Search results
Search for "similarity" in Full Text gives 294 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- similarity of the successive evolution and of the transient spectra detected in the two cases indicates that a similar reactivity is induced upon one-photon and two-photon excitation, further confirming the two-photon photoswitching ability of hydrazone 1 already inferred by previous measurements [29]. Using
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- on the prototypical arylazopyrazole 1 [35] (Scheme 1) and a previously reported [48] metal–organic cage 2 (see Figure 1). We have recently demonstrated that various azobenzenes formed 2:1 inclusion complexes with 2 [49] and hypothesized, based on the structural similarity between azobenzenes and
Excited state dynamics for visible-light sensitization of a photochromic benzil-subsituted phenoxyl-imidazolyl radical complex
Beilstein J. Org. Chem. 2019, 15, 2369–2379, doi:10.3762/bjoc.15.229

- constant at the early stage of the transient absorption spectra. The EAS with time constants of 2.5 ns and 2.0 μs are safely assigned to the absorption spectra of the S1 and the T1 states, respectively, because of the similarity of the spectra to those reported previously [39][40]. In the Benzil-PIC system
- ≈2 ps [45]. The similarity of the time constant of the bond breaking to that of EAS1 supports that the C–N bond is cleaved by the direct excitation of the PIC unit. The spectral evolution from EAS2 (1.4 ps) to EAS3 (38 ps, green in Figure 3d) shows the continuous spectral shift due to the benzil unit
Isolation and biosynthesis of an unsaturated fatty acid with unusual methylation pattern from a coral-associated bacterium Microbulbifer sp.
Beilstein J. Org. Chem. 2019, 15, 2327–2332, doi:10.3762/bjoc.15.225

- dilution was spread onto marine agar 2216 (Difco). The plates were kept at 23 °C, and a single colony was repeatedly transferred onto the same agar medium to obtain the pure isolate of strain C4-6. The strain was identified as a member of the genus Microbulbifer on the basis of 99.0% similarity in the 16S
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- E1/21, two new absorption bands were observed in this solution (Figure 4a). Based on its similarity to the reported NAr3•+ derivatives [56], the first band at 760 nm derives from the π–π* transition of the NAr3•+ moiety of 1b+ [56]. However, the second band in the NIR region significantly differs in
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- photoswitchable, compounds 4a and 4b were synthesized to test the influence of a rigid conformation around the C=C double bond on sirtuin inhibition. Interestingly, this increased rigidity provokes a complete loss of activity against Sirt1. Despite the fact, that all mammalian sirtuins possess profound similarity
- the LC–HRMS, clearly confirming the experimentally found blue shift of 0.75 eV and 0.54 eV compared to compounds (E)-2b and (Z)-2b, respectively. Also here, we conclude that the calculations clearly support the formation of compounds 8a and/or 8b. However, due to the similarity of the spectra of 8a
- and 8b, calculations do not allow to predict which of the two isomers was present in the respective fraction analysed. Regarding the high similarity between 8a/8b and selisistat, it was likely that these cyclized compounds could possess biological activity against sirtuins, too. On the other hand they
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
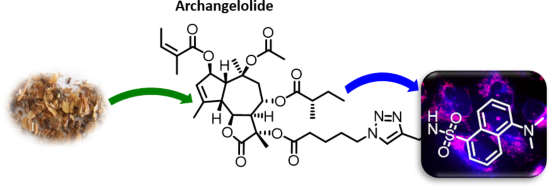
- of action are unknown. Therefore, based on the structural similarity between compound 1 and 2, we suspected that new interesting and relevant information on this biologically largely undescribed SL could be identified. In order to study the action of these commercially unavailable compounds
- trilobolide (2), from the seeds of Laserpitium archangelica Wulfen, we extended the knowledge on the first one, so far almost undescribed. We revealed that despite a high degree of structural similarity with two cytotoxic SLs, compound 2 and thapsigargin, compound 1 exhibited cytotoxicity neither in the
Halide metathesis in overdrive: mechanochemical synthesis of a heterometallic group 1 allyl complex
Beilstein J. Org. Chem. 2019, 15, 1856–1863, doi:10.3762/bjoc.15.181

