclization [20]. Ligand-controlled palladium(II)-catalyzed regiodivergent carbonylation of alkynes [21]. Catalyst-controlled

Graphical Abstract

Scheme 1: Ligand-controlled regiodivergent C1 insertion into arynes [19].

Scheme 2: Ligand effect in homogenous gold catalysis enabling regiodivergent π-bond-activated cyclization [20].

Scheme 3: Ligand-controlled palladium(II)-catalyzed regiodivergent carbonylation of alkynes [21].

Scheme 4: Catalyst-controlled annulations of strained cyclic allenes with π-allyl palladium complexes and pro...

Scheme 5: Ring expansion of benzosilacyclobutenes with alkynes [23].

Scheme 6: Photoinduced regiodivergent and enantioselective cross-coupling [24].

Scheme 7: Catalyst-controlled regiodivergent and enantioselective formal hydroamination of N,N-disubstituted ...

Scheme 8: Catalyst-tuned regio- and enantioselective C(sp3)–C(sp3) coupling [31].

Scheme 9: Catalyst-controlled annulations of bicyclo[1.1.0]butanes with vinyl azides [32].

Scheme 10: Solvent-driven reversible macrocycle-to-macrocycle interconversion [39].

Scheme 11: Unexpected solvent-dependent reactivity of cyclic diazo imides and mechanism [40].

Scheme 12: Palladium-catalyzed annulation of prochiral N-arylphosphonamides with aromatic iodides [41].

Scheme 13: Time-dependent enantiodivergent synthesis [42].

Scheme 14: Time-controlled palladium-catalyzed divergent synthesis of silacycles via C–H activation [43].

Scheme 15: Proposed mechanism for the time-controlled palladium-catalyzed divergent synthesis of silacycles [43].

Scheme 16: Metal-free temperature-controlled regiodivergent borylative cyclizations of enynes [45].

Scheme 17: Nickel-catalyzed switchable site-selective alkene hydroalkylation by temperature regulation [46].

Scheme 18: Copper-catalyzed decarboxylative amination/hydroamination sequence [48].

Scheme 19: Proposed mechanism of copper-catalyzed decarboxylative amination/hydroamination sequence [48].

Scheme 20: Enantioselective chemodivergent three-component radical tandem reactions [49].

Scheme 21: Substrate-controlled synthesis of indoles and 3H-indoles [52].

Scheme 22: Controlled mono- and double methylene insertions into nitrogen–boron bonds [53].

Scheme 23: Copper-catalyzed substrate-controlled carbonylative synthesis of α-keto amides and amides [54].

Scheme 24: Divergent sulfur(VI) fluoride exchange linkage of sulfonimidoyl fluorides and alkynes [55].

Scheme 25: Modular and divergent syntheses of protoberberine and protonitidine alkaloids [56].
Advances in radical peroxidation with hydroperoxides
- Oleg V. Bityukov,
- Pavel Yu. Serdyuchenko,
- Andrey S. Kirillov,
- Gennady I. Nikishin,
- Vera A. Vil’ and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2024, 20, 2959–3006, doi:10.3762/bjoc.20.249
- bond, revealing the amino acyl radical, which is then added to the double bond. Tetra-n-butylammonium bromide (TBAB)-catalyzed carbonylation–peroxidation of styrene derivatives 149 with TBHP and aldehydes 150, which allows for the synthesis of β-peroxy ketones 151, was described (Scheme 47) [111]. tert
- -Butoxy and tert-butylperoxy radicals are generated through the redox reaction of bromine. Vanadium(IV) oxychloride (VOCl2) was found to be an efficient catalyst to achieve peroxidation–carbonylation of styrenes 152 with TBHP and aldehydes 153 to give β-peroxy ketones 154 (Scheme 48) [112]. V(IV)OCl2 is

Graphical Abstract

Scheme 1: Organic peroxide initiators in polymer chemistry.

Scheme 2: Synthesis of organic peroxides.

Scheme 3: Richness of radical cascades with species formed from hydroperoxides in redox conditions.

Scheme 4: Co-catalyzed allylic peroxidation of alkenes 1 and 3 by TBHP.

Scheme 5: Allylic peroxidation of alkenes 6 by Pd(II)TBHP.

Scheme 6: Cu(I)-catalyzed allylic peroxidation.

Scheme 7: Enantioselective peroxidation of alkenes 10 with TBHP in the presence of copper(I) compounds.

Scheme 8: Oxidation of α-pinene (12) by the Cu(I)/TBHP system.

Scheme 9: Introduction of the tert-butylperoxy fragment into the α-position of cyclic ketones 15 and 17.

Scheme 10: α-Peroxidation of β-dicarbonyl compounds 19 using the Cu(II)/TBHP system.

Scheme 11: Co-catalyzed peroxidation of cyclic compounds 21 with TBHP.

Scheme 12: Co-, Mn- and Fe-catalyzed peroxidation of 2-oxoindoles 23, barbituric acids 25, and 4-hydroxycoumar...

Scheme 13: Cu-catalyzed and metal-free peroxidation of barbituric acid derivatives 31 and 3,4-dihydro-1,4-benz...

Scheme 14: Electrochemical peroxidation of 1,3-dicarbonyl compounds 35.

Scheme 15: Peroxidation of β-dicarbonyl compounds, cyanoacetic esters and malonic esters 37 by the TBAI/TBHP s...

Scheme 16: Cu-catalyzed peroxidation of malonodinitriles and cyanoacetic esters 39 with TBHP.

Scheme 17: Mn-catalyzed remote peroxidation via trifluromethylation of double bond.

Scheme 18: Cu-catalyzed remote peroxidation via trifluromethylthiolation of double bond.

Scheme 19: Fe-, Mn-, and Ru-catalyzed peroxidation of alkylaromatics 45, 47, 49, and 51 with TBHP.

Scheme 20: Cu-catalyzed peroxidation of diphenylacetonitrile (53) with TBHP.

Scheme 21: Cu-catalyzed peroxidation of benzyl cyanides 60 with TBHP.

Scheme 22: Synthesis of tert-butylperoxy esters 63 from benzyl alcohols 62 using the TBAI/TBHP system.

Scheme 23: Enantioselective peroxidation of 2-phenylbutane (64) with TBHP and chiral Cu(I) complex.

Scheme 24: Photochemical synthesis of peroxides 67 from carboxylic acids 66.

Scheme 25: Photochemical peroxidation of benzylic C(sp3)–H.

Scheme 26: Cu- and Ru-catalyzed peroxidation of alkylamines with TBHP.

Scheme 27: Peroxidation of amides 76 with the TBAI/TBHP system.

Scheme 28: Fe-catalyzed functionalization of ethers 78 with TBHP.

Scheme 29: Synthesis of 4-(tert-butylperoxy)-5-phenyloxazol-2(3H)-ones 82 from benzyl alcohols 80 and isocyana...

Scheme 30: Fe- and Co-catalyzed peroxidation of alkanes with TBHP.

Scheme 31: Rh-catalyzed tert-butylperoxy dienone synthesis with TBHP.

Scheme 32: Rh- and Cu-catalyzed phenolic oxidation with TBHP.

Scheme 33: Metal-free peroxidation of phenols 94.

Scheme 34: Cu-catalyzed alkylation–peroxidation of acrylonitrile.

Scheme 35: Cu-catalyzed cycloalkylation–peroxidation of coumarins 99.

Scheme 36: Metal-free cycloalkylation–peroxidation of coumarins 102.

Scheme 37: Difunctionalization of indene 104 with tert-butylperoxy and alkyl groups.

Scheme 38: Acid-catalyzed radical addition of ketones (108, 111) and TBHP to alkenes 107 and acrylates 110.

Scheme 39: Cu-catalyzed alkylation–peroxidation of alkenes 113 with TBHP and diazo compounds 114.

Scheme 40: Cobalt(II)-catalyzed addition of TBHP and 1,3-dicarbonyl compound 116 to alkenes 117.

Scheme 41: Cu(0)- or Co(II)-catalyzed addition of TBHP and alcohols 120 to alkenes 119.

Scheme 42: Fe-catalyzed functionalization of allenes 122 with TBHP.

Scheme 43: Fe-catalyzed alkylation–peroxidation of alkenes 125 and 127.

Scheme 44: Fe- and Co-catalyzed alkylation–peroxidation of alkenes 130, 133 and 134 with TBHP and aldehydes as...

Scheme 45: Carbonylation–peroxidation of alkenes 137, 140, 143 with hydroperoxides and aldehydes.

Scheme 46: Carbamoylation–peroxidation of alkenes 146 with formamides and TBHP.

Scheme 47: TBAB-catalyzed carbonylation–peroxidation of alkenes.

Scheme 48: VOCl2-catalyzed carbonylation–peroxidation of alkenes 152.

Scheme 49: Acylation–peroxidation of alkenes 155 with aldehydes 156 and TBHP using photocatalysis.

Scheme 50: Cu-catalyzed peroxidation of styrenes 158.

Scheme 51: Fe-catalyzed acylation-peroxidation of alkenes 161 with carbazates 160 and TBHP.

Scheme 52: Difunctionalization of alkenes 163, 166 with TBHP and (per)fluoroalkyl halides.

Scheme 53: Difunctionalization of alkenes 169 and 172 with hydroperoxides and sodium (per)fluoromethyl sulfina...

Scheme 54: Trifluoromethylation–peroxidation of styrenes 175 using MOF Cu3(BTC)2 as a catalyst.

Scheme 55: Difunctionalization of alkenes 178 with tert-butylperoxy and dihalomethyl fragments.

Scheme 56: Difunctionalization of alkenes 180 with the tert-butylperoxy and dihalomethyl moieties.

Scheme 57: The nitration–peroxidation of alkenes 182 with t-BuONO and TBHP.

Scheme 58: Azidation–peroxidation of alkenes 184 with TMSN3 and TBHP.

Scheme 59: Co-catalyzed bisperoxidation of butadiene 186.

Scheme 60: Bisperoxidation of styrene (189) and acrylonitrile (192) with TBHP by Minisci.

Scheme 61: Mn-catalyzed synthesis of bis(tert-butyl)peroxides 195 from styrenes 194.

Scheme 62: Bisperoxidation of arylidene-9H-fluorenes 196 and 3-arylidene-2-oxoindoles 198 with TBHP under Mn-c...

Scheme 63: Synthesis of bisperoxides from styrenes 200 and 203 using the Ru and Rh catalysis.

Scheme 64: Iodine-catalyzed bisperoxidation of styrenes 206.

