Search results
Search for "1,2-diols" in Full Text gives 21 result(s) in Beilstein Journal of Organic Chemistry.
Synthesis of ether lipids: natural compounds and analogues
- Marco Antônio G. B. Gomes,
- Alicia Bauduin,
- Chloé Le Roux,
- Romain Fouinneteau,
- Wilfried Berthe,
- Mathieu Berchel,
- Hélène Couthon and
- Paul-Alain Jaffrès
Beilstein J. Org. Chem. 2023, 19, 1299–1369, doi:10.3762/bjoc.19.96

Graphical Abstract

Figure 1: Chemical structure of some natural ether lipids (ELs).

Figure 2: Synthesis of lyso-PAF and PAF from 1-O-alkylglycerol [64].

Figure 3: Synthesis of lyso-PAF from 1,3-benzylideneglycerol 3.1 [69].

Figure 4: A) Synthesis of the two enantiomers of octadecylglycerol (4.6 and 4.10) from ᴅ-mannitol (4.1); B) s...

Figure 5: Four-step synthesis of PAF 5.6 from (S)-glycidol [73].

Figure 6: Synthesis of 1-O-alkylglycerol A) from solketal, B) from ᴅ- or ʟ-tartaric acid and the intermediate ...

Figure 7: Synthesis of EL building blocks starting from substituted glycidol 7.1a–c [82].

Figure 8: Synthesis of PAF 8.5 by using phosphoramidite 8.2 [86].

Figure 9: Synthesis of oleyl-PAF 9.7 from ʟ-serine [88].

Figure 10: Synthesis of racemic analogues of lyso-PAF 10.8 and PAF 10.9 featuring a phenyl group between the g...

Figure 11: Synthesis of racemic deoxy-lyso-PAF 11.7 and deoxy-PAF 11.8 [91].

Figure 12: Synthesis of racemic thio-PAF 12.8 [93].

Figure 13: Racemic synthesis of 13.6 to illustrate the modification of the glycerol backbone by adding a methy...

Figure 14: Racemic synthesis of 14.5 as an illustration of the introduction of methyl substituents on the glyc...

Figure 15: Synthesis of functionalized sn-2-acyl chains of PC-EL; A) Steglich esterification or acylation reac...

Figure 16: Synthesis of racemic mc-PAF (16.3), a carbamate analogue of PAF [102].

Figure 17: A) Synthesis of (R)-17.2 and (S)-17.6 starting from (S)-solketal (17.1); B) synthesis of N3-PAF (17...

Figure 18: Modification of the phosphocholine polar head to produce PAF analogues [81].

Figure 19: Racemic PAF analogues 19.3 and 19.5 characterized by the absence of the phosphate group [107].

Figure 20: Synthesis of PIP3-PAF (20.7) [108].

Figure 21: Large-scale synthesis of C18-edelfosine (21.8) [116].

Figure 22: Synthesis of C16-edelfosine (22.10) starting from isopropylidene-ʟ-glyceric acid methyl ester (22.1...

Figure 23: Phosphocholine moiety installation by the use of chlorophosphite 23.2 as key reagent [119].

Figure 24: Synthesis of rac-1-alkyl-2-O-methylglycerol (AMG) [120].

Figure 25: Synthesis of stereocontrolled 1-alkyl-2-O-methyl glycerol 25.9 (AMG) from dimethyl ᴅ-tartrate [81].

Figure 26: A) Racemic synthesis of thioether 26.4 [129,130], B) structure of sulfone analogue 26.5 [129].

Figure 27: Stereocontrolled synthesis of C18-edelfosine thioether analogue 27.8 [118].

Figure 28: Synthesis of thioether 28.4 that include a thiophosphate function [134].

Figure 29: Synthesis of ammonium thioether 29.4 and 29.6 [135].

Figure 30: Synthesis of the N-methylamino analogue of edelfosine 30.6 (BN52211) [138].

Figure 31: Synthesis of 1-desoxy analogues of edelfosine; A) with a saturated alkyl chain; B) synthesis of the...

Figure 32: Stereocontrolled synthesis of edelfosine analogue (S)-32.8 featuring a C18:1 lipid chain [142].

Figure 33: Synthesis of edelfosine analogues with modulation of the lipid chain; A) illustration with the synt...

Figure 34: Synthesis of phospholipid featuring a carbamate function to link the lipid chain to the glycerol un...

Figure 35: Synthesis of sesquiterpene conjugates of phospho glycero ether lipids [148].

Figure 36: Racemic synthesis of methyl-substituted glycerol analogues 36.7 and 36.10: A) synthesis of diether ...

Figure 37: Racemic synthesis of ilmofosine (37.6) [155,156].

Figure 38: A) Stereoselective synthesis of 38.5 via a stereoselective hydroboration reaction; B) synthesis of ...

Figure 39: Racemic synthesis of SRI62-834 (39.6) featuring a spiro-tetrahydrofurane heterocycle in position 2 ...

Figure 40: Racemic synthesis of edelfosine analogue 40.5 featuring an imidazole moiety in sn-2 position [160].

Figure 41: Racemic synthesis of fluorine-functionalized EL: A) Synthesis of 41.6 and B) synthesis of 41.8 [161-163].

Figure 42: A) Synthesis of the β-keto-ester 42.6 that also features a decyl linker between the phosphate and t...

Figure 43: Synthesis of phosphonate-based ether lipids; A) edelfosine phosphonate analogue 43.7 and B) thioeth...

Figure 44: Enantioselective synthesis of phosphonates 44.3 and 44.4 [171].

Figure 45: Racemic synthesis of phosphinate-based ether lipid 45.10 [172].

Figure 46: Racemic synthesis of edelfosine arsonium analogue 46.5 [173].

Figure 47: Synthesis of edelfosine dimethylammonium analogue 47.2 [118].

Figure 48: Synthesis of rac-C18-edelfosine methylammonium analogue 48.4 [176].

Figure 49: A) Synthesis of edelfosine N-methylpyrrolidinium analogue 49.2 or N-methylmorpholinium analogue 49.3...

Figure 50: A) Synthesis of edelfosine’s analogue 50.4 with a PE polar group; B) illustration of a pyridinium d...

Figure 51: A) Synthesis of 51.4 featuring a thiazolium cationic moiety; B) synthesis of thiazolium-based EL 51...

Figure 52: Synthesis of cationic ether lipids 52.3, 52.4 and 52.6 [135,183].

Figure 53: Synthesis of cationic carbamate ether lipid 53.5 [184].

Figure 54: Synthesis of cationic sulfonamide 54.5 [185].

Figure 55: Chemical structure of ONO-6240 (55.1) and SRI-63-119 (55.2).

Figure 56: Synthesis of non-ionic ether lipids 56.2–56.9 [188].

Figure 57: Synthesis of ether lipid conjugated to foscarnet 57.6 [189].

Figure 58: A) Synthesis of ether lipid conjugated to arabinofuranosylcytosine; B) synthesis of AZT conjugated ...

Figure 59: Synthesis of quercetin conjugate to edelfosine [191].

Figure 60: Synthesis of 60.8 (Glc-PAF) [194].

Figure 61: A) Synthesis of amino ether lipid 61.7 functionalized with a rhamnose unit and its amide analogue 6...

Figure 62: A) Synthesis of glucose ether lipid 62.4; B) structure of ether lipid 62.5 possessing a maltose uni...

Figure 63: A) Synthesis of glucuronic methyl ester 63.8; B) structure of cellobiose 63.9 and maltose 63.10 ana...

Figure 64: A) Synthesis of maltosyl glycerolipid 64.7; B) structure of lactose analogue 64.8 prepared followin...

Figure 65: A) Asymmetric synthesis of the aglycone moiety starting from allyl 4-methoxyphenyl ether; B) glycos...

Figure 66: A) Synthesis of ohmline possessing a lactose moiety. B) Structure of other glyco glycero lipids pre...

Figure 67: A) Synthesis of lactose-glycerol ether lipid 67.5; B) analogues possessing a maltose (67.6) or meli...