- that both [KA']∞ and [CsKA'2] display three such angles, two of which are relatively similar at ca. 135–140°, and a third that is substantially more bent (<120°) (see the Supporting Information File 1, Figure S2, for a visualization of the similarity). The significance of this is that [KA'] can be
The cyclopropylcarbinyl route to γ-silyl carbocations
Beilstein J. Org. Chem. 2019, 15, 1769–1780, doi:10.3762/bjoc.15.170
- ]. The similarity of products formed from acetolysis of 32 and 42 implies that the same cation rearrangement manifold is involved. Scheme 9 gives a mechanistic rationale for these products. Capture of an unrearranged discrete cyclopropylcarbinyl cation 43 gives the major product 38, while migration of
Fluorine-containing substituents: metabolism of the α,α-difluoroethyl thioether motif
Beilstein J. Org. Chem. 2019, 15, 1441–1447, doi:10.3762/bjoc.15.144
- other areas of bioactives discovery research, to complement the widely used ROCF3 and RSCF3 groups and there is a clear similarity to the ‘polar lipophilic’ groups ROCF2H, and RSCF2H [26]. Experimental Microorganism growth Cunninghamella elegans DSM1908 was grown on Saboraud dextrose agar gel plates for
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- waves in the cyclic voltammograms, the LUMO energy levels were calculated from Egopt and the HOMO energy and decrease with increasing amount of selenium atoms in the heterotriacenes. Because of the structural similarity of heterotriacenes 1–4 to 2,2’-bithiophene and 2,2’-biselenophene, which can be
Catalytic asymmetric oxo-Diels–Alder reactions with chiral atropisomeric biphenyl diols
Beilstein J. Org. Chem. 2019, 15, 955–962, doi:10.3762/bjoc.15.92
- supramolecular helices or dimers through intermolecular hydrogen bonding of two axially chiral biphenyl hybrid diols (1 and 2 in Scheme 1) which contain point chirality at the side arms and axial chirality at the biphenyl backbone [37]. We envisage the structural similarity and the ability of our scaffold to
An efficient synthesis of the guaiane sesquiterpene (−)-isoguaiene by domino metathesis
Beilstein J. Org. Chem. 2019, 15, 858–862, doi:10.3762/bjoc.15.83
- roots of Parthenium hysterophorus [5]. A recent enantioselective synthesis of (−)-isoguaiene (1) from (+)-dihydrocarvone [6] enabled an unambiguous assignment of its absolute configuration as depicted in Figure 1. Due to the structural similarity of 1 and the trisnorsesquiterpene clavukerin A (2), we
Selective benzylic C–H monooxygenation mediated by iodine oxides
Beilstein J. Org. Chem. 2019, 15, 602–609, doi:10.3762/bjoc.15.55
- the use of a catalytic system using HNO3, dioxygen, iodine and NHPI in the acetoxylation of C–H bonds [47]. Given this similarity, we believe that the two systems are operating by similar mechanisms. In the Minisci system, a phthalimide N-oxyl (PINO) radical, which is formed by the oxidation of NHPI
Computational characterization of enzyme-bound thiamin diphosphate reveals a surprisingly stable tricyclic state: implications for catalysis
Beilstein J. Org. Chem. 2019, 15, 145–159, doi:10.3762/bjoc.15.15

- , albeit more indirect, comes from an inhibition study using omeprazole, which was predicted to possibly interact with ThDP-dependent enzymes. The prediction was based on the similarity of omeprazole to the tricyclic form of thiamin. This was confirmed experimentally when omeprazole was subsequently shown
Synthesis, biophysical properties, and RNase H activity of 6’-difluoro[4.3.0]bicyclo-DNA
Beilstein J. Org. Chem. 2019, 15, 79–88, doi:10.3762/bjoc.15.9
- opposite the modified part and 2’-O-methyl RNA flanks. The same construct was previously applied for the evaluation of 2’F-tc-ANA [35] and a similar one for CeNA [52]. For the assay E. coli RNase H was used due to its commercial availability and its similarity to the human enzyme [22]. The cleavage pattern
Lectins of Mycobacterium tuberculosis – rarely studied proteins
Beilstein J. Org. Chem. 2019, 15, 1–15, doi:10.3762/bjoc.15.1