Scheme 65: Synthesis of di-tert-butylperoxyoxoindoles 210 from acrylic acid anilides 209 using a Pd(II)/TBHP o...

Scheme 66: Pinolation/peroxidation of styrenes 211 catalyzed by Cu(I).

Scheme 67: TBAI-catalyzed acyloxylation–peroxidation of alkenes 214 with carboxylic acids and TBHP.

Scheme 68: Difunctionalization of alkenes 217 with TBHP and water or alcohols.

Scheme 69: TBAI-catalyzed hydroxyperoxidation of 1,3-dienes 220.

Scheme 70: Hydroxyperoxidation of 1,3-dienes 220.

Scheme 71: Iodination/peroxidation of alkenes 223 with I2 and hydroperoxides.

Scheme 72: The reactions of cyclic enol ethers 226 and 228 with I2/ROOH system.

Scheme 73: Synthesis of 1-(tert-butylperoxy)-2-iodoethanes 231.

Scheme 74: Synthesis of 1-iodo-2-(tert-butylperoxy)ethanes 233.

Scheme 75: Cu-catalyzed phosphorylation–peroxidation of alkenes 234.

Scheme 76: Co-catalyzed phosphorylation–peroxidation of alkenes 237.

Scheme 77: Ag-catalyzed sulfonylation–peroxidation of alkenes 241.

Scheme 78: Co-catalyzed sulfonylation–peroxidation of alkenes 244.

Scheme 79: Synthesis of α/β-peroxysulfides 248 and 249 from styrenes 247.

Scheme 80: Cu-catalyzed trifluoromethylthiolation–peroxidation of alkenes 250 and allenes 252.

Scheme 81: Photocatalytic sulfonyl peroxidation of alkenes 254 via deamination of N-sulfonyl ketimines 255.

Scheme 82: Photoredox-catalyzed 1,4-peroxidation–sulfonylation of enynones 257.

Scheme 83: Cu-catalyzed silylperoxidation of α,β-unsaturated compounds 260 and enynes 261.

Scheme 84: Fe-catalyzed silyl peroxidation of alkenes.

Scheme 85: Cu-catalyzed germyl peroxidation of alkenes 267.

Scheme 86: TBAI-catalyzed intramolecular cyclization of diazo compounds 269 with further peroxidation.

Scheme 87: Co-catalyzed three-component coupling of benzamides 271, diazo compounds 272 and TBHP.

Scheme 88: Co-catalyzed esterification-peroxidation of diazo compounds 274 with TBHP and carboxylic acids 275.

Scheme 89: Cu-catalyzed alkylation–peroxidation of α-carbonylimines 277 or ketones 280.

Scheme 90: Mn-catalyzed ring-opening peroxidation of cyclobutanols 282 with TBHP.

Scheme 91: Peroxycyclization of tryptamines 284 with TBHP.

Scheme 92: Radical cyclization–peroxidation of homotryptamines 287.

Scheme 93: Iodine-catalyzed oxidative coupling of indoles 288, cyanoacetic esters and TBHP.

Scheme 94: Summary of metal-catalyzed peroxidation processes.
Efficacy of radical reactions of isocyanides with heteroatom radicals in organic synthesis
- Akiya Ogawa and
- Yuki Yamamoto
Beilstein J. Org. Chem. 2024, 20, 2114–2128, doi:10.3762/bjoc.20.182
- ), but is also widely used in transition-metal-catalyzed carbonylation reactions [1][2]. However, carbon monoxide is a flammable gas with a wide explosive range, although colorless and odorless, and requires special care in handling due to its high toxicity. In addition, when carbon monoxide is used in a
- ] because isocyanide is susceptible to multiple imidoylation [9][10][11], whereas carbon monoxide is less susceptible to multiple carbonylation. Therefore, precise control of the reaction is required for selective formation of the monoimidoylation product. Regarding the radical reaction of isocyanides, the

Graphical Abstract

Figure 1: Resonance structures and reactivity of carbon monoxide.

Figure 2: Resonance structures and reactivity of isocyanides.

Scheme 1: Possible three pathways of the E• formation for imidoylation.

Scheme 2: Radical addition of thiols to isocyanides.

Scheme 3: Selective thioselenation and catalytic dithiolation of isocyanides.

Scheme 4: Synthesis of carbacephem framework.

Scheme 5: Sequential addition of (PhSe)2 to ethyl propiolate and isocyanide.

Scheme 6: Isocyanide insertion reaction into carbon-tellurium bonds.

Scheme 7: Radical addition to isocyanides with disubstituted phosphines.

Scheme 8: Radical addition to phenyl isocyanides with diphosphines.

Scheme 9: Radical reaction of tin hydride and hydrosilane toward isocyanide.

Scheme 10: Isocyanide insertion into boron compounds.

Scheme 11: Isocyanide insertion into cyclic compounds containing boron units.

Scheme 12: Photoinduced hydrodefunctionalization of isocyanides.

Scheme 13: Tin hydride-mediated indole synthesis and cross-coupling.

Scheme 14: 2-Thioethanol-mediated radical cyclization of alkenyl isocyanide.

Scheme 15: Thiol-mediated radical cyclization of o-alkenylaryl isocyanide.

Scheme 16: (PhTe)2-assisted dithiolative cyclization of o-alkenylaryl isocyanide.

Scheme 17: Trapping imidoyl radicals with heteroatom moieties.

Scheme 18: Trapping imidoyl radicals with isocyano group.

Scheme 19: Quinoline synthesis via aza-Bergman cyclization.

Scheme 20: Phenanthridine synthesis via radical cyclization of 2-isocyanobiaryls.

Scheme 21: Phenanthridine synthesis by radical reactions with AIBN, DBP and TTMSS.

Scheme 22: Phenanthridine synthesis by oxidative cyclization of 2-isocyanobiaryls.

Scheme 23: Phenanthridine synthesis using a photoredox system.

Scheme 24: Phenanthridine synthesis induced by phosphorus-centered radicals.

Scheme 25: Phenanthridine synthesis induced by sulfur-centered radicals.

Scheme 26: Phenanthridine synthesis induced by boron-centered radicals.

Scheme 27: Phenanthridine synthesis by oxidative cyclization of 2-aminobiaryls.
Syntheses and medicinal chemistry of spiro heterocyclic steroids
- Laura L. Romero-Hernández,
- Ana Isabel Ahuja-Casarín,
- Penélope Merino-Montiel,
- Sara Montiel-Smith,
- José Luis Vega-Báez and
- Jesús Sandoval-Ramírez
Beilstein J. Org. Chem. 2024, 20, 1713–1745, doi:10.3762/bjoc.20.152
- with similar yields. In 2006, Akita et al. used an intramolecular Knoevenagel–Claisen type condensation between a β-ketoester and an acetate residue to synthesize spiro-2H-furan-3-ones [24]. For instance, the intermediate orthoester 35 was obtained in 86% yield after a cyclization–carbonylation

Graphical Abstract

Figure 1: Steroidal spiro heterocycles with remarkable pharmacological activity.

Scheme 1: Synthesis of the spirooxetanone 2. a) t-BuOK, THF, rt, 16%.

Scheme 2: Synthesis of the 17-spirooxetane derivative 7. a) HC≡C(CH2)2CH2OTBDPS, n-BuLi, THF, BF3·Et2O, −78 °...

Scheme 3: Pd-catalyzed carbonylation of steroidal alkynols to produce α-methylene-β-lactones at C-3 and C-17 ...

Scheme 4: Catalyst-free protocol to obtain functionalized spiro-lactones by an intramolecular C–H insertion. ...

Scheme 5: One-pot procedure from dienamides to spiro-β-lactams. a) 1. Ac2O, DMAP, Et3N, CH2Cl2, 2. malononitr...

Scheme 6: Spiro-γ-lactone 20 afforded from 7α-alkanamidoestrone derivative 17. a) HC≡CCH2OTHP, n-BuLi, THF, –...

Scheme 7: Synthesis of the 17-spiro-γ-lactone 23, a key intermediate to obtain spironolactone. a) Ethyl propi...

Scheme 8: Synthetic pathway to obtain 17-spirodihydrofuran-3(2H)-ones from 17-oxosteroids. a) 1-Methoxypropa-...

Scheme 9: One-pot procedure to obtain 17-spiro-2H-furan-3-one compounds. a) NaH, diethyl oxalate, benzene, rt...

Scheme 10: Synthesis of 17-spiro-2H-furan-3-one derivatives. a) RCH=NOH, N-chlorosuccinimide/CHCl3, 99%; b) H2...

Scheme 11: Intramolecular condensation of a γ-acetoxy-β-ketoester to synthesize spirofuranone 37. a) (CH3CN)2P...

Scheme 12: Synthesis of spiro 2,5-dihydrofuran derivatives. a) Allyl bromide, DMF, NaH, 0 °C to rt, 93%; b) G-...

Scheme 13: First reported synthesis of C-16 dispiropyrrolidine derivatives. a) Sarcosine, isatin, MeOH, reflux...

Scheme 14: Cycloadducts 47 with antiproliferative activity against human cancer cell lines. a) 1,4-Dioxane–MeO...

Scheme 15: Spiropyrrolidine compounds generated from (E)-16-arylidene steroids and different ylides. a) Acenap...

Scheme 16: 3-Spiropyrrolidines 52a–c obtained from ketones 50a–c. a) p-Toluenesulfonyl hydrazide, MeOH, rt; b)...

Scheme 17: 16-Spiropyrazolines from 16-methylene-13α-estrone derivatives. a) AgOAc, toluene, rt, 78–81%.

Scheme 18: 6-Spiroimidazolines 57 synthesized by a one-pot multicomponent reaction. a) R3-NC, T3P®, DMSO, 70 °...

Scheme 19: Synthesis of spiro-1,3-oxazolines 60, tested as progesterone receptor antagonist agents. a) CF3COCF3...

Scheme 20: Synthesis of spiro-1,3-oxazolidin-2-ones 63 and 66a,b. a) RNH2, EtOH, 70 °C, 70–90%; b) (CCl3O)2CO,...

Scheme 21: Formation of spiro 1,3-oxazolidin-2-one and spiro 2-substituted amino-4,5-dihydro-1,3-oxazoles from ...

Scheme 22: Synthesis of diastereomeric spiroisoxazolines 74 and 75. a) Ar-C(Cl)=N-OH, DIPEA, toluene, rt, 74 (...