Figure 68: Synthesis of digalactosyl EL 68.6, A) by using trityl, benzyl and acetyl protecting groups, B) by u...

Figure 69: A) Synthesis of α-ohmline; B) structure of disaccharide ether lipids prepared by using similar meth...

Figure 70: Synthesis of lactose ether lipid 70.3 and its analogue 70.6 featuring a carbamate function as linke...

Figure 71: Synthesis of rhamnopyranoside diether 71.4 [196].

Figure 72: Synthesis of 1-O-hexadecyl-2-O-methyl-3-S-(α-ᴅ-1'-thioglucopyranosyl)-sn-glycerol (72.5) [225].

Figure 73: A) Preparation of lipid intermediate 73.4; B) synthesis of 2-desoxy-C-glycoside 73.10 [226].

Figure 74: Synthesis of galactose-pyridinium salt 74.3 [228].

Figure 75: Synthesis of myo-inositol derivative Ino-C2-PAF (75.10) [230].

Figure 76: A) Synthesis of myo-inositol phosphate building block 76.7; B) synthesis of myo-inositolphosphate d...

Figure 77: A) Synthesis of phosphatidyl-3-desoxy-inositol 77.4; B) synthesis of phosphono-3-desoxyinositol 77.9...

Figure 78: A) Structure of diether phosphatidyl-myo-inositol-3,4-diphosphate 78.1; B) synthesis of phosphatidy...

Figure 79: A) Synthesis of diether-phosphatidyl derivative 79.4 featuring a hydroxymethyl group in place of a ...

Figure 80: Synthesis of Glc-amine-PAF [78].

Figure 81: Synthesis of glucosamine ether lipid 81.4 and its analogues functionalized in position 3 of the ami...

Figure 82: Synthesis of fully deprotected aminoglucoside ether lipid 82.5 [246].

Figure 83: Synthesis of C-aminoglycoside 83.12 using Ramberg–Bäcklund rearrangement as a key step [250].

Figure 84: A) List of the most important glyco lipids and amino glyco lipids included in the study of Arthur a...

Figure 85: Synthesis of mannosamine ether lipid 85.6 [254].

Figure 86: A) Synthesis of glucosamine ether lipids with a non-natural ʟ-glucosamine moiety; B) synthesis of e...

Figure 87: A) Structure of the most efficient anticancer agents 87.1–87.4 featuring a diamino glyco ether lipi...

Figure 88: A) Synthesis of diamino glyco ether lipid 87.4; B) synthesis of bis-glycosylated ether lipid 88.10 [256]....

Figure 89: Synthesis of triamino ether lipid 89.4 [260].

Figure 90: Synthesis of chlorambucil conjugate 90.7 [261].

Figure 91: Three main methods for the preparation of glycerol ether lipid 91.3; A) from solketal and via a tri...

Figure 92: Four different methods for the installation of the phosphocholine polar head group; A) method using...

Figure 93: Illustration of two methods for the installation of saccharides or aminosaccharides; A) O-glycosyla...
Electrochemical formal homocoupling of sec-alcohols
- Kosuke Yamamoto,
- Kazuhisa Arita,
- Masashi Shiota,
- Masami Kuriyama and
- Osamu Onomura
Beilstein J. Org. Chem. 2022, 18, 1062–1069, doi:10.3762/bjoc.18.108
- anode-free electrochemical protocol for the synthesis of pinacol-type vic-1,2-diols from sec-alcohols, namely benzyl alcohol derivatives and ethyl lactate. The corresponding vic-1,2-diols are obtained in moderate to good yields, and good to high levels of stereoselectivity are observed for sec-benzyl
- -valent metal reductants, such as Al, Ti, V, Zn, and Sm (Scheme 1a). Although these protocols have proven to be a reliable strategy to access vic-1,2-diols, producing a large amount of metal waste may be a major drawback especially in a large-scale synthesis. Thus, the improved procedures using a
- ][16][17][18]. In addition to the reductive coupling of carbonyl compounds, oxidative homocoupling reactions of benzyl alcohols under transition metal- or semiconductor-based photoredox catalysis have been demonstrated as attractive approaches to access vic-1,2-diols [19][20][21][22][23

Graphical Abstract

Scheme 1: Strategies for the synthesis of vic-1,2-diols.

Scheme 2: Substrate scope. Reaction conditions: 1 (1.0 mmol), Et4NBr (0.1 equiv), imidazole (0.05 equiv), MeC...

Scheme 3: Investigation of cross-coupling reaction.

Scheme 4: Large-scale experiment.

Scheme 5: Control experiments. aDetermined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. b...

Scheme 6: Proposed mechanism.
BINOL as a chiral element in mechanically interlocked molecules
- Matthias Krajnc and
- Jochen Niemeyer
Beilstein J. Org. Chem. 2022, 18, 508–523, doi:10.3762/bjoc.18.53

- desymmetrization of meso-1,2-diols [58]. The [2]rotaxane (R)-42 was synthesized by interaction of the ammonium salt 41 with the BINOL-based macrocycle (R)-12 and end-capping with 3,5-di-tert-butylbenzoic acid (see Figure 10). In the asymmetric desymmetrization reaction of meso-hydrobenzoin, rotaxane (R)-42 gave
- desymmetrization reaction of meso-1,2-diols with rotaxane (R)-42. Synthesis of Niemeyer´s axially chiral [2]catenane (S,S)-47. Results for the enantioselective transfer hydrogenation of 2-phenylquinoline with catalysts (S,S)-47, (S)-48, and (S)-49. Synthesis of Niemeyer´s chiral [2]rotaxanes (S)-56/57. Results for

Graphical Abstract

Figure 1: Molecular structures of (R)-BINOL (left) and (S)-BINOL (right).

Figure 2: Synthesis of Sauvage´s [2]catenanes (S,S)-5 and (S,S)-6 containing two BINOL units by the passive m...

Figure 3: Synthesis of Saito´s [2]rotaxane (R)-10 from a BINOL-based macrocycle by the active metal template ...

Figure 4: Synthesis of Stoddart´s [2]rotaxane (rac)-14 by an ammonium crown ether template.

Figure 5: Synthesis of Stoddart´s BINOL-containing [2]catenanes 18/20/22/24 by π–π recognition.

Figure 6: Synthesis of Takata´s rotaxanes featuring chiral centers on the axle: a) rotaxane (R,R,R/S)-27 obta...

Figure 7: Takata´s chiral polyacetylenes 32/33 featuring BINOL-based [2]rotaxane side chains.

Figure 8: Synthesis of Takata´s chiral thiazolium [2]rotaxanes (R)-35a/b and (R)-38.

Figure 9: Results for the asymmetric benzoin condensation of benzaldehyde (39) with catalysts (R)-35a/b and (R...

Figure 10: Synthesis of Takata´s pyridine-based [2]rotaxane (R)-42.

Figure 11: The asymmetric desymmetrization reaction of meso-1,2-diols with rotaxane (R)-42.

Figure 12: Synthesis of Niemeyer´s axially chiral [2]catenane (S,S)-47.

Figure 13: Results for the enantioselective transfer hydrogenation of 2-phenylquinoline with catalysts (S,S)-47...

Figure 14: Synthesis of Niemeyer´s chiral [2]rotaxanes (S)-56/57.

Figure 15: Results for the enantioselective Michael addition with different rotaxane catalysts (S)-56a/56b/57a/...

Figure 16: Synthesis of Beer´s [2]rotaxanes 64a/b for anion recognition.

Figure 17: Association constants of different anions (used as the Bu4N+ salts) to the [2]rotaxanes (S)-64a/b a...

Figure 18: Synthesis of Beer´s [3]rotaxane (S)-68.

Figure 19: Association constants of different anions (used as the Bu4N+-salts) to the [2]rotaxane (S)-68 and a...
Double-headed nucleosides: Synthesis and applications
- Vineet Verma,
- Jyotirmoy Maity,
- Vipin K. Maikhuri,
- Ritika Sharma,
- Himal K. Ganguly and
- Ashok K. Prasad
Beilstein J. Org. Chem. 2021, 17, 1392–1439, doi:10.3762/bjoc.17.98

Graphical Abstract

Figure 1: Double-headed nucleosides. B1 and B2 = nucleobases or heterocyclic/carbocyclic moieties; L = linker....