- products of Rv1753 and Rv2082 were reported to have 27% and 25% amino acid sequence similarity to the ALS1 gene from Candida albicans, which encodes the candida adhesin [78]. This lectin is cell surface-localized and mediates adherence of the fungus to endothelial and epithelial cells [86][87]. Fucose
- Rv2082, and an associated ALS-like lectin function is only speculation. Mannose-sensitive hemagglutinin Two Mtb gene products, encoded by Rv2813 and Rv3659, were classified as MSHA-like proteins, with the highest amino acid similarity directed to MshM (41%) and MshE (26%), respectively, of the marine
- homodimers, homotrimers, and higher-ordered oligomers, which increases their avidity for multivalent ligands. C-Type lectins play key roles in cell–cell interactions, such as host–pathogen interactions, and phagocytosis [92]. The Mtb gene product of Rv2075c shows partial amino acid sequence similarity to
N-Acylated amino acid methyl esters from marine Roseobacter group bacteria
Beilstein J. Org. Chem. 2018, 14, 2964–2973, doi:10.3762/bjoc.14.276
- and ESI mass spectra revealed fragmentation patterns helpful for the detection of similar compounds derived from other amino acids. Some of these compounds showed antimicrobial activity. The structural similarity of N-acylated amino acid methyl esters and similar lipophilicity to AHLs might indicate a
- retention index for iso-C15:0 and anteiso-C15:0-NAME are Ic = 2352 and Ic = 2365, respectively, while all other methyl branch locations had a lower value. The close similarity of Inat = 2354 and Ic = 2352 suggested the methyl branch to be located in the iso-position. Consequently, iso-C15:0-NAME (6) was
- against E. coli TolC, and it was the only compound that showed moderate cytotoxicity on a human cancer cell line. The close similarity of NAMEs, NAVMEs, and NAGMEs to AHLs might indicate a function as signalling compounds, although experiments with NAMEs and AHL reporter assays did not reveal any activity
The activity of indenylidene derivatives in olefin metathesis catalysts
Beilstein J. Org. Chem. 2018, 14, 2956–2963, doi:10.3762/bjoc.14.275
- 29.6% for SIMes-based catalysts 1, 3 and 5, respectively (see Figure 1). The same trend applies to the IMes-based catalysts 2, 4 and 6, with %VBur values of 30.0%, 29.7% and 29.6%, respectively. On the other hand, as expected since there is similarity in the part bonded to the metal [49], no
Protein–protein interactions in bacteria: a promising and challenging avenue towards the discovery of new antibiotics
Beilstein J. Org. Chem. 2018, 14, 2881–2896, doi:10.3762/bjoc.14.267

- was screened by X-ray crystallography leading to the identification of four fragment hits. In an attempt to improve their binding affinities, another library was searched for compounds displaying similarity to these initial hits. After a docking-based screening followed by a fluorescence polarization
Synthesis of a tyrosinase inhibitor by consecutive ethenolysis and cross-metathesis of crude cashew nutshell liquid
Beilstein J. Org. Chem. 2018, 14, 2737–2744, doi:10.3762/bjoc.14.252
- are certainly drawbacks for larger scale production (Scheme 2) [27]. Due to the structural similarity of ginkgolic and anacardic acids, we believed that a particularly desirable synthesis of 2-hydroxy-6-tridecylbenzoic acid (3) would involve CNSL as the substrate basis. However, the functionalization
Synthesis and biological evaluation of 1,2-disubstituted 4-quinolone analogues of Pseudonocardia sp. natural products
Beilstein J. Org. Chem. 2018, 14, 2680–2688, doi:10.3762/bjoc.14.245
- analogues was observed to inhibit production of the virulence factor pyocyanin in the human pathogen Pseudomonas aeruginosa, which may be a result of their similarity to the Pseudomonas quinolone signal (PQS) quorum sensing autoinducer. This provided new insights regarding the effect of N-substitution in
- was noted for the compounds possessing a linear octanyl side chain. We speculate that this is due to the similarity to the heptanyl chain present in the native PQS ligand. This observation is highly intriguing, as HHQ (Figure 1), which differs from these analogues only by the lack of an N-substituent
Non-native autoinducer analogs capable of modulating the SdiA quorum sensing receptor in Salmonella enterica serovar Typhimurium
Beilstein J. Org. Chem. 2018, 14, 2651–2664, doi:10.3762/bjoc.14.243

- correlation is interesting because sdiA is the descendent of a horizontal gene transfer of rhlR [63]. Indeed, the sequence of SdiA from Salmonella is 45% identical to RhlR, more than it is to LasR (27%), QscR (33%), TraR (23%), LuxR (27%), or CviR (32%) [21]. In further support of a possible similarity
Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers
Beilstein J. Org. Chem. 2018, 14, 2627–2645, doi:10.3762/bjoc.14.241

- similarity to the signal molecule synthase were investigated. Here, substrates of chalcone synthase CHS2 from Medicago sativa were tested for their ability to block PqsD function. Indeed, caffeic acid analogues, such as 15, were identified as hits and further characterized as channel blockers as described
- before [59]. Further interesting starting points for the discovery of PqsD inhibitors have been provided by a dedicated screening campaign involving fragment-based hit discovery, in silico screening and a similarity-guided approach starting from FabH inhibitors [60]. The most potent hit 16 of this study
Synthesis of 3-aminocoumarin-N-benzylpyridinium conjugates with nanomolar inhibitory activity against acetylcholinesterase
Beilstein J. Org. Chem. 2018, 14, 2545–2552, doi:10.3762/bjoc.14.231
- acetylcholinesterase complexed with donepezil (PDB code 4ey7) [18] retrieved from the Protein Data Bank was used in the study because of its high similarity (approximately 88% similarity), good resolution (at 2.35 Å) and ligand state with donepezil of Human AChE structure. Molecular docking of AChE via the GOLD v5.2.2










































































































































































































