Scheme 23: Spiro 1,3-thiazolidine derivatives 77–79 obtained from 2α-bromo-5α-cholestan-3-one 76. a) 2-aminoet...

Scheme 24: Method for the preparation of derivative 83. a) Benzaldehyde, MeOH, reflux, 77%; b) thioglycolic ac...

Scheme 25: Synthesis of spiro 1,3-thiazolidin-4-one derivatives from steroidal ketones. a) Aniline, EtOH, refl...

Scheme 26: Synthesis of spiro N-aryl-1,3-thiazolidin-4-one derivatives 91 and 92. a) Sulfanilamide, DMF, reflu...

Scheme 27: 1,2,4-Trithiolane dimers 94a–e selectively obtained from carbonyl derivatives. a) LR, CH2Cl2, reflu...

Scheme 28: Spiro 1,2,4-triazolidin-3-ones synthesized from semicarbazones. a) H2O2, CHCl3, 0 °C, 82–85%.

Scheme 29: Steroidal spiro-1,3,4-oxadiazoline 99 obtained in two steps from cholest-5-en-3-one (97). a) NH2NHC...

Scheme 30: Synthesis of spiro-1,3,4-thiadiazoline 101 by cyclization and diacetylation of thiosemicarbazone 100...

Scheme 31: Mono- and bis(1,3,4-thiadiazolines) obtained from estrane and androstane derivatives. a) H2NCSNHNH2...

Scheme 32: Different reaction conditions to synthesize spiro-1,3,2-oxathiaphospholanes 108 and 109.

Scheme 33: Spiro-δ-lactones derived from ADT and epi-ADT as inhibitors of 17β-HSDs. a) CH≡C(CH2)2OTHP, n-BuLi,...

Scheme 34: Spiro-δ-lactams 123a,b obtained in a five-step reaction sequence. a) (R)-(+)-tert-butylsulfinamide,...

Scheme 35: Steroid-coumarin conjugates as fluorescent DHT analogues to study 17-oxidoreductases for androgen m...

Scheme 36: 17-Spiro estradiolmorpholinones 130 bearing two types of molecular diversity. a) ʟ- or ᴅ-amino acid...

Scheme 37: Steroidal spiromorpholinones as inhibitors of enzyme 17β-HSD3. a) Methyl ester of ʟ- or ᴅ-leucine, ...

Scheme 38: Steroidal spiro-morpholin-3-ones achieved by N-alkylation or N-acylation of amino diols 141, follow...

Scheme 39: Straightforward method to synthesize a spiromorpholinone derivative from estrone. a) BnBr, K2CO3, CH...

Scheme 40: Pyrazolo[4,3-e][1,2,4]-triazine derivatives 152–154. a) 4-Aminoantipyrine, EtOH/DMF, reflux, 82%; b...

Scheme 41: One-pot procedure to synthesize spiro-1,3,4-thiadiazine derivatives. a) NH2NHCSCONHR, H2SO4, dioxan...

Scheme 42: 1,2,4-Trioxanes with antimalarial activity. a) 1. O2, methylene blue, CH3CN, 500 W tungsten halogen...

Scheme 43: Tetraoxanes 167 and 168 synthesized from ketones 163, 165 and 166. a) NaOH, iPrOH/H2O, 80 °C, 93%; ...

Scheme 44: 1,2,4,5-Tetraoxanes bearing a steroidal moiety and a cycloalkane. a) 30% H2O2/CH2Cl2/CH3CN, HCl, rt...

Scheme 45: Spiro-1,3,2-dioxaphosphorinanes obtained from estrone derivatives. a) KBH4, MeOH, THF or CH2Cl2; b)...

Scheme 46: Synthesis of steroidal spiro-ε-lactone 183. a) 1. Jones reagent, acetone, 0 °C to rt, 2. ClCOCOCl, ...

Scheme 47: Synthesis of spiro-2,3,4,7-tetrahydrooxepines 185 and 187 derived from mestranol and lynestrenol (38...
Carbonylative synthesis and functionalization of indoles
- Alex De Salvo,
- Raffaella Mancuso and
- Xiao-Feng Wu
Beilstein J. Org. Chem. 2024, 20, 973–1000, doi:10.3762/bjoc.20.87
- -Str. 29a, 18059 Rostock, Germany Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Liaoning, China 10.3762/bjoc.20.87 Abstract Carbonylation processes have become widely recognized as a versatile, convenient, and low-cost method for
- recent achievements on the synthesis and functionalization of indole derivatives via carbonylative approaches. Keywords: carbonylation; functionalization; indole; metal catalyst; organometallic chemistry; Introduction Indole is a heterocyclic compound consisting of a benzene ring fused with a pyrrole
- 1883 and involves its synthesis from phenylhydrazine and an aldehyde or ketone using an appropriate acid catalyst [8]. In the following years, new processes were developed for the synthesis of indole such as the Castro, Bischler, and Larock synthesis etc. [2][9][10]. Carbonylation reactions represent a

Graphical Abstract

Scheme 1: Pd(0)-catalyzed domino C,N-coupling/carbonylation/Suzuki coupling reaction for the synthesis of 2-a...

Scheme 2: Pd(0)-catalyzed single isonitrile insertion: synthesis of 1-(3-amino)-1H-indol-2-yl)-1-ketones.

Scheme 3: Pd(0)-catalyzed gas-free carbonylation of 2-alkynylanilines to 1-(1H-indol-1-yl)-2-arylethan-1-ones....

Scheme 4: Pd(II)-catalyzed heterocyclization/alkoxycarbonylation of 2-alkynylaniline imines.

Scheme 5: Pd(II)-catalyzed heterocyclization/alkoxycarbonylation of 2-alkynylanilines to N-substituted indole...

Scheme 6: Synthesis of indol-2-acetic esters by Pd(II)-catalyzed carbonylation of 1-(2-aminoaryl)-2-yn-1-ols.

Scheme 7: Pd(II)-catalyzed carbonylative double cyclization of suitably functionalized 2-alkynylanilines to 3...

Scheme 8: Indole synthesis by deoxygenation reactions of nitro compounds reported by Cenini et al. [21].

Scheme 9: Indole synthesis by reduction of nitro compounds: approach reported by Watanabe et al. [22].

Scheme 10: Indole synthesis from o-nitrostyrene compounds as reported by Söderberg and co-workers [23].

Scheme 11: Synthesis of fused indoles (top) and natural indoles present in two species of European Basidiomyce...

Scheme 12: Synthesis of 1,2-dihydro-4(3H)-carbazolones through N-heteroannulation of functionalized 2-nitrosty...

Scheme 13: Synthesis of indoles from o-nitrostyrenes by using Pd(OAc)2 and Pd(tfa)2 in conjunction with bident...

Scheme 14: Synthesis of substituted 3-alkoxyindoles via palladium-catalyzed reductive N-heteroannulation.

Scheme 15: Synthesis of 3-arylindoles by palladium-catalyzed C–H bond amination via reduction of nitroalkenes.

Scheme 16: Synthesis of 2,2′-bi-1H-indoles, 2,3′-bi-1H-indoles, 3,3′-bi-1H-indoles, indolo[3,2-b]indoles, indo...

Scheme 17: Pd-catalyzed reductive cyclization of 1,2-bis(2-nitrophenyl)ethene and 1,1-bis(2-nitrophenyl)ethene...

Scheme 18: Flow synthesis of 2-substituted indoles by reductive carbonylation.

Scheme 19: Pd-catalyzed synthesis of variously substituted 3H-indoles from nitrostyrenes by using Mo(CO)6 as C...

Scheme 20: Synthesis of indoles from substituted 2-nitrostyrenes (top) and ω-nitrostyrenes (bottom) via reduct...

Scheme 21: Synthesis of indoles from substituted 2-nitrostyrenes with formic acid as CO source.

Scheme 22: Ni-catalyzed carbonylative cyclization of 2-nitroalkynes and aryl iodides (top) and the Ni-catalyze...

Scheme 23: Mechanism of the Ni-catalyzed carbonylative cyclization of 2-nitroalkynes and aryl iodides (top) an...

Scheme 24: Route to indole derivatives through Rh-catalyzed benzannulation of heteroaryl propargylic esters fa...

Scheme 25: Pd-catalyzed cyclization of 2-(2-haloaryl)indoles reported by Yoo and co-workers [54], Guo and co-worke...

Scheme 26: Approach for the synthesis of 6H-isoindolo[2,1-a]indol-6-ones reported by Huang and co-workers [57].

Scheme 27: Zhou group’s method for the synthesis of 6H-isoindolo[2,1-a]indol-6-ones.

Scheme 28: Synthesis of 6H-isoindolo[2,1-a]indol-6-ones from o-1,2-dibromobenzene and indole derivatives by us...

Scheme 29: Pd(OAc)2-catalyzed Heck cyclization of 2-(2-bromophenyl)-1-alkyl-1H-indoles reported by Guo et al. [55]....

Scheme 30: Synthesis of indolo[1,2-a]quinoxalinone derivatives through Pd/Cu co-catalyzed carbonylative cycliz...

Scheme 31: Pd-catalyzed carbonylative cyclization of o-indolylarylamines and N-monosubstituted o-indolylarylam...

Scheme 32: Pd-catalyzed diasteroselective carbonylative cyclodearomatization of N-(2-bromobenzoyl)indoles with...

Scheme 33: Pd(0)-catalyzed synthesis of CO-linked heterocyclic scaffolds from alkene-indole derivatives and 2-...

Scheme 34: Proposed mechanism for the Pd(0)-catalyzed synthesis of CO-linked heterocyclic scaffolds.

Scheme 35: Pd-catalyzed C–H and N–H alkoxycarbonylation of indole derivatives to indole-3-carboxylates and ind...

Scheme 36: Rh-catalyzed C–H alcoxycarbonylation of indole derivatives to indole-3-carboxylates reported by Li ...

Scheme 37: Pd-catalyzed C–H alkoxycarbonylation of indole derivatives with alcohols and phenols to indole-3-ca...

Scheme 38: Synthesis of N-methylindole-3-carboxylates from N-methylindoles and phenols through metal-catalyst-...

Scheme 39: Synthesis of indol-3-α-ketoamides (top) and indol-3-amides (bottom) via direct double- and monoamin...

Scheme 40: The direct Sonogashira carbonylation coupling reaction of indoles and alkynes via Pd/CuI catalysis ...

Scheme 41: Synthesis of indole-3-yl aryl ketones reported by Zhao and co-workers [73] (path a) and Zhang and co-wo...