Scheme 1: Synthesis of 2′-(pyrimidin-1-yl)methyl- or 2′-(purin-9-yl)methyl-substituted double-headed nucleosi...

Scheme 2: Synthesis of double-headed nucleoside 7 having two cytosine moieties.

Scheme 3: Synthesis of double-headed nucleoside 2′-deoxy-2′-C-(2-(thymine-1-yl)ethyl)-uridine (11).

Scheme 4: Double-headed nucleosides 14 and 15 obtained by click reaction.

Scheme 5: Synthesis of the double-headed nucleoside 19.

Scheme 6: Synthesis of the double-headed nucleosides 24 and 25.

Scheme 7: Synthesis of double-headed nucleosides 28 and 29.

Scheme 8: Synthesis of double-headed nucleoside 33.

Scheme 9: Synthesis of double-headed nucleoside 37.

Scheme 10: Synthesis of the double-headed nucleoside 1-(5′-O-(4,4′-dimethoxytrityl)-2′-C-((4-(pyren-1-yl)-1,2,...

Scheme 11: Synthesis of triazole-containing double-headed ribonucleosides 46a–c and 50a–e.

Scheme 12: Synthesis of double-headed nucleosides 54a–g.

Scheme 13: Synthesis of double-headed nucleosides 59 and 60.

Scheme 14: Synthesis of the double-headed nucleosides 63 and 64.

Scheme 15: Synthesis of double-headed nucleosides 66a–c.

Scheme 16: Synthesis of benzoxazole-containing double-headed nucleosides 69 and 71 from 5′-amino-5′-deoxynucle...

Scheme 17: Synthesis of 4′-C-((N6-benzoyladenin-9-yl)methyl)thymidine (75) and 4′-C-((thymin-1-yl)methyl)thymi...

Scheme 18: Synthesis of double-headed nucleosides 5′-(adenine-9-yl)-5′-deoxythymidine (79) and 5′-(adenine-9-y...

Scheme 19: Synthesis of double-headed nucleosides 85–87 via reversed nucleosides methodology.

Scheme 20: Double-headed nucleosides 91 and 92 derived from ω-terminal-acetylenic sugar derivatives 90a,b.

Scheme 21: Synthesis of double-headed nucleosides 96a–g.

Scheme 22: Synthesis of double-headed nucleosides 100 and 103.

Scheme 23: Double-headed nucleosides 104 and 105 with a triazole motif.

Scheme 24: Synthesis of the double-headed nucleosides 107 and 108.

Scheme 25: Synthesis of double-headed nucleoside 110 with additional nucleobase in 5′-(S)-C-position joined th...

Scheme 26: Synthesis of double-headed nucleosides 111–113 with additional nucleobases in the 5′-(S)-C-position...

Scheme 27: Synthesis of double-headed nucleoside 114 by click reaction.

Scheme 28: Synthesis of double-headed nucleosides 118 with an additional nucleobase at the 5′-(S)-C-position.

Scheme 29: Synthesis of bicyclic double-headed nucleoside 122.

Scheme 30: Synthesis of double-headed nucleosides 125a–c derived from 2′-amino-LNA.

Scheme 31: Double-headed nucleoside 127 obtained by click reaction.

Scheme 32: Synthesis of double-headed nucleoside 130.

Scheme 33: Double-headed nucleosides 132a–d and 134a–d synthesized by Sonogashira cross coupling reaction.

Scheme 34: Synthesis of double-headed nucleosides 137 and 138 via Suzuki coupling.

Scheme 35: Synthesis of double-headed nucleosides 140 and 141 via Sonogashira cross coupling reaction.

Scheme 36: Synthesis of double-headed nucleoside 143.

Scheme 37: Synthesis of the double-headed nucleoside 146.

Scheme 38: Synthesis of 5-C-alkynyl-functionalized double-headed nucleosides 151a–d.

Scheme 39: Synthesis of 5-C-triazolyl-functionalized double-headed nucleosides 154a, b.

Scheme 40: Synthesis of double-headed nucleosides 157a–c.

Scheme 41: Synthesis of double-headed nucleoside 159, phosphoramidite 160 and the corresponding nucleotide mon...

Scheme 42: Synthesis of double-headed nucleoside 163, phosphoramidite 164 and the corresponding nucleotide mon...

Scheme 43: Synthesis of double-headed nucleoside 167, phosphoramidite 168, and the corresponding nucleotide mo...

Scheme 44: Synthesis of double-headed nucleoside 171, phosphoramidite 172, and the corresponding nucleotide mo...

Scheme 45: Synthesis of double-headed nucleoside 175, phosphoramidite 176, and the corresponding nucleotide mo...

Scheme 46: Synthesis of double-headed nucleoside 178.

Scheme 47: Synthesis of the double-headed nucleosides 181 and 183.

Scheme 48: Alternative synthesis of the double-headed nucleoside 183.

Scheme 49: Synthesis of double-headed nucleoside 188 through thermal [2 + 3] sydnone–alkyne cycloaddition reac...

Scheme 50: Synthesis of the double-headed nucleosides 190 and 191.

Scheme 51: Synthesis of 1-((5S)-2,3,4-tri-O-acetyl-5-(2,6-dichloropurin-9-yl)-β-ᴅ-xylopyranosyl)uracil (195).

Scheme 52: Synthesis of hexopyranosyl double-headed pyrimidine homonucleosides 200a–c.

Figure 2: 3′-C-Ethynyl-β-ᴅ-allopyranonucleoside derivatives 201a–f.

Scheme 53: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 203–207.

Scheme 54: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleosides 208 and 209.

Scheme 55: Synthesis of 3′-C-(1,4-disubstituted-1,2,3-triazolyl)-double-headed pyranonucleoside 210.

Scheme 56: Synthesis of double-headed acyclic nucleosides (2S,3R)-1,4-bis(thymine-1-yl)butane-2,3-diol (213a) ...

Scheme 57: Synthesis of double-headed acyclic nucleosides (2R,3S)-1,4-bis(thymine-1-yl)butane-2,3-diol (213c) ...

Scheme 58: Synthesis of double-headed acetylated 1,3,4-oxadiazino[6,5-b]indolium-substituted C-nucleosides 218b...

Scheme 59: Synthesis of double-headed acyclic nucleoside 222.

Scheme 60: Synthesis of functionalized 1,2-bis(1,2,4-triazol-3-yl)ethane-1,2-diols 223a–f.

Scheme 61: Synthesis of acyclic double-headed 1,2,4-triazino[5,6-b]indole C-nucleosides 226–231.

Scheme 62: Synthesis of double-headed 1,3,4-thiadiazoline, 1,3,4-oxadiazoline, and 1,2,4-triazoline acyclo C-n...

Scheme 63: Synthesis of double-headed acyclo C-nucleosides 240–242.

Scheme 64: Synthesis of double-headed acyclo C-nucleoside 246.

Scheme 65: Synthesis of acyclo double-headed nucleoside 250.

Scheme 66: Synthesis of acyclo double-headed nucleoside 253.

Scheme 67: Synthesis of acyclo double-headed nucleosides 259a–d.

Scheme 68: Synthesis of acyclo double-headed nucleoside 261.
Stereoselective synthesis and transformation of pinane-based 2-amino-1,3-diols
- Ákos Bajtel,
- Mounir Raji,
- Matti Haukka,
- Ferenc Fülöp and
- Zsolt Szakonyi
Beilstein J. Org. Chem. 2021, 17, 983–990, doi:10.3762/bjoc.17.80
- aminohydroxylation process starting from allylic carbamates usually carried out in the presence of potassium osmate [24][25][26][27][28]. In recent years, we have extensively studied the stereoselective synthesis, as well as catalytic and pharmacological applications of monoterpene-based 3-amino-1,2-diols, which are
- -fused oxazolidine 17 was obtained regioselectively (Scheme 5), as it was indicated by clear HMBC correlations between the CH2 of the oxazolidine ring and the anellation carbons, in contrast to the results observed in the case of the regioisomeric 3-amino-1,2-diols, where spiro-oxazolidines formed
- observed in the reaction of aminodiols 13 and 16 with aldehydes (see Scheme 4 and Scheme 5), but it is similar to that observed in our earlier study with pinane-based 3-amino-1,2-diols [41]. During the NMR spectroscopic study of 21A in CDCl3 for 30 days, an unknown slow ring–ring tautomerization was

Graphical Abstract

Figure 1: Biologically active 2-amino-1,3-diols.