Scheme 42: Pd-catalyzed carbonylative synthesis of BIMs from aryl iodides and N-substituted and NH-free indole...

Scheme 43: Cu-catalyzed direct double-carbonylation and monocarbonylation of indoles and alcohols with hexaket...

Scheme 44: Rh-catalyzed direct C–H alkoxycarbonylation of indoles to indole-2-carboxylates [79] (top) and Co-catal...

Scheme 45: Pd-catalyzed carbonylation of NH free-haloindoles.
Metal-catalyzed coupling/carbonylative cyclizations for accessing dibenzodiazepinones: an expedient route to clozapine and other drugs
- Amina Moutayakine and
- Anthony J. Burke
Beilstein J. Org. Chem. 2024, 20, 193–204, doi:10.3762/bjoc.20.19
- carried out with Cu. The aminocarbonylation reaction which was introduced by Schoenberg and Heck in 1974 is an efficient catalytic route to carboxamides [11]. It was a major step forward and has been amply applied in a number of carbonylation reactions over the years [12]. In 2011, Buchwald and
- driving forces for the development of the work discussed in this report. Results and Discussion Synthesis of o-(2-bromophenyl)aminoaniline via Buchwald–Hartwig C–N coupling One-pot synthesis of dibenzodiazepinones Our preliminary attempt to synthesize DBDAPs via B–H amination and carbonylation was carried

Graphical Abstract

Figure 1: Biologically active dibenzodiazepinones.

Scheme 1: Different synthetic routes to DBDAPs (a–c), including our novel approach (d).

Scheme 2: One-pot synthesis of 5H-dibenzo[b,e][1,4]diazepin-11-ol (5).

Scheme 3: Scope of the Chan–Lam coupling between o-phenylenediamines and 2-bromophenylboronic acids (please n...

Scheme 4: Scope of the synthesis of DBDAPs. Please note that product 4g contained some unidentified impuritie...

Scheme 5: Proposed mechanism.
Sequential hydrozirconation/Pd-catalyzed cross coupling of acyl chlorides towards conjugated (2E,4E)-dienones
- Benedikt Kolb,
- Daniela Silva dos Santos,
- Sanja Krause,
- Anna Zens and
- Sabine Laschat
Beilstein J. Org. Chem. 2023, 19, 176–185, doi:10.3762/bjoc.19.17
- example, the sequential hydrozirconation/carbonylation of propargylic ethers 18 reported by Donato [58] yielded α,β-unsaturated lactones 19. Beside the hydrozirconation/acylation sequence of nitriles utilizing acid chlorides published by Majoral/Floreancig [59][60], Cox revealed that terminal alkynes 16

Graphical Abstract

Scheme 1: Examples of biologically active compounds with (2Ε,4E)-unsaturated ketone units.

Scheme 2: Selected examples for the synthesis of conjugated dienones from the literature [6-21].

Scheme 3: Previous work of hydrozirconations with Schwartz's reagent and our work [54,55,57,58,61,62].

Scheme 4: Synthesis of substituted enynes 25f–o via Corey–Fuchs reaction and Hunsdiecker reaction.

Scheme 5: Synthesis of non-natural (a) and natural (b) dienone-containing terpenes: synthesis of β-ionone (3)....
Recent advances in the tandem annulation of 1,3-enynes to functionalized pyridine and pyrrole derivatives
- Yi Liu,
- Puying Luo,
- Yang Fu,
- Tianxin Hao,
- Xuan Liu,
- Qiuping Ding and
- Yiyuan Peng
Beilstein J. Org. Chem. 2021, 17, 2462–2476, doi:10.3762/bjoc.17.163
- structural motifs to provide the functionalized pyridine and pyrrole derivatives. The functionalization reactions cover iodination, bromination, trifluoromethylation, azidation, carbonylation, arylation, alkylation, selenylation, sulfenylation, amidation, esterification, and hydroxylation. We also briefly
- functionalizations of pyrrole derivatives, such as iodination, bromination, trifluoromethylation, azidation, carbonylation, arylation, and alkylation. The proposed mechanism generally involves two kinds of intramolecular cyclizations: one is 6-endo-dig cyclization to promote the formation of pyridine ring

Graphical Abstract

Scheme 1: Ag/I2-mediated electrophilic annulation of 2-en-4-ynyl azides 1.

Scheme 2: The proposed mechanism of Ag-catalyzed aza-annulation.

Scheme 3: The proposed mechanism of I2-mediated aza-annulation.

Scheme 4: Copper-catalyzed amination of (E)-2-en-4-ynyl azides 1.

Scheme 5: The proposed mechanism of copper-catalyzed amination.

Scheme 6: The derivatization of sulfonated aminonicotinates.

Scheme 7: Copper-catalyzed chalcogenoamination of (E)-2-en-4-ynyl azides 1.

Scheme 8: The possible mechanism of chalcogenoamination.

Scheme 9: The derivatization of 5‑selenyl- and 5-sulfenyl-substituted nicotinates.

Scheme 10: The tandem reaction of nitriles, Reformatsky reagents, and 1,3-enynes.

Scheme 11: Nickel-catalyzed [4 + 2]-cycloaddition of 3-azetidinones with 1,3-enynes.

Scheme 12: Electrophilic iodocyclization of 2-nitro-1,3-enynes to pyrroles.

Scheme 13: Electrophilic halogenation of 2-trifluoromethyl-1,3-enynes to pyrroles.

Scheme 14: Copper-catalyzed cascade cyclization of 2-nitro-1,3-enynes with amines.

Scheme 15: Tandem cyclization of 2-nitro-1,3-enynes, Togni reagent II, and amines.

Scheme 16: Tandem cyclization of 2-nitro-1,3-enynes, TMSN3, and amines.

Scheme 17: Cascade cyclization of 6-hydroxyhex-2-en-4-ynals to pyrroles.

Scheme 18: Au/Ag-catalyzed oxidative aza-annulation of 1,3-enynyl azides.

Scheme 19: The plausible mechanism of Au/Ag-catalyzed oxidative aza-annulation.

Scheme 20: Synthesis of 2-tetrazolyl-substituted 3-acylpyrroles from enynals.

Scheme 21: CuH-catalyzed coupling reaction of 1,3-enynes and nitriles to pyrroles.

Scheme 22: The mechanism of CuH-catalyzed coupling of 1,3-enynes and nitriles to pyrroles.
Advances in mercury(II)-salt-mediated cyclization reactions of unsaturated bonds
- Sumana Mandal,
- Raju D. Chaudhari and
- Goutam Biswas
Beilstein J. Org. Chem. 2021, 17, 2348–2376, doi:10.3762/bjoc.17.153

- furylmercurials 91 via syn-addition of acetylene, which on carbonylation yielded the furan-containing carbonyl compound 92 (Scheme 30) [83]. It was proposed that initially mercuration of acetylene bonds via mercurinium like ions or π-complex takes place. Then the structure was stabilized through hydrogen bonding

Graphical Abstract

Scheme 1: Schematic representation of Hg(II)-mediated addition to an unsaturated bond.

Scheme 2: First report of Hg(II)-mediated synthesis of 2,5-dioxane derivatives from allyl alcohol.

Scheme 3: Stepwise synthesis of 2,6-distubstituted dioxane derivatives.

Scheme 4: Cyclization of carbohydrate alkene precursor.

Scheme 5: Hg(II)-mediated synthesis of C-glucopyranosyl derivatives.

Scheme 6: Synthesis of C-glycosyl amino acid derivative using Hg(TFA)2.

Scheme 7: Hg(OAc)2-mediated synthesis of α-ᴅ-ribose derivative.

Scheme 8: Synthesis of β-ᴅ-arabinose derivative 18.

Scheme 9: Hg(OAc)2-mediated synthesis of tetrahydrofuran derivatives.

Scheme 10: Synthesis of Hg(TFA)2-mediated bicyclic nucleoside derivative.

Scheme 11: Synthesis of pyrrolidine and piperidine derivatives.

Scheme 12: HgCl2-mediated synthesis of diastereomeric pyrrolidine derivatives.

Scheme 13: HgCl2-mediated cyclization of alkenyl α-aminophosphonates.

Scheme 14: Cyclization of 4-cycloocten-1-ol with Hg(OAc)2 forming fused bicyclic products.

Scheme 15: trans-Amino alcohol formation through Hg(II)-salt-mediated cyclization.

Scheme 16: Hg(OAc)2-mediated 2-aza- or 2-oxa-bicyclic ring formations.

Scheme 17: Hg(II)-salt-induced cyclic peroxide formation.

Scheme 18: Hg(OAc)2-mediated formation of 1,2,4-trioxanes.

Scheme 19: Endocyclic enol ether derivative formation through Hg(II) salts.

Scheme 20: Synthesis of optically active cyclic alanine derivatives.

Scheme 21: Hg(II)-salt-mediated formation of tetrahydropyrimidin-4(1H)-one derivatives.

Scheme 22: Cyclization of ether derivatives to form stereoselective oxazolidine derivatives.

Scheme 23: Cyclization of amide derivatives induced by Hg(OAc)2.

Scheme 24: Hg(OAc)2/Hg(TFA)2-promoted cyclization of salicylamide-derived amidal auxiliary derivatives.

Scheme 25: Hg(II)-salt-mediated cyclization to form dihydrobenzopyrans.

Scheme 26: HgCl2-induced cyclization of acetylenic silyl enol ether derivatives.

Scheme 27: Synthesis of exocyclic and endocyclic enol ether derivatives.

Scheme 28: Cyclization of trans-acetylenic alcohol by treatment with HgCl2.

Scheme 29: Synthesis of benzofuran derivatives in presence of HgCl2.

Scheme 30: a) Hg(II)-salt-mediated cyclization of 4-hydroxy-2-alkyn-1-ones to furan derivatives and b) its mec...

Scheme 31: Cyclization of arylacetylenes to synthesize carbocyclic and heterocyclic derivatives.

Scheme 32: Hg(II)-salt-promoted cyclization–rearrangement to form heterocyclic compounds.

Scheme 33: a) HgCl2-mediated cyclization reaction of tethered alkyne dithioacetals; and b) proposed mechanism.

Scheme 34: Cyclization of aryl allenic ethers on treatment with Hg(OTf)2.

Scheme 35: Hg(TFA)2-mediated cyclization of allene.

Scheme 36: Hg(II)-catalyzed intramolecular trans-etherification reaction of 2-hydroxy-1-(γ-methoxyallyl)tetrah...

Scheme 37: a) Cyclization of alkene derivatives by catalytic Hg(OTf)2 salts and b) mechanism of cyclization.

Scheme 38: a) Synthesis of 1,4-dihydroquinoline derivatives by Hg(OTf)2 and b) plausible mechanism of formatio...

Scheme 39: Synthesis of Hg(II)-salt-catalyzed heteroaromatic derivatives.

Scheme 40: Hg(II)-salt-catalyzed synthesis of dihydropyranone derivatives.

Scheme 41: Hg(II)-salt-catalyzed cyclization of alkynoic acids.

Scheme 42: Hg(II)-salt-mediated cyclization of alkyne carboxylic acids and alcohol to furan, pyran, and spiroc...

Scheme 43: Hg(II)-salt-mediated cyclization of 1,4-dihydroxy-5-alkyne derivatives.

Scheme 44: Six-membered morpholine derivative formation by catalytic Hg(II)-salt-induced cyclization.

Scheme 45: Hg(OTf)2-catalyzed hydroxylative carbocyclization of 1,6-enyne.

Scheme 46: a) Hg(OTf)2-catalyzed hydroxylative carbocyclization of 1,6-enyne. b) Proposed mechanism.

Scheme 47: a) Synthesis of carbocyclic derivatives using a catalytic amount of Hg(II) salt. b) Proposed mechan...

Scheme 48: Cyclization of 1-alkyn-5-ones to 2-methylfuran derivatives.

Scheme 49: Hg(NO3)2-catalyzed synthesis of 2-methylenepiperidine.

Scheme 50: a) Preparation of indole derivatives through cycloisomerization of 2-ethynylaniline and b) its mech...

Scheme 51: a) Hg(OTf)2-catalyzed synthesis of 3-indolinones and 3-coumaranones and b) simplified mechanism.

Scheme 52: a) Hg(OTf)2-catalyzed one pot cyclization of nitroalkyne and b) its plausible mechanism.

Scheme 53: Synthesis of tricyclic heterocyclic scaffolds.

Scheme 54: HgCl2-mediated cyclization of 2-alkynylphenyl alkyl sulfoxide.

Scheme 55: a) Hg(OTf)2-catalyzed cyclization of allenes and alkynes. b) Proposed mechanism of cyclization.

Scheme 56: Stereoselective synthesis of tetrahydropyran derivatives.

Scheme 57: a) Hg(ClO4)2-catalyzed cyclization of α-allenol derivatives. b) Simplified mechanism.

Scheme 58: Hg(TFA)2-promoted cyclization of a γ-hydroxy alkene derivative.

Scheme 59: Synthesis Hg(II)-salt-mediated cyclization of allyl alcohol for the construction of ventiloquinone ...

Scheme 60: Hg(OAc)2-mediated cyclization as a key step for the synthesis of hongconin.

Scheme 61: Examples of Hg(II)-salt-mediated cyclized ring formation in the syntheses of (±)-fastigilin C and (...

Scheme 62: Formal synthesis of (±)-thallusin.

Scheme 63: Total synthesis of hippuristanol and its analog.

Scheme 64: Total synthesis of solanoeclepin A.

Scheme 65: a) Synthesis of Hg(OTf)2-catalyzed azaspiro structure for the formation of natural products. b) Pro...
On the application of 3d metals for C–H activation toward bioactive compounds: The key step for the synthesis of silver bullets
- Renato L. Carvalho,
- Amanda S. de Miranda,
- Mateus P. Nunes,
- Roberto S. Gomes,
- Guilherme A. M. Jardim and
- Eufrânio N. da Silva Júnior
Beilstein J. Org. Chem. 2021, 17, 1849–1938, doi:10.3762/bjoc.17.126


Graphical Abstract

Scheme 1: Schematic overview of transition metals studied in C–H activation processes.

Scheme 2: (A) Known biological activities related to benzimidazole-based compounds; (B and C) an example of a...

Scheme 3: (A) Known biological activities related to quinoline-based compounds; (B and C) an example of a sca...

Scheme 4: (A) Known biological activities related to sulfur-containing compounds; (B and C) an example of a s...

Scheme 5: (A) Known biological activities related to aminoindane derivatives; (B and C) an example of a scand...

Scheme 6: (A) Known biological activities related to norbornane derivatives; (B and C) an example of a scandi...

Scheme 7: (A) Known biological activities related to aniline derivatives; (B and C) an example of a titanium-...

Scheme 8: (A) Known biological activities related to cyclohexylamine derivatives; (B) an example of an intram...

Scheme 9: (A) Known biologically active benzophenone derivatives; (B and C) photocatalytic oxidation of benzy...

Scheme 10: (A) Known bioactive fluorine-containing compounds; (B and C) vanadium-mediated C(sp3)–H fluorinatio...

Scheme 11: (A) Known biologically active Lythraceae alkaloids; (B) synthesis of (±)-decinine (30).

Scheme 12: (A) Synthesis of (R)- and (S)-boehmeriasin (31); (B) synthesis of phenanthroindolizidines by vanadi...

Scheme 13: (A) Known bioactive BINOL derivatives; (B and C) vanadium-mediated oxidative coupling of 2-naphthol...

Scheme 14: (A) Known antiplasmodial imidazopyridazines; (B) practical synthesis of 41.

Scheme 15: (A) Gold-catalyzed drug-release mechanism using 2-alkynylbenzamides; (B and C) chromium-mediated al...

Scheme 16: (A) Examples of anti-inflammatory benzaldehyde derivatives; (B and C) chromium-mediated difunctiona...

Scheme 17: (A and B) Manganese-catalyzed chemoselective intramolecular C(sp3)–H amination; (C) late-stage modi...

Scheme 18: (A and B) Manganese-catalyzed C(sp3)–H amination; (C) late-stage modification of a leelamine deriva...

Scheme 19: (A) Known bioactive compounds containing substituted N-heterocycles; (B and C) manganese-catalyzed ...

Scheme 20: (A) Known indoles that present GPR40 full agonist activity; (B and C) manganese-catalyzed C–H alkyl...

Scheme 21: (A) Examples of known biaryl-containing drugs; (B and C) manganese-catalyzed C–H arylation through ...

Scheme 22: (A) Known zidovudine derivatives with potent anti-HIV properties; (B and C) manganese-catalyzed C–H...

Scheme 23: (A and B) Manganese-catalyzed C–H organic photo-electrosynthesis; (C) late-stage modification.

Scheme 24: (A) Example of a known antibacterial silylated dendrimer; (B and C) manganese-catalyzed C–H silylat...

Scheme 25: (A and B) Fe-based small molecule catalyst applied for selective aliphatic C–H oxidations; (C) late...

Scheme 26: (A) Examples of naturally occurring gracilioethers; (B) the first total synthesis of gracilioether ...

Scheme 27: (A and B) Selective aliphatic C–H oxidation of amino acids; (C) late-stage modification of proline-...

Scheme 28: (A) Examples of Illicium sesquiterpenes; (B) first chemical synthesis of (+)-pseudoanisatin (80) in...

Scheme 29: (A and B) Fe-catalyzed deuteration; (C) late-stage modification of pharmaceuticals.

Scheme 30: (A and B) Biomimetic Fe-catalyzed aerobic oxidation of methylarenes to benzaldehydes (PMHS, polymet...

Scheme 31: (A) Known tetrahydroquinolines with potential biological activities; (B and C) redox-selective Fe c...

Scheme 32: (A) Known drugs containing a benzofuran unit; (B and C) Fe/Cu-catalyzed tandem O-arylation to acces...

Scheme 33: (A) Known azaindolines that act as M4 muscarinic acetylcholine receptor agonists; (B and C) intramo...

Scheme 34: (A) Known indolinones with anticholinesterase activity; (B and C) oxidative C(sp3)–H cross coupling...

Scheme 35: (A and B) Cobalt-catalyzed C–H alkenylation of C-3-peptide-containing indoles; (C) derivatization b...

Scheme 36: (A) Cobalt-Cp*-catalyzed C–H methylation of known drugs; (B and C) scope of the o-methylated deriva...

Scheme 37: (A) Known lasalocid A analogues; (B and C) three-component cobalt-catalyzed C–H bond addition; (D) ...

Scheme 38: (A and B) Cobalt-catalyzed C(sp2)–H amidation of thiostrepton.

Scheme 39: (A) Known 4H-benzo[d][1,3]oxazin-4-one derivatives with hypolipidemic activity; (B and C) cobalt-ca...

Scheme 40: (A and B) Cobalt-catalyzed C–H arylation of pyrrole derivatives; (C) application for the synthesis ...

Scheme 41: (A) Known 2-phenoxypyridine derivatives with potent herbicidal activity; (B and C) cobalt-catalyzed...

Scheme 42: (A) Natural cinnamic acid derivatives; (B and C) cobalt-catalyzed C–H carboxylation of terminal alk...

Scheme 43: (A and B) Cobalt-catalyzed C–H borylation; (C) application to the synthesis of flurbiprofen.

Scheme 44: (A) Benzothiazoles known to present anticonvulsant activities; (B and C) cobalt/ruthenium-catalyzed...

Scheme 45: (A and B) Cobalt-catalyzed oxygenation of methylene groups towards ketone synthesis; (C) synthesis ...

Scheme 46: (A) Known anticancer tetralone derivatives; (B and C) cobalt-catalyzed C–H difluoroalkylation of ar...

Scheme 47: (A and B) Cobalt-catalyzed C–H thiolation; (C) application in the synthesis of quetiapine (153).

Scheme 48: (A) Known benzoxazole derivatives with anticancer, antifungal, and antibacterial activities; (B and...

Scheme 49: (A and B) Cobalt-catalyzed C–H carbonylation of naphthylamides; (C) BET inhibitors 158 and 159 tota...

Scheme 50: (A) Known bioactive pyrrolo[1,2-a]quinoxalin-4(5H)-one derivatives; (B and C) cobalt-catalyzed C–H ...

Scheme 51: (A) Known antibacterial cyclic sulfonamides; (B and C) cobalt-catalyzed C–H amination of propargyli...

Scheme 52: (A and B) Cobalt-catalyzed intramolecular 1,5-C(sp3)–H amination; (C) late-stage functionalization ...

Scheme 53: (A and B) Cobalt-catalyzed C–H/C–H cross-coupling between benzamides and oximes; (C) late-state syn...

Scheme 54: (A) Known anticancer natural isoquinoline derivatives; (B and C) cobalt-catalyzed C(sp2)–H annulati...