Scheme 1: Stereoselective synthesis of the pinane-fused oxazolidin-2-one 9.

Figure 2: NOESY experiments and X-ray structure elucidation of oxazolidin-2-one 9.

Scheme 2: Stereoselective synthesis of the pinane spiro-fused oxazolidin-2-one 12.

Scheme 3: Parallel synthesis of 2-amino-1,3-diols.

Scheme 4: Synthesis of N-benzyl-2-amino-1,3-diol 16.

Scheme 5: Synthesis of 2-amino-1,3-diols.

Figure 3: NOESY experiments and X-ray structure proofment of the structure of oxazolidine 17.

Scheme 6: Synthesis of 2-phenyliminooxazolidines.

Figure 4: Proposed pathway for the ring–ring tautomerism.
Designed whole-cell-catalysis-assisted synthesis of 9,11-secosterols
- Marek Kõllo,
- Marje Kasari,
- Villu Kasari,
- Tõnis Pehk,
- Ivar Järving,
- Margus Lopp,
- Arvi Jõers and
- Tõnis Kanger
Beilstein J. Org. Chem. 2021, 17, 581–588, doi:10.3762/bjoc.17.52
- 1,2-diols. Starting with a compound already possessing a hydroxy group at the position C11, only hydroxylation at C9 is needed. Commercially available corticosteroid cortisol already possesses a hydroxy group at the position C11, and therefore only hydroxylation at C9 is needed, making cortisol (1) an

Graphical Abstract

Figure 1: A) Tetracyclic core of steroids and possible sites of bond cleavages for secosteroids. B)The first ...

Scheme 1: Retrosynthetic analysis of 9,11-secosterols.

Scheme 2: Synthesis of starting materials. Reagents and conditions: i) NaBH4, EtOH/CH2Cl2 1:1, 2 h, rt, then ...

Scheme 3: Oxidation of diols 5 and 6 with NaOCl·5H2O.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
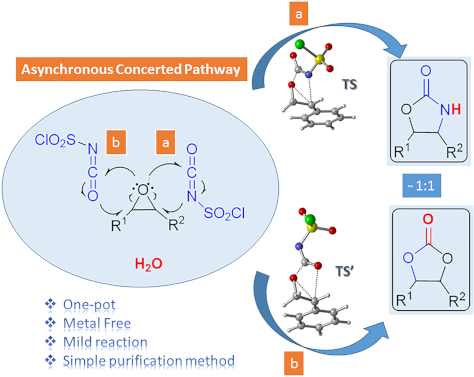
- shorter reaction times under mild conditions using a simple purification method. Apparently, our protocol describes a reasonable methodology for the conversion of epoxides to protected 1,2-diols and 2-amino alcohols. Attention is drawn on these 1,2-oxygen and/or nitrogen units since they are present in
- using a safe, inexpensive, metal-free reagent, a simple purification method and shorter reaction times via a one-pot reaction. The study presents a useful method for one-pot conversion of epoxides to protected 1,2-diols and 2-amino alcohols in one reaction. In the computational part of the study, the

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- energy [54]. The aromatic carbonyl compounds were dissolved in isopropanol and exposed to direct sunlight for 7–10 days to give the corresponding 1,2-diols 92 in high to moderate yield. The excitation of the carbonyl compound 87 was followed by hydrogen atom abstraction from the solvent 89, affording the

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
- Balaram S. Takale,
- Ruchita R. Thakore,
- Elham Etemadi-Davan and
- Bruce H. Lipshutz
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- silyl ethers 89–92 (Scheme 18), thus showcasing the synergistic relationship between Pd and Cu catalysis [43]. Driven by the success of earlier results, the authors utilized 78 for reductive couplings between ketones 93 and imines 97 as electrophiles to form unsymmetrical 1,2-diols 94–96 and 1,2-amino

Graphical Abstract

Scheme 1: Pharmaceuticals possessing a silicon or boron atom.

Scheme 2: The first Cu-catalyzed C(sp3)–Si bond formation.

Scheme 3: Conversion of benzylic phosphate 6 to the corresponding silane.

Scheme 4: Conversion of alkyl triflates to alkylsilanes.

Scheme 5: Conversion of secondary alkyl triflates to alkylsilanes.

Scheme 6: Conversion of alkyl iodides to alkylsilanes.

Scheme 7: Trapping of intermediate radical through cascade reaction.

Scheme 8: Radical pathway for conversion of alkyl iodides to alkylsilanes.

Scheme 9: Conversion of alkyl ester of N-hydroxyphthalimide to alkylsilanes.

Scheme 10: Conversion of gem-dibromides to bis-silylalkanes.

Scheme 11: Conversion of imines to α-silylated amines (A) and the reaction pathway (B).

Scheme 12: Conversion of N-tosylimines to α-silylated amines.

Scheme 13: Screening of diamine ligands.

Scheme 14: Conversion of N-tert-butylsulfonylimines to α-silylated amines.

Scheme 15: Conversion of aldimines to nonracemic α-silylated amines.

Scheme 16: Conversion of N-tosylimines to α-silylated amines.

Scheme 17: Reaction pathway [A] and conversion of aldehydes to α-silylated alcohols [B].

Scheme 18: Conversion of aldehydes to benzhydryl silyl ethers.

Scheme 19: Conversion of ketones to 1,2-diols (A) and conversion of imines to 1,2-amino alcohols (B).

Scheme 20: Ligand screening (A) and conversion of aldehydes to α-silylated alcohols (B).

Scheme 21: Conversion of aldehydes to α-silylated alcohols.

Scheme 22: 1,4-Additions to α,β-unsaturated ketones.

Scheme 23: 1,4-Additions to unsaturated ketones to give β-silylated derivatives.

Scheme 24: Additions onto α,β-unsaturated lactones to give β-silylated lactones.

Scheme 25: Conversion of α,β-unsaturated to β-silylated lactams.

Scheme 26: Conversion of N-arylacrylamides to silylated oxindoles.

Scheme 27: Conversion of α,β-unsaturated carbonyl compounds to silylated tert-butylperoxides.

Scheme 28: Catalytic cycle for Cu(I) catalyzed α,β-unsaturated compounds.

Scheme 29: Conversion of p-quinone methides to benzylic silanes.

Scheme 30: Conversion of α,β-unsaturated ketimines to regio- and stereocontrolled allylic silanes.

Scheme 31: Conversion of α,β-unsaturated ketimines to enantioenriched allylic silanes.

Scheme 32: Regioselective conversion of dienedioates to allylic silanes.

Scheme 33: Conversion of alkenyl-substituted azaarenes to β-silylated adducts.

Scheme 34: Conversion of conjugated benzoxazoles to enantioenriched β-silylated adducts.

Scheme 35: Conversion of α,β-unsaturated carbonyl indoles to α-silylated N-alkylated indoles.

Scheme 36: Conversion of β-amidoacrylates to α-aminosilanes.

Scheme 37: Conversion of α,β-unsaturated ketones to enantioenriched β-silylated ketones, nitriles, and nitro d...

Scheme 38: Regio-divergent silacarboxylation of allenes.

Scheme 39: Silylation of diazocarbonyl compounds, (A) asymmetric and (B) racemic.

Scheme 40: Enantioselective hydrosilylation of alkenes.

Scheme 41: Conversion of 3-acylindoles to indolino-silanes.

Scheme 42: Proposed mechanism for the silylation of 3-acylindoles.

Scheme 43: Silyation of N-chlorosulfonamides.

Scheme 44: Conversion of acyl silanes to α-silyl alcohols.

Scheme 45: Conversion of N-tosylaziridines to β-silylated N-tosylamines.

Scheme 46: Conversion of N-tosylaziridines to silylated N-tosylamines.

Scheme 47: Conversion of 3,3-disubstituted cyclopropenes to silylated cyclopropanes.

Scheme 48: Conversion of conjugated enynes to 1,3-bis(silyl)propenes.

Scheme 49: Proposed sequence for the Cu-catalyzed borylation of substituted alkenes.

Scheme 50: Cu-catalyzed synthesis of nonracemic allylic boronates.

Scheme 51: Cu–NHC catalyzed synthesis of α-substituted allylboronates.

Scheme 52: Synthesis of α-chiral (γ-alkoxyallyl)boronates.

Scheme 53: Cu-mediated formation of nonracemic cis- or trans- 2-substituted cyclopropylboronates.

Scheme 54: Cu-catalyzed synthesis of γ,γ-gem-difluoroallylboronates.

Scheme 55: Cu-catalyzed hydrofunctionalization of internal alkenes and vinylarenes.

Scheme 56: Cu-catalyzed Markovnikov and anti-Markovnikov borylation of alkenes.

Scheme 57: Cu-catalyzed borylation/ortho-cyanation/Cope rearrangement.

Scheme 58: Borylfluoromethylation of alkenes.

Scheme 59: Cu-catalyzed synthesis of tertiary nonracemic alcohols.

Scheme 60: Synthesis of densely functionalized and synthetically versatile 1,2- or 4,3-borocyanated 1,3-butadi...

Scheme 61: Cu-catalyzed trifunctionalization of allenes.

Scheme 62: Cu-catalyzed selective arylborylation of arenes.

Scheme 63: Asymmetric borylative coupling between styrenes and imines.

Scheme 64: Regio-divergent aminoboration of unactivated terminal alkenes.

Scheme 65: Cu-catalyzed 1,4-borylation of α,β-unsaturated ketones.

Scheme 66: Cu-catalyzed protodeboronation of α,β-unsaturated ketones.

Scheme 67: Cu-catalyzed β-borylation of α,β-unsaturated imines.

Scheme 68: Cu-catalyzed synthesis of β-trifluoroborato carbonyl compounds.

Scheme 69: Asymmetric 1,4-borylation of α,β-unsaturated carbonyl compounds.

Scheme 70: Cu-catalyzed ACB and ACA reactions of α,β-unsaturated 2-acyl-N-methylimidazoles.

Scheme 71: Cu-catalyzed diborylation of aldehydes.

Scheme 72: Umpolung pathway for chiral, nonracemic tertiary alcohol synthesis (top) and proposed mechanism for...

Scheme 73: Cu-catalyzed synthesis of α-hydroxyboronates.

Scheme 74: Cu-catalyzed borylation of ketones.

Scheme 75: Cu-catalyzed borylation of unactivated alkyl halides.

Scheme 76: Cu-catalyzed borylation of allylic difluorides.

Scheme 77: Cu-catalyzed borylation of cyclic and acyclic alkyl halides.

Scheme 78: Cu-catalyzed borylation of unactivated alkyl chlorides and bromides.

Scheme 79: Cu-catalyzed decarboxylative borylation of carboxylic acids.

Scheme 80: Cu-catalyzed borylation of benzylic, allylic, and propargylic alcohols.
Recent advances in photocatalyzed reactions using well-defined copper(I) complexes
- Mingbing Zhong,
- Xavier Pannecoucke,
- Philippe Jubault and
- Thomas Poisson
Beilstein J. Org. Chem. 2020, 16, 451–481, doi:10.3762/bjoc.16.42
- reaction. In the presence of 2 mol % of the catalyst, the Hantzsch ester (HEH), as a hydrogen atom donor, under blue light irradiation, a large panel of ketones and aldehydes was readily converted into the corresponding 1,2-diols in moderate to excellent yields. The functional group tolerance of the

Graphical Abstract

Scheme 1: [Cu(I)(dap)2]Cl-catalyzed ATRA reaction under green light irradiation.

Scheme 2: Photocatalytic allylation of α-haloketones.

Scheme 3: [Cu(I)(dap)2]Cl-photocatalyzed chlorosulfonylation and chlorotrifluoromethylation of alkenes.

Scheme 4: Photocatalytic perfluoroalkylchlorination of electron-deficient alkenes using the Sauvage catalyst.

Scheme 5: Photocatalytic synthesis of fluorinated sultones.

Scheme 6: Photocatalyzed haloperfluoroalkylation of alkenes and alkynes.

Scheme 7: Chlorosulfonylation of alkenes catalyzed by [Cu(I)(dap)2]Cl. aNo Na2CO3 was added. b1 equiv of Na2CO...

Scheme 8: Copper-photocatalyzed reductive allylation of diaryliodonium salts.

Scheme 9: Copper-photocatalyzed azidomethoxylation of olefins.

Scheme 10: Benzylic azidation initiated by [Cu(I)(dap)2]Cl.

Scheme 11: Trifluoromethyl methoxylation of styryl derivatives using [Cu(I)(dap)2]PF6. All redox potentials ar...

Scheme 12: Trifluoromethylation of silyl enol ethers.

Scheme 13: Synthesis of annulated heterocycles upon oxidation with the Sauvage catalyst.

Scheme 14: Oxoazidation of styrene derivatives using [Cu(dap)2]Cl as a precatalyst.

Scheme 15: [Cu(I)(dpp)(binc)]PF6-catalyzed ATRA reaction.

Scheme 16: Allylation reaction of α-bromomalonate catalyzed by [Cu(I)(dpp)(binc)]PF6 following an ATRA mechani...

Scheme 17: Bromo/tribromomethylation reaction using [Cu(I)(dmp)(BINAP)]PF6.

Scheme 18: Chlorotrifluoromethylation of alkenes catalyzed by [Cu(I)(N^N)(xantphos)]PF6.

Scheme 19: Chlorosulfonylation of styrene and alkyne derivatives by ATRA reactions.

Scheme 20: Reduction of aryl and alkyl halides with the complex [Cu(I)(bcp)(DPEPhos)]PF6. aIrradiation was car...

Scheme 21: Meerwein arylation of electron-rich aromatic derivatives and 5-exo-trig cyclization catalyzed by th...

Scheme 22: [Cu(I)(bcp)(DPEPhos)]PF6-photocatalyzed synthesis of alkaloids. aYield over two steps (cyclization ...

Scheme 23: Copper-photocatalyzed decarboxylative amination of NHP esters.

Scheme 24: Photocatalytic decarboxylative alkynylation using [Cu(I)(dq)(binap)]BF4.

Scheme 25: Copper-photocatalyzed alkylation of glycine esters.

Scheme 26: Copper-photocatalyzed borylation of organic halides. aUnder continuous flow conditions.

Scheme 27: Copper-photocatalyzed α-functionalization of alcohols with glycine ester derivatives.

Scheme 28: δ-Functionalization of alcohols using [Cu(I)(dmp)(xantphos)]BF4.

Scheme 29: Photocatalytic synthesis of [5]helicene and phenanthrene.

Scheme 30: Oxidative carbazole synthesis using in situ-formed [Cu(I)(dmp)(xantphos)]BF4.

Scheme 31: Copper-photocatalyzed functionalization of N-aryl tetrahydroisoquinolines.

Scheme 32: Bicyclic lactone synthesis using a copper-photocatalyzed PCET reaction.

Scheme 33: Photocatalytic Pinacol coupling reaction catalyzed by [Cu(I)(pypzs)(BINAP)]BF4. The ligands of the ...

Scheme 34: Azide photosensitization using a Cu-based photocatalyst.
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
- Munmun Ghosh,
- Valmik S. Shinde and
- Magnus Rueping
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- electrochemical asymmetric oxidation of 1,2-diols 74 and amino alcohols 77 in presence of a Br- mediator using Cu(OTf)2 and (R,R)-Ph-BOX 76 as the catalytic system (Scheme 29). The method enabled an asymmetric synthesis of α-hydroxycycloalkanones 75 and α-amino esters 78 along with the kinetic resolution of

Graphical Abstract

Figure 1: General classification of asymmetric electroorganic reactions.