Scheme 55: (A) Enantioselective intramolecular nickel-catalyzed C–H activation; (B) bioactive obtained motifs;...

Scheme 56: (A and B) Nickel-catalyzed α-C(sp3)–H arylation of ketones; (C) application of the method using kno...

Scheme 57: (A and B) Nickel-catalyzed C(sp3)–H acylation of pyrrolidine derivatives; (C) exploring the use of ...

Scheme 58: (A) Nickel-catalyzed C(sp3)–H arylation of dioxolane; (B) library of products obtained from biologi...

Scheme 59: (A) Intramolecular enantioselective nickel-catalyzed C–H cycloalkylation; (B) product examples, inc...

Scheme 60: (A and B) Nickel-catalyzed C–H deoxy-arylation of azole derivatives; (C) late-stage functionalizati...

Scheme 61: (A and B) Nickel-catalyzed decarbonylative C–H arylation of azole derivatives; (C) application of t...

Scheme 62: (A and B) Another important example of nickel-catalyzed C–H arylation of azole derivatives; (C) app...

Scheme 63: (A and B) Another notable example of a nickel-catalyzed C–H arylation of azole derivatives; (C) lat...

Scheme 64: (A and B) Nickel-based metalorganic framework (MOF-74-Ni)-catalyzed C–H arylation of azole derivati...

Scheme 65: (A) Known commercially available benzothiophene-based drugs; (B and C) nickel-catalyzed C–H arylati...

Scheme 66: (A) Known natural tetrahydrofuran-containing substances; (B and C) nickel-catalyzed photoredox C(sp3...

Scheme 67: (A and B) Another notable example of a nickel-catalyzed photoredox C(sp3)–H alkylation/arylation; (...

Scheme 68: (A) Electrochemical/nickel-catalyzed C–H alkoxylation; (B) achieved scope, including three using na...

Scheme 69: (A) Enantioselective photoredox/nickel catalyzed C(sp3)–H arylation; (B) achieved scope, including ...

Scheme 70: (A) Known commercially available trifluoromethylated drugs; (B and C) nickel-catalyzed C–H trifluor...

Scheme 71: (A and B) Stereoselective nickel-catalyzed C–H difluoroalkylation; (C) late-stage functionalization...

Scheme 72: (A) Cu-mediated ortho-amination of oxalamides; (B) achieved scope, including derivatives obtained f...

Scheme 73: (A) Electro-oxidative copper-mediated amination of 8-aminoquinoline-derived amides; (B) achieved sc...

Scheme 74: (A and B) Cu(I)-mediated C–H amination with oximes; (C) derivatization using telmisartan (241) as s...

Scheme 75: (A and B) Cu-mediated amination of aryl amides using ammonia; (C) late-stage modification of proben...

Scheme 76: (A and B) Synthesis of purine nucleoside analogues using copper-mediated C(sp2)–H activation.

Scheme 77: (A) Copper-mediated annulation of acrylamide; (B) achieved scope, including the synthesis of the co...

Scheme 78: (A) Known bioactive compounds containing a naphthyl aryl ether motif; (B and C) copper-mediated eth...

Scheme 79: (A and B) Cu-mediated alkylation of N-oxide-heteroarenes; (C) late-stage modification.

Scheme 80: (A) Cu-mediated cross-dehydrogenative coupling of polyfluoroarenes and alkanes; (B) scope from know...

Scheme 81: (A) Known anticancer acrylonitrile compounds; (B and C) Copper-mediated cyanation of unactivated al...

Scheme 82: (A) Cu-mediated radiofluorination of 8-aminoquinoline-derived aryl amides; (B) achieved scope, incl...

Scheme 83: (A) Examples of natural β-carbolines; (B and C) an example of a zinc-catalyzed C–H functionalizatio...

Scheme 84: (A) Examples of anticancer α-aminophosphonic acid derivatives; (B and C) an example of a zinc-catal...
Unexpected rearrangements and a novel synthesis of 1,1-dichloro-1-alkenones from 1,1,1-trifluoroalkanones with aluminium trichloride
- Beatrice Lansbergen,
- Catherine S. Meister and
- Michael C. McLeod
Beilstein J. Org. Chem. 2021, 17, 404–409, doi:10.3762/bjoc.17.36
- trichloride; dichloroalkenes; Friedel–Crafts alkylation; rearrangement; trifluoroalkanes; Introduction 1,1-Dichloro-1-alkenes are valuable synthetic intermediates and have been employed in Pd-mediated cross couplings of one or both chlorine atoms [1][2][3][4][5][6][7], carbonylation reactions [8], and C–H

Graphical Abstract

Figure 1: Examples of biologically active 1,1-dichloro-1-alkenes.

Figure 2: a) Common methods for the preparation of 1,1-dichloro-1-alkenes from aldehydes. b) This work: a two...

Scheme 1: The initial attempt for the conversion of 1,1,1-trifluoroalkanone 5a to 1,1-dichloroalkenone 6a.

Scheme 2: Substrate scope for the reaction of 1,1,1 trifluoroalkanones with AlCl3. aPurification (normal or r...

Scheme 3: Proposed mechanism for the formation of dichloroalkene 6 from trifluoroalkanone 5.

Scheme 4: Formation of bicyclic ketones 9 and 10 from 1,1,1-trifluoroalkanone 5b using 7 equivalents of AlCl3....

Scheme 5: Conversion of 1,1,1-trifluoroalkanone 5n to acid chloride 13 with AlCl3, and possible mechanism.

Scheme 6: Conversion of 1,1,1-trifluoroalkane 16 to bicycle 17 upon treatment with AlCl3, and possible mechan...
19F NMR as a tool in chemical biology
- Diana Gimenez,
- Aoife Phelan,
- Cormac D. Murphy and
- Steven L. Cobb
Beilstein J. Org. Chem. 2021, 17, 293–318, doi:10.3762/bjoc.17.28


Graphical Abstract

Figure 1: Selected examples of 19F-labelled amino acid analogues used as probes in chemical biology.

Figure 2: (a) Sequences of the antimicrobial peptide MSI-78 and pFtBSer-containing analogs and cartoon repres...

Figure 3: (a) Chemical structures of a selection of trifluoromethyl tags. (b) Comparative analysis showing th...

Figure 4: (a) First bromodomain of Brd4 with all three tryptophan residues displayed in blue and labelled by ...

Figure 5: (a) Enzymatic hydroxylation of GBBNF in the presence of hBBOX (b) 19F NMR spectra showing the conve...

Figure 6: (a) In-cell enzymatic hydrolysis of the fluorinated anandamide analogue ARN1203 catalyzed by hFAAH....

Figure 7: (a) X-ray crystal structure of CAM highlighting the location the phenylalanine residues replaced by...

Figure 8: 19F PREs of 4-F, 5-F, 6-F, 7-FTrp49 containing MTSL-modified S52CCV-N. The 19F NMR resonances of ox...

Figure 9: 19F NMR as a direct probe of Ud NS1A ED homodimerization. Schematic representation showing the loca...

Figure 10: (a) Representative spectrum of a 182 μM sample of Aβ1-40-tfM35 at varying times indicating the majo...

Figure 11: Illustration of the conformational switch induced by SDS in 4-tfmF-labelled α-Syn. Also shown are t...

Figure 12: (a) Structural models of the Myc‐Max (left), Myc‐Max‐DNA (middle) and Myc‐Max‐BRCA1 complexes (righ...

Figure 13: (a) Side (left) and bottom (right) views of the pentameric apo ELIC X-ray structure (PDB ID: 3RQU) ...

Figure 14: (a) General structure of a selection of recently developed 19F-labelled nucleotides for their use a...

Figure 15: Monitoring biotransformation of the fluorinated pesticide cyhalothrin by the fungus C. elegans. The...

Figure 16: Following the biodegradation of emerging fluorinated pollutants by 19F NMR. The spectra are from cu...

Figure 17: Discovery of new fluorinated natural products by 19F NMR. The spectrum is of the culture supernatan...

Figure 18: Application of 19F NMR to investigate the biosynthesis of nucleocidin. The spectra are from culture...

Figure 19: Detection of new fluorofengycins (indicated by arrows) in culture supernatants of Bacillus sp. CS93...

Figure 20: Measurement of β-galactosidase activity in MCF7 cancer cells expressing lacZ using 19F NMR. The deg...

Figure 21: Detection of ions using 19F NMR. (a) Structure of TF-BAPTA and its 19F iCEST spectra in the presenc...

Figure 22: (a) The ONOO−-mediated decarbonylation of 5-fluoroisatin and 6-fluoroisatin. The selectivity of (b)...
The preparation and properties of 1,1-difluorocyclopropane derivatives
- Kymbat S. Adekenova,
- Peter B. Wyatt and
- Sergazy M. Adekenov
Beilstein J. Org. Chem. 2021, 17, 245–272, doi:10.3762/bjoc.17.25
- , carbanion, and radical chemistry. Furthermore, gem-difluorocyclopropanes readily go through carbonylation, dehalogenation, and annulation, resulting in various useful materials. 2.1 Thermal rearrangements The substitution of hydrogen with fluorine in cyclopropane leads to a significant weakening of the C–C

Graphical Abstract

Scheme 1: Synthesis of 1,1-difluoro-2,3-dimethylcyclopropane (2).

Scheme 2: Cyclopropanation via dehydrohalogenation of chlorodifluoromethane.

Scheme 3: Difluorocyclopropanation of methylstyrene 7 using dibromodifluoromethane and zinc.

Scheme 4: Synthesis of difluorocyclopropanes from the reaction of dibromodifluoromethane and triphenylphosphi...

Scheme 5: Generation of difluorocarbene in a catalytic two-phase system and its addition to tetramethylethyle...

Scheme 6: The reaction of methylstyrene 7 with chlorodifluoromethane (11) in the presence of a tetraarylarson...

Scheme 7: Pyrolysis of sodium chlorodifluoroacetate (12) in refluxing diglyme in the presence of alkene 13.

Scheme 8: Synthesis of boron-substituted gem-difluorocyclopropanes 16.

Scheme 9: Addition of sodium bromodifluoroacetate (17) to alkenes.

Scheme 10: Addition of sodium bromodifluoroacetate (17) to silyloxy-substituted cyclopropanes 20.

Scheme 11: Synthesis of difluorinated nucleosides.

Scheme 12: Addition of butyl acrylate (26) to difluorocarbene generated from TFDA (25).

Scheme 13: Addition of difluorocarbene to propargyl esters 27 and conversion of the difluorocyclopropenes 28 t...