Scheme 1: Asymmetric reduction of 4-acetylpyridine using a modified graphite cathode.

Scheme 2: Asymmetric hydrogenation of ketones using Raney nickel powder electrodes modified with optically ac...

Scheme 3: Asymmetric reduction of prochiral activated olefins with a poly-ʟ-valine-coated graphite cathode.

Scheme 4: Asymmetric reduction of prochiral carbonyl compounds, oximes and gem-dibromides on a poly-ʟ-valine-...

Scheme 5: Asymmetric hydrogenation of prochiral ketones with poly[RuIII(L)2Cl2]+-modified carbon felt cathode...

Scheme 6: Asymmetric hydrogenation of α-keto esters using chiral polypyrrole film-coated cathode incorporated...

Scheme 7: Quinidine and cinchonidine alkaloid-induced asymmetric electroreduction of acetophenone.

Scheme 8: Asymmetric electroreduction of 4- and 2-acetylpyridines at a mercury cathode in the presence of a c...

Scheme 9: Enantioselective reduction of 4-methylcoumarin in the presence of catalytic yohimbine.

Scheme 10: Cinchonine-induced asymmetric electrocarboxylation of 4-methylpropiophenone.

Scheme 11: Enantioselective hydrogenation of methyl benzoylformate using an alkaloid entrapped silver cathode.

Scheme 12: Alkaloid-induced enantioselective hydrogenation using a Cu nanoparticle cathode.

Scheme 13: Alkaloid-induced enantioselective hydrogenation of aromatic ketones using a bimetallic Pt@Cu cathod...

Scheme 14: Enantioselective reduction of ketones at mercury cathode using N,N'-dimethylquininium tetrafluorobo...

Scheme 15: Asymmetric synthesis of an amino acid using an electrode modified with amino acid oxidase and elect...

Scheme 16: Asymmetric oxidation of p-tolyl methyl sulfide using chemically modified graphite anode.

Scheme 17: Asymmetric oxidation of unsymmetric sulfides using poly(amino acid)-coated electrodes.

Scheme 18: Enantioselective, electocatalytic oxidative coupling on TEMPO-modified graphite felt electrode in t...

Scheme 19: Asymmetric electrocatalytic oxidation of racemic alcohols on a TEMPO-modified graphite felt electro...

Scheme 20: Asymmetric electrocatalytic lactonization of diols on TEMPO-modified graphite felt electrodes.

Scheme 21: Asymmetric electrochemical pinacolization in a chiral solvent.

Scheme 22: Asymmetric electroreduction using a chiral supporting electrolyte.

Scheme 23: Asymmetric anodic oxidation of enol acetates using chiral supporting electrolytes.

Scheme 24: Kinetic resolution of primary amines using a chiral N-oxyl radical mediator.

Scheme 25: Chiral N-oxyl-radical-mediated kinetic resolution of secondary alcohols via electrochemical oxidati...

Scheme 26: Chiral iodoarene-mediated asymmetric electrochemical lactonization.

Scheme 27: Os-catalyzed electrochemical asymmetric dihydroxylation of olefins using the Sharpless ligand and i...

Scheme 28: Asymmetric electrochemical epoxidation of olefins catalyzed by a chiral Mn-salen complex.

Scheme 29: Asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.

Scheme 30: Mechanism of asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper cat...

Scheme 31: Enantioselective electrocarboxylation catalyzed by an electrogenerated chiral [CoI(salen)]− complex....

Scheme 32: Asymmetric oxidative cross coupling of 2-acylimidazoles with silyl enol ethers.

Scheme 33: Ni-catalyzed asymmetric electroreductive cleavage of allylic β-keto ester 89.

Scheme 34: Asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.

Scheme 35: Mechanism of asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst....

Scheme 36: Asymmetric epoxidation by electrogenerated percarbonate and persulfate ions in the presence of chir...

Scheme 37: α-Oxyamination of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 38: The α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 39: Mechanism of α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 40: Electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.

Scheme 41: Mechanism of electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehyd...

Scheme 42: Asymmetric cross-dehydrogenative coupling of tertiary amines with simple ketones via an electrochem...

Scheme 43: Electroenzymatic asymmetric reduction using enoate reductase.

Scheme 44: Assymetric reduction using alcohol dehydrogenase as the electrocatalyst.

Scheme 45: Asymmetric electroreduction catalyzed by thermophilic NAD-dependent alcohol dehydrogenase.

Scheme 46: Asymmetric epoxidation of styrene by electrochemical regeneration of flavin-dependent monooxygenase....

Scheme 47: Asymmetric electroreduction using a chloroperoxidase catalyst.

Scheme 48: Asymmetric electrochemical transformation mediated by hydrophobic vitamin B12.

Scheme 49: Diastereoselective cathodic reduction of phenylglyoxalic acids substituted with amines as chiral au...

Scheme 50: Ni-catalyzed asymmetric electroreductive cross coupling of aryl halides with α-chloropropanoic acid...

Scheme 51: Electrochemical Mannich addition of silyloxyfuran to in situ-generated N-acyliminium ions.

Scheme 52: Stereoselective electroreductive homodimerization of cinnamates attached to a camphor-derived chira...

Scheme 53: Diastereoselective electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives.

Scheme 54: Electrocatalytic stereoselective conjugate addition of chiral β-dicarbonyl compounds to methyl viny...

Scheme 55: Stereoselective electrochemical carboxylation of chiral cinnamic acid derivatives under a CO2 atmos...

Scheme 56: Electrochemical diastereoselective α-alkylation of pyrrolidines attached with phosphorus-derived ch...

Scheme 57: Electrogenerated cyanomethyl anion-induced synthesis of chiral cis-β-lactams from amides bearing ch...

Scheme 58: Diastereoselective anodic oxidation followed by intramolecular cyclization of ω-hydroxyl amides bea...

Scheme 59: Electrochemical deprotonation of Ni(II) glycinate containing (S)-BPB as a chiral auxiliary: diaster...

Scheme 60: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compound...

Scheme 61: Asymmetric total synthesis of ropivacaine and its analogues using a electroorganic reaction as a ke...

Scheme 62: Asymmetric total synthesis of (−)-crispine A and its natural enantiomer via anodic cyanation of tet...

Scheme 63: Asymmetric oxidative electrodimerization of cinnamic acid derivatives as key step for the synthesis...
α-Photooxygenation of chiral aldehydes with singlet oxygen
- Dominika J. Walaszek,
- Magdalena Jawiczuk,
- Jakub Durka,
- Olga Drapała and
- Dorota Gryko
Beilstein J. Org. Chem. 2019, 15, 2076–2084, doi:10.3762/bjoc.15.205
- University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland 10.3762/bjoc.15.205 Abstract Organocatalytic α-oxygenation of chiral aldehydes with photochemically generated singlet oxygen allows synthesizing chiral 3-substituted 1,2-diols. Stereochemical results indicate that the reaction in the presence
- of diarylprolinol silyl ethers is highly diastereoselective and that the configuration of a newly created stereocenter at the α-position depends predominantly on the catalyst structure. The absolute configuration of chiral 1,2-diols has been unambiguously established based on electronic circular
- dichroism (ECD) and TD-DFT methods. Keywords: 1,2-diols; ECD; enamines; organocatalysis; porphyrins; silyl ethers of diarylprolinols; singlet oxygen; Introduction Carbonyl compounds are one of the most important building blocks in organic synthesis. As a consequence, there is a constant need for new

Graphical Abstract

Scheme 1: Asymmetric α-photooxygenation of chiral aldehydes.

Scheme 2: α-Photooxygenation of β-substituted aldehydes.

Scheme 3: Synthesis and α-photooxygenation of 3,4-diphenylbutanal (1).

Scheme 4: Stereoselective α-photooxygenation of 3,4-diphenylbutanal (1) with 1O2.

Scheme 5: Schematic representation of the in situ methodology and preferred conformation of diols with Mo2 co...

Figure 1: ECD spectra of diols syn-6 and anti’-6 recorded a) with 19 in DMSO and b) in acetonitrile compared ...

Scheme 6: Asymmetric synthesis of 3,4-diphenylbutane-1,2-diol.
Systematic synthetic study of four diastereomerically distinct limonene-1,2-diols and their corresponding cyclic carbonates
- Hiroshi Morikawa,
- Jun-ichi Yamaguchi,
- Shun-ichi Sugimura,
- Masato Minamoto,
- Yuuta Gorou,
- Hisatoyo Morinaga and
- Suguru Motokucho
Beilstein J. Org. Chem. 2019, 15, 130–136, doi:10.3762/bjoc.15.13
- significance, largely because LM derivatives with their versatile functionality can be effectively utilised in organic and polymer chemistry. (4R)-Limonene-1,2-diols (LMdiols) are some of the most important LM derivatives because they act as precursors of bioactive molecules [12][13][14]. Furthermore, LMdiol

Graphical Abstract

Figure 1: Diastereomers of LM5CCs, 1a–d.

Figure 2: Diastereomers of LMdiols, 2a–d.

Scheme 1: Dihydroxylation of (a) (R)-limonene and (b) (R)-dihydrolimonene.

Scheme 2: Reduction of LM5CC (1a) and carbonation of LMdiol (2a).

Scheme 3: Overall synthetic routes to LM5CCs and LMdiols. The 93% value (standing for *) from 1d to 2d was ci...

Scheme 4: Plausible conformations of the intermediate in the reaction of LMdiol (2b) with triphosgene.

Figure 3: 1H NMR spectra of LM5CCs 1a–d in CDCl3.

Figure 4: 1H NMR spectra of LMdiols 2a–d in CDCl3.

Figure 5: Plausible conformations of LM5CCs and LMdiols in CDCl3.
Synthesis of eunicellane-type bicycles embedding a 1,3-cyclohexadiene moiety
- Alex Frichert,
- Peter G. Jones and
- Thomas Lindel
Beilstein J. Org. Chem. 2018, 14, 2461–2467, doi:10.3762/bjoc.14.222
- two conformers in CDCl3. Thus, it was to be explored how open-ring cyclohexadiene precursors would be synthesized and behave under McMurry conditions, and how stable the resulting [8.4.0]bicycles would be. Normally, McMurry conditions lead to the formation of alkenes, but medium-sized ring 1,2-diols

Graphical Abstract

Figure 1: Bicyclic eunicellane-type diterpenes.

Figure 2: Synthetic eunicellane-type compounds with benzene partial structure.

Scheme 1: Access to ketoester 14 that did not cyclize to the ethyl vinyl ether under McMurry conditions.

Scheme 2: Synthesis of the 1,3-cyclohexadiene-containing eunicellane-type [8.4.0]bicycle 18 by McMurry coupli...

Figure 3: Preferred conformations of diastereomeric diols 18 and 19 including decisive NOESY correlations.

Scheme 3: Assembly of the envisaged cyclization precursor 27.

Scheme 4: Structure analysis of diastereomeric cyanohydrins 29 and 30.

Scheme 5: Formation of allenes 32 and 34 from sterically crowded propargylic alcohol 31.
Electrochemical Corey–Winter reaction. Reduction of thiocarbonates in aqueous methanol media and application to the synthesis of a naturally occurring α-pyrone
- Ernesto Emmanuel López-López,
- José Alvano Pérez-Bautista,
- Fernando Sartillo-Piscil and
- Bernardo A. Frontana-Uribe
Beilstein J. Org. Chem. 2018, 14, 547–552, doi:10.3762/bjoc.14.41
- ; thiocarbonates; Findings The Corey–Winter reaction (also known as the Corey–Winter reductive olefination) is a chemical transformation that permits the conversion of 1,2-diols A into E-alkenes C via the formation and reduction of a cyclic thiocarbonate intermediate B (Scheme 1) [1][2]. In general this reaction
- convert thiocarbonates derived from 1,2-diols containing the 6-pentyl-2H-pyran-2-one framework to trans-alkenes by means of electrochemical reduction in an H-type separated cell was developed. The thiocarbonate functional group can be reduced using a vitreous carbon electrode in MeOH/H2O 80:20 with AcOH

Graphical Abstract

Scheme 1: The Corey–Winter reaction in general.

Scheme 2: Proposed route for the synthesis of metabolites isolated from Trichoderma and Penicillium species f...

Scheme 3: Preparation of thiocarbonate precursor 6 from pyrone dioxolane 7.

Figure 1: Cyclic voltammetry of thiocarbonates 4 (left) and 6 (right); c = 1 × 10−3 M, N2 bubbling 5 min, WE ...

Scheme 4: Putative reaction mechanism of the electrochemical Corey–Winter reaction.
Remarkable functions of sn-3 hydroxy and phosphocholine groups in 1,2-diacyl-sn-glycerolipids to induce clockwise (+)-helicity around the 1,2-diacyl moiety: Evidence from conformation analysis by 1H NMR spectroscopy
- Yoshihiro Nishida,
- Mengfei Yuan,
- Kazuo Fukuda,
- Kaito Fujisawa,
- Hirofumi Dohi and
- Hirotaka Uzawa
Beilstein J. Org. Chem. 2017, 13, 1999–2009, doi:10.3762/bjoc.13.196

- Equation 2 [18]. From the vicinal coupling constants (3J Hz) of H1proR and H1proS signals, the fractional populations (%) of the three staggered conformers are calculated. Equation 1 is a standard equation, in which the three staggered conformers have the dihedral angles of ± 60° or 180° around 1,2-diols

Graphical Abstract

Figure 1: (a) Structures of cell-membrane glycerophospholpids with a common asymmetric 1,2-diacyl-sn-glycerol...

Scheme 1: The four 1,2-dipalmitoyl-sn-glycerols 1–4 examined in this study.

Figure 2: 1H NMR spectra of 1,2-dipalmitin (3) in CDCl3 after partial isomerization into the 1,3-isomer. (a) ...

Figure 3: Fractional populations (%) of the three staggered conformers around the sn-1,2 C–C single bond in 1...

Scheme 2: Structures of glycerophospholipids with a common structural skeleton of 1,2-dipalmitoyl-sn-glycerol...

Figure 4: Partial 1H NMR spectrum of 4 in a mixture of CDCl3 and methanol-d4 (C/M = 10:1, v/v).

Figure 5: Linear relation between the helical disparity (%) and gt(+) population (%) as observed for the heli...

Figure 6: An empirical diagram showing helical conformational properties around 1,2-diacyl moiety in asymmetr...

Scheme 3: Chirally 2H-labelled tripalmitins (1S)- and (1R)-1-[2H]-1 [23].
Ionic liquids as transesterification catalysts: applications for the synthesis of linear and cyclic organic carbonates
- Maurizio Selva,
- Alvise Perosa,
- Sandro Guidi and
- Lisa Cattelan
Beilstein J. Org. Chem. 2016, 12, 1911–1924, doi:10.3762/bjoc.12.181
- transesterification activity of [P8881][CH3OCO2]-based ionic liquids was also tested on bio-based diols possessing primary and secondary hydroxy groups. Although a number of different products is expectable, the organocatalysts allowed highly selective reactions. For example, 1,2-diols afforded exclusively the

Graphical Abstract

Scheme 1: The transesterification of diethyl oxalate (DEO) with phenol catalyzed by MoO3/SiO2.

Scheme 2: Transesterification of a triglyceride (TG) with DMC for biodiesel production using KOH as the base ...

Scheme 3: Top: Green methylation of phosphines and amines by dimethyl carbonate (Q = N, P). Bottom: anion met...

Figure 1: Structures of some representative SILs and PILs systems. MCF is a silica-based mesostructured mater...

Scheme 4: Synthesis of the acid polymeric IL. EGDMA: ethylene glycol dimethacrylate.

Scheme 5: The transesterification of sec-butyl acetate with MeOH catalyzed by some acidic imidazolium ILs.