Scheme 14: The generation of difluorocyclopropanes using MDFA 30.

Scheme 15: gem-Difluorocyclopropanation of styrene (32) using difluorocarbene generated from TMSCF3 (31) under...

Scheme 16: Synthesis of a gem-difluorocyclopropane derivative using HFPO (41) as a source of difluorocarbene.

Scheme 17: Cyclopropanation of (Z)-2-butene in the presence of difluorodiazirine (44).

Scheme 18: The cyclopropanation of 1-octene (46) using Seyferth's reagent (45) as a source of difluorocarbene.

Scheme 19: Alternative approaches for the difluorocarbene synthesis from trimethyl(trifluoromethyl)tin (48).

Scheme 20: Difluorocyclopropanation of cyclohexene (49).

Scheme 21: Synthesis of difluorocyclopropane derivative 53 using bis(trifluoromethyl)cadmium (51) as the diflu...

Scheme 22: Addition of difluorocarbene generated from tris(trifluoromethyl)bismuth (54).

Scheme 23: Addition of a stable (trifluoromethyl)zinc reagent to styrenes.

Scheme 24: The preparation of 2,2-difluorocyclopropanecarboxylic acids of type 58.

Scheme 25: Difluorocyclopropanation via Michael cyclization.

Scheme 26: Difluorocyclopropanation using N-acylimidazolidinone 60.

Scheme 27: Difluorocyclopropanation through the cyclization of phenylacetonitrile (61) and 1,2-dibromo-1,1-dif...

Scheme 28: gem-Difluoroolefins 64 for the synthesis of functionalized cyclopropanes 65.

Scheme 29: Preparation of aminocyclopropanes 70.

Scheme 30: Synthesis of fluorinated methylenecyclopropane 74 via selenoxide elimination.

Scheme 31: Reductive dehalogenation of (1R,3R)-75.

Scheme 32: Synthesis of chiral monoacetates by lipase catalysis.

Scheme 33: Transformation of (±)-trans-81 using Rhodococcus sp. AJ270.

Scheme 34: Transformation of (±)-trans-83 using Rhodococcus sp. AJ270.

Scheme 35: Hydrogenation of difluorocyclopropenes through enantioselective hydrocupration.

Scheme 36: Enantioselective transfer hydrogenation of difluorocyclopropenes with a Ru-based catalyst.

Scheme 37: The thermal transformation of trans-1,2-dichloro-3,3-difluorocyclopropane (84).

Scheme 38: cis–trans-Epimerization of 1,1-difluoro-2,3-dimethylcyclopropane.

Scheme 39: 2,2-Difluorotrimethylene diradical intermediate.

Scheme 40: Ring opening of stereoisomers 88 and 89.

Scheme 41: [1,3]-Rearrangement of alkenylcyclopropanes 90–92.

Scheme 42: Thermolytic rearrangement of 2,2-difluoro-1-vinylcyclopropane (90).

Scheme 43: Thermal rearrangement for ethyl 3-(2,2-difluoro)-3-phenylcyclopropyl)acrylates 93 and 95.

Scheme 44: Possible pathways of the ring opening of 1,1-difluoro-2-vinylcyclopropane.

Scheme 45: Equilibrium between 1,1-difluoro-2-methylenecyclopropane (96) and (difluoromethylene)cyclopropane 97...

Scheme 46: Ring opening of substituted 1,1-difluoro-2,2-dimethyl-3-methylenecyclopropane 98.

Scheme 47: 1,1-Difluorospiropentane rearrangement.

Scheme 48: Acetolysis of (2,2-difluorocyclopropyl)methyl tosylate (104) and (1,1-difluoro-2-methylcyclopropyl)...

Scheme 49: Ring opening of gem-difluorocyclopropyl ketones 106 and 108 by thiolate nucleophiles.

Scheme 50: Hydrolysis of gem-difluorocyclopropyl acetals 110.

Scheme 51: Ring-opening reaction of 2,2-difluorocyclopropyl ketones 113 in the presence of ionic liquid as a s...

Scheme 52: Ring opening of gem-difluorocyclopropyl ketones 113a by MgI2-initiated reaction with diarylimines 1...

Scheme 53: Ring-opening reaction of gem-difluorocyclopropylstannanes 117.

Scheme 54: Preparation of 1-fluorovinyl vinyl ketone 123 and the synthesis of 2-fluorocyclopentenone 124. TBAT...

Scheme 55: Iodine atom-transfer ring opening of 1,1-difluoro-2-(1-iodoalkyl)cyclopropanes 125a–c.

Scheme 56: Ring opening of bromomethyl gem-difluorocyclopropanes 130 and formation of gem-difluoromethylene-co...

Scheme 57: Ring-opening aerobic oxidation reaction of gem-difluorocyclopropanes 132.

Scheme 58: Dibrominative ring-opening functionalization of gem-difluorocyclopropanes 134.

Scheme 59: The selective formation of (E,E)- and (E,Z)-fluorodienals 136 and 137 from difluorocyclopropyl acet...

Scheme 60: Proposed mechanism for the reaction of difluoro(methylene)cyclopropane 139 with Br2.

Scheme 61: Thermal rearrangement of F2MCP 139 and iodine by CuI catalysis.

Scheme 62: Synthesis of 2-fluoropyrroles 142.

Scheme 63: Ring opening of gem-difluorocyclopropyl ketones 143 mediated by BX3.

Scheme 64: Lewis acid-promoted ring-opening reaction of 2,2-difluorocyclopropanecarbonyl chloride (148).

Scheme 65: Ring-opening reaction of the gem-difluorocyclopropyl ketone 106 by methanolic KOH.

Scheme 66: Hydrogenolysis of 1,1-difluoro-3-methyl-2-phenylcyclopropane (151).

Scheme 67: Synthesis of monofluoroalkenes 157.

Scheme 68: The stereoselective Ag-catalyzed defluorinative ring-opening diarylation of 1-trimethylsiloxy-2,2-d...

Scheme 69: Synthesis of 2-fluorinated allylic compounds 162.

Scheme 70: Pd-catalyzed cross-coupling reactions of gem-difluorinated cyclopropanes 161.

Scheme 71: The (Z)-selective Pd-catalyzed ring-opening sulfonylation of 2-(2,2-difluorocyclopropyl)naphthalene...

Figure 1: Structures of zosuquidar hydrochloride and PF-06700841.

Scheme 72: Synthesis of methylene-gem-difluorocyclopropane analogs of nucleosides.

Figure 2: Anthracene-difluorocyclopropane hybrid derivatives.

Figure 3: Further examples of difluorcyclopropanes in modern drug discovery.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
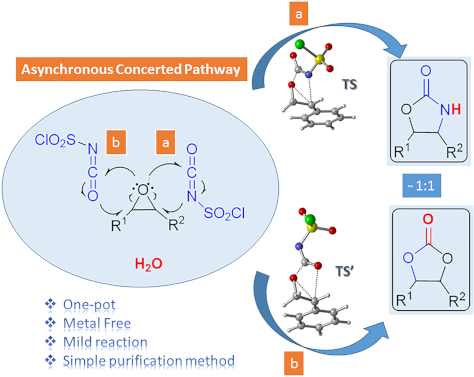
- oxazolidinones and five-membered cyclic carbonates of various structures. The most well-known strategies for the synthesis of oxazolidinones are the reaction of an amino alcohol with phosgene [5][22], the carbonylation reaction of β-amino alcohols with CO2 or dialkyl carbonates [23][24][25][26][27], the

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
- Lucas Guillemard and
- Joanna Wencel-Delord
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- ) intermediate occurs prior to the one-electron oxidation by the photoexcited catalyst. This contribution inspired also other research groups to investigate synergistic catalyses using Pd-based C–H catalysts. In 2016, Lei’s group reported the aerobic intramolecular oxidative carbonylation of enamides for the

Graphical Abstract

Figure 1: Concept of dual synergistic catalysis.

Figure 2: Classification of catalytic systems involving two catalysts.

Figure 3: General mechanism for the dual nickel/photoredox catalytic system.

Figure 4: General mechanisms for C–H activation catalysis involving different reoxidation strategies.

Figure 5: Indole synthesis via dual C–H activation/photoredox catalysis.

Figure 6: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 7: Oxidative Heck reaction on arenes via the dual catalysis.

Figure 8: Proposed mechanism for the Heck reaction on arenes via dual catalysis.

Figure 9: Oxidative Heck reaction on phenols via the dual catalysis.

Figure 10: Proposed mechanism for the Heck reaction on phenols via dual catalysis.

Figure 11: Carbazole synthesis via dual C–H activation/photoredox catalysis.

Figure 12: Proposed mechanism for the carbazole synthesis via dual catalysis.

Figure 13: Carbonylation of enamides via the dual C–H activation/photoredox catalysis.

Figure 14: Proposed mechanism for carbonylation of enamides via dual catalysis.

Figure 15: Annulation of benzamides via the dual C–H activation/photoredox catalysis.

Figure 16: Proposed mechanism for the annulation of benzamides via dual catalysis.

Figure 17: Synthesis of indoles via the dual C–H activation/photoredox catalysis.

Figure 18: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 19: General concept of dual catalysis merging C–H activation and photoredox catalysis.

Figure 20: The first example of dual catalysis merging C–H activation and photoredox catalysis.

Figure 21: Proposed mechanism for the C–H arylation with diazonium salts via dual catalysis.

Figure 22: Dual catalysis merging C–H activation/photoredox using diaryliodonium salts.

Figure 23: Direct arylation via the dual catalytic system reported by Xu.

Figure 24: Direct arylation via dual catalytic system reported by Balaraman.

Figure 25: Direct arylation via dual catalytic system reported by Guo.

Figure 26: C(sp3)–H bond arylation via the dual Pd/photoredox catalytic system.

Figure 27: Acetanilide derivatives acylation via the dual C–H activation/photoredox catalysis.

Figure 28: Proposed mechanism for the C–H acylation with α-ketoacids via dual catalysis.

Figure 29: Acylation of azobenzenes via the dual catalysis C–H activation/photoredox.

Figure 30: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 31: Proposed mechanism for the C2-acylation of indoles with aldehydes via dual catalysis.

Figure 32: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 33: Perfluoroalkylation of arenes via the dual C–H activation/photoredox catalysis.

Figure 34: Proposed mechanism for perfluoroalkylation of arenes via dual catalysis.

Figure 35: Sulfonylation of 1-naphthylamides via the dual C–H activation/photoredox catalysis.

Figure 36: Proposed mechanism for sulfonylation of 1-naphthylamides via dual catalysis.

Figure 37: meta-C–H Alkylation of arenes via visible-light metallaphotocatalysis.

Figure 38: Alternative procedure for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 39: Proposed mechanism for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 40: C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 41: Proposed mechanism for C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 42: Undirected C–H aryl–aryl cross coupling via dual gold/photoredox catalysis.

Figure 43: Proposed mechanism for the undirected C–H aryl–aryl cross-coupling via dual catalysis.

Figure 44: Undirected C–H arylation of (hetero)arenes via dual manganese/photoredox catalysis.

Figure 45: Proposed mechanism for the undirected arylation of (hetero)arenes via dual catalysis.

Figure 46: Photoinduced C–H arylation of azoles via copper catalysis.

Figure 47: Photo-induced C–H chalcogenation of azoles via copper catalysis.

Figure 48: Decarboxylative C–H adamantylation of azoles via dual cobalt/photoredox catalysis.

Figure 49: Proposed mechanism for the C–H adamantylation of azoles via dual catalysis.

Figure 50: General mechanisms for the “classical” (left) and Cu-free variant (right) Sonogoshira reaction.

Figure 51: First example of a dual palladium/photoredox catalysis for Sonogashira-type couplings.

Figure 52: Arylation of terminal alkynes with diazonium salts via dual gold/photoredox catalysis.

Figure 53: Proposed mechanism for the arylation of terminal alkynes via dual catalysis.

Figure 54: C–H Alkylation of alcohols promoted by H-atom transfer (HAT).

Figure 55: Proposed mechanism for the C–H alkylation of alcohols promoted by HAT.

Figure 56: C(sp3)–H arylation of latent nucleophiles promoted by H-atom transfer.

Figure 57: Proposed mechanism for the C(sp3)–H arylation of latent nucleophiles promoted by HAT.

Figure 58: Direct α-arylation of alcohols promoted by H-atom transfer.

Figure 59: Proposed mechanism for the direct α-arylation of alcohols promoted by HAT.

Figure 60: C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 61: Proposed mechanism for the C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 62: C–H functionalization of nucleophiles via excited ketone/nickel dual catalysis.

Figure 63: Proposed mechanism for the C–H functionalization enabled by excited ketones.

Figure 64: Selective sp3–sp3 cross-coupling promoted by H-atom transfer.

Figure 65: Proposed mechanism for the selective sp3–sp3 cross-coupling promoted by HAT.

Figure 66: Direct C(sp3)–H acylation of amines via dual Ni/photoredox catalysis.

Figure 67: Proposed mechanism for the C–H acylation of amines via dual Ni/photoredox catalysis.

Figure 68: C–H hydroalkylation of internal alkynes via dual Ni/photoredox catalysis.

Figure 69: Proposed mechanism for the C–H hydroalkylation of internal alkynes.

Figure 70: Alternative procedure for the C–H hydroalkylation of ynones, ynoates, and ynamides.

Figure 71: Allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 72: Proposed mechanism for the allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 73: Asymmetric allylation of aldehydes via dual Cr/photoredox catalysis.

Figure 74: Proposed mechanism for the asymmetric allylation of aldehydes via dual catalysis.

Figure 75: Aldehyde C–H functionalization promoted by H-atom transfer.

Figure 76: Proposed mechanism for the C–H functionalization of aldehydes promoted by HAT.

Figure 77: Direct C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 78: Proposed mechanism for the C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 79: Direct C–H trifluoromethylation of strong aliphatic bonds promoted by HAT.

Figure 80: Proposed mechanism for the C–H trifluoromethylation of strong aliphatic bonds.
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- ) [36]. The authors hypothesized that the C–O bond cleavage could be achieved via cobalt-mediated alcohol carbonylation followed by radical decarboxylation of the alkoxycarbonyl intermediate. In a proof-of-concept study, they proceeded with the carbonylation of 1-phenylethanol using Co(II) tetrakis(4

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Rhodium-catalyzed reductive carbonylation of aryl iodides to arylaldehydes with syngas
- Zhenghui Liu,
- Peng Wang,
- Zhenzhong Yan,
- Suqing Chen,
- Dongkun Yu,
- Xinhui Zhao and
- Tiancheng Mu
Beilstein J. Org. Chem. 2020, 16, 645–656, doi:10.3762/bjoc.16.61
- of China, Beijing 100872, China 10.3762/bjoc.16.61 Abstract The reductive carbonylation of aryl iodides to aryl aldehydes possesses broad application prospects. We present an efficient and facile Rh-based catalytic system composed of the commercially available Rh salt RhCl3·3H2O, PPh3 as phosphine
- ligand, and Et3N as the base, for the synthesis of arylaldehydes via the reductive carbonylation of aryl iodides with CO and H2 under relatively mild conditions with a broad substrate range affording the products in good to excellent yields. Systematic investigations were carried out to study the
- pressure, cheap ligand and low metal dosage could significantly improve the practicability in both chemical researches and industrial applications. Keywords: cost-effective ligand; industrial catalysis; reductive carbonylation; rhodium catalyst; syngas; Introduction The exploration of environmentally

Graphical Abstract

Figure 1: Rhodium-catalyzed reductive carbonylation of iodobenzene with CO and H2 to afford benzaldehyde. a) ...

Scheme 1: Scaled-up experiment of the reductive carbonylation of iodobenzene to benzaldehyde under the optimi...

Scheme 2: Catalytic species participating in the catalytic process.

Scheme 3: Substrate scope for the Rh-catalyzed reductive carbonylation of aryl iodides using CO and H2. React...

Scheme 4: Isotope-labeling experiments.

Scheme 5: Proposed reaction mechanism for the Rh-catalyzed reductive carbonylation of aryl iodides using CO a...
Visible-light-induced addition of carboxymethanide to styrene from monochloroacetic acid
- Kaj M. van Vliet,
- Nicole S. van Leeuwen,
- Albert M. Brouwer and
- Bas de Bruin
Beilstein J. Org. Chem. 2020, 16, 398–408, doi:10.3762/bjoc.16.38
- useful for the formation of new C–C bonds. Previous works from our group presented cobalt-catalyzed radical cyclization and carbonylation reactions [12][13][14][15][16]. Photoredox catalysis provides a way to generate radical intermediates from simple organic molecules by single-electron redox processes

Graphical Abstract

Figure 1: A part of the industry around monochloroacetic acid.

Scheme 1: Redox based activation of haloacetic acid.

Figure 2: Cyclic voltammogram of monochloroacetic acid and ferrocene with 0.1 M [TBA][PF6] in MeCN. The poten...

Scheme 2: Initial attempts for lactone formation by photoredox catalysis.

Scheme 3: The photoredox reaction of TEMPO with monochloroacetic acid catalyzed by fac-[Ir(ppy)3].

Figure 3: EPR spectra measured (black) and simulated (red) based on the structure of the oxidized photoredox ...

Scheme 4: Two possible acid-assisted, reductive activation pathways of monochloroacetic acid (A–H = acid).

Figure 4: Reaction mixtures after overnight irradiation of (A) 4-chloro-4-phenylbutanoic acid (3) and fac-[Ir...

Scheme 5: Substrate scope of styrene derivatives in the photoredox reaction with monochloroacetic acid. Yield...

Scheme 6: Proposed reaction mechanism.

Scheme 7: The photoredox formation of 1-(chloromethoxy)-2,2,6,6-tetramethylpiperidine.
A review of the total syntheses of triptolide
- Xiang Zhang,
- Zaozao Xiao and
- Hongtao Xu
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- -phenylethylamine (49) to generate key tricyclic intermediates 51 and 52, a Pd(II)-catalyzed carbonylation–lactonization reaction of 9 to construct the butenolide (D-ring), and a Friedel–Crafts isopropylation to install the C-13 isopropyl group. Still, the construction of the C-5 trans junction A-/B-ring was
- conversion of 89 to vinyl triflate 90, followed by reduction and palladium-catalyzed carbonylation–lactone formation to give key intermediate 8 [75][76][77]. After that, the three successive epoxides were installed by known procedures with some modification. The highlights were the introduction of the second
- and its relatives from commercially available acid 32 (Figure 2, route J and Scheme 8) [46]. This synthesis highlights the utilization of an indium(III)-catalyzed cationic polycyclization of 17 and a palladium-catalyzed carbonylation–in situ lactone formation to construct the key intermediate 94

Graphical Abstract

Figure 1: Structures of triptolide (1), triptonide (2), tripdiolide (3), 16-hydroxytriptolide (4), triptrioli...

Figure 2: Syntheses of triptolide.

Scheme 1: Berchtold’s synthesis of triptolide.

Scheme 2: Li’s formal synthesis of triptolide.

Scheme 3: van Tamelen’s asymmetric synthesis of triptonide and triptolide.

Scheme 4: Van Tamelen’s (method II) formal synthesis of triptolide.

Scheme 5: Sherburn’s formal synthesis of triptolide.

Scheme 6: van Tamelen’s biogenetic type total synthesis of triptolide.

Scheme 7: Yang’s total synthesis of triptolide.

Scheme 8: Key intermediates or transformations of routes J–N.
Vicinal difunctionalization of alkenes by four-component radical cascade reaction of xanthogenates, alkenes, CO, and sulfonyl oxime ethers
- Shuhei Sumino,
- Takahide Fukuyama,
- Mika Sasano,
- Ilhyong Ryu,
- Antoine Jacquet,
- Frédéric Robert and
- Yannick Landais
Beilstein J. Org. Chem. 2019, 15, 1822–1828, doi:10.3762/bjoc.15.176
- tributyltin radical with 1a. The electrophilic α-carbonyl radical A does not react with CO even at high CO pressure [6], and therefore selectively adds to electron-rich olefin 2a to form a carbon-centered radical B. The radical B, regarded as a nucleophilic radical, then undergoes radical carbonylation with
- radical which sustains the radical chain. Since radical B can also add to sulfonyl oxime ether 3a, we used high CO pressure conditions to encourage the radical carbonylation to form acyl radical C. Conclusion In summary, we demonstrated that a four-component radical cascade reaction, between xanthogenates

Graphical Abstract

Scheme 1: Concept: Alkene difuctionalization by four-component radical reaction using xanthates, alkenes, CO ...

Figure 1: Vicinal difunctionalization of alkenes by four-component radical cascade reaction using xanthogenat...

Figure 2: Proposed radical chain mechanism.
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165


Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.