Figure 2: Representative examples of ionic liquids for biodiesel production.

Scheme 6: Top: phosgenation of methanol; middle: EniChem and Ube processes; bottom: Asahi process for the pro...

Scheme 7: The transesterification in the synthesis of organic carbonates.

Scheme 8: The transesterification of DMC with alcohols and diols.

Scheme 9: Transesterification of glycerol with DMC in the presence of 1-n-butyl-3-methylimidazolium-2-carboxy...

Scheme 10: Synthesis of the BMIM-2-CO2 catalyst from butylimidazole and DMC.

Scheme 11: Plausible cooperative (nucleophilic–electrophilic) mechanism for the transesterification of glycero...

Scheme 12: Synthesis of diazabicyclo[5.4.0]undec-7-ene-based ionic liquids.

Scheme 13: Synthesis of the DABCO–DMC ionic liquid.

Scheme 14: Cooperative mechanism of ionic liquid-catalyzed glycidol production.

Scheme 15: [TMA][OH]-catalyzed synthesis of glycidol (GD) from glycerol and dimethyl carbonate [46].

Scheme 16: [BMIM]OH-catalyzed synthesis of DPC from DMC and 1-pentanol.

Figure 3: Representative examples of ionic liquids for biodiesel production.

Figure 4: Acyclic non-symmetrical organic carbonates synthetized with 1-(trimethoxysilyl)propyl-3-methylimida...

Scheme 17: A simplified reaction mechanism for DMC production.

Scheme 18: [P8881][MeOCO2] metathesis with acetic acid and phenol.

Figure 5: Examples of carbonates obtained through transesterification using phosphonium salts as catalysts.

Scheme 19: Examples of carbonates obtained from different bio-based diols using [P8881][CH3OCO2] as catalyst.

Scheme 20: Ambiphilic catalysis for transesterification reactions in the presence of carbonate phosphonium sal...
Selected synthetic strategies to cyclophanes
- Sambasivarao Kotha,
- Mukesh E. Shirbhate and
- Gopalkrushna T. Waghule
Beilstein J. Org. Chem. 2015, 11, 1274–1331, doi:10.3762/bjoc.11.142

- , dibromide 83 was converted to bis-aldehyde 84. Finally, McMurry coupling of dialdehyde 84 provided the cyclophane derivative 85 (28%). Yamoto and co-workers have reported the synthesis of medium-sized cyclophanes, [2.n]metacyclophane-1,2-diols 86 and 87 by using the McMurry coupling as a key step (Figure 8

Graphical Abstract

Figure 1: General representation of cyclophanes.

Figure 2: cyclophanes one or more with heteroatom.

Figure 3: Metathesis catalysts 12–17 and C–C coupling catalyst 18.

Figure 4: Natural products containing the cyclophane skeleton.

Figure 5: Turriane family of natural products.

Scheme 1: Synthesis of [3]ferrocenophanes through Mannich reaction. Reagents and conditions: (i) excess HNMe2...

Scheme 2: Synthesis of cyclophanes through Michael addition. Reagents and conditions: (i) xylylene dibromide,...

Scheme 3: Synthesis of normuscopyridine analogue 37 through an oxymercuration–oxidation strategy. Reagents an...

Scheme 4: Synthesis of tribenzocyclotriyne 39 through Castro–Stephens coupling reaction. Reagents and conditi...

Scheme 5: Synthesis of cyclophane 43 through Glaser–Eglinton coupling. Reagents and conditions: (i) 9,10-bis(...

Scheme 6: Synthesis of the macrocyclic C-glycosyl cyclophane through Glaser coupling. Reagents and conditions...

Scheme 7: Synthesis of cyclophane-containing complex 49 through Glaser–Eglinton coupling reaction. Reagents a...

Scheme 8: Synthesis of cyclophane 53 through Glaser–Eglinton coupling. Reagents and conditions: (i) K2CO3, ac...

Figure 6: Cyclophanes 54–56 that have been synthesized through Glaser–Eglinton coupling.

Figure 7: Synthesis of tetrasubstituted [2.2]paracyclophane 57 and chiral cyclophyne 58 through Eglinton coup...

Scheme 9: Synthesis of cyclophane through Glaser–Hay coupling reaction. Reagents and conditions: (i) CuCl2 (1...

Scheme 10: Synthesis of seco-C/D ring analogs of ergot alkaloids through intramolecular Heck reaction. Reagent...

Scheme 11: Synthesis of muscopyridine 73 via Kumada coupling. Reagents and conditions: (i) 72, THF, ether, 20 ...

Scheme 12: Synthesis of the cyclophane 79 via McMurry coupling. Reagents and conditions: (i) 75, decaline, ref...

Scheme 13: Synthesis of stilbenophane 81 via McMurry coupling. Reagents and conditions: (i) TiCl4, Zn, pyridin...

Scheme 14: Synthesis of stilbenophane 85 via McMurry coupling. Reagents and conditions: (i) NBS (2 equiv), ben...

Figure 8: List of cyclophanes prepared via McMurry coupling reaction as a key step.

Scheme 15: Synthesis of paracyclophane by cross coupling involving Pd(0) catalyst. Reagents and conditions: (i...

Scheme 16: Synthesis of the cyclophane 112 via the pinacol coupling and 113 by RCM. Reagents and conditions: (...

Scheme 17: Synthesis of cyclophane derivatives 122a–c via Sonogoshira coupling. Reagents and conditions: (i) C...

Scheme 18: Synthesis of cyclophane 130 via Suzuki–Miyaura reaction as a key step. Reagents and conditions: (i)...

Scheme 19: Synthesis of the mycocyclosin via Suzuki–Miyaura cross coupling. Reagents and conditions: (i) benzy...

Scheme 20: Synthesis of cyclophanes via Wurtz coupling reaction Reagents and conditions: (i) PhLi, Et2O, C6H6,...

Scheme 21: Synthesis of non-natural glycophanes using alkyne metathesis. Reagents and conditions: (i) G-I (12)...

Figure 9: Synthesis of cyclophanes via ring-closing alkyne metathesis.

Scheme 22: Synthesis of crownophanes by cross-enyne metathesis. Reagents and conditions: (i) G-II (13), 5 mol ...

Scheme 23: Synthesis of (−)-cylindrocyclophanes A (156) and (−)-cylindrocyclophanes F (155). Reagents and cond...

Scheme 24: Synthesis of cyclophane 159 derivatives via SM cross-coupling and RCM. Reagents and conditions: (i)...

Scheme 25: Sexithiophene synthesis via cross metathesis. Reagents and conditions: (i) 161, Pd(PPh3)4, K2CO3, T...

Scheme 26: Synthesis of pyrrole-based cyclophane using enyne metathesis. Reagents and conditions: (i) Se, chlo...

Scheme 27: Synthesis of macrocyclic derivatives by RCM. Reagents and conditions: (i) G-I/G-II, CH2Cl2, 0.005 M...

Scheme 28: Synthesis of enantiopure β-lactam-based dienyl bis(dihydrofuran) 179. Reagents and conditions: (i) ...

Scheme 29: Synthesis of a [1.1.6]metaparacyclophane derivative 183 via SM cross coupling. Reagents and conditi...

Scheme 30: Synthesis of a [1.1.6]metaparacyclophane derivative 190 via SM cross coupling. Reagents and conditi...

Scheme 31: Template-promoted synthesis of cyclophanes involving RCM. Reagents and conditions: (i) acenaphthene...

Scheme 32: Synthesis of [3.4]cyclophane derivatives 200 via SM cross coupling and RCM. Reagents and conditions...

Figure 10: Examples for cyclophanes synthesized by RCM.

Scheme 33: Synthesis of the longithorone C framework assisted by fluorinated auxiliaries. Reagents and conditi...

Scheme 34: Synthesis of the longithorone framework via RCM. Reagents and conditions: (i) 213, NaH, THF, rt, 10...

Scheme 35: Synthesis of floresolide B via RCM as a key step. Reagents and conditions: (i) G-II (13, 0.1 equiv)...

Scheme 36: Synthesis of normuscopyridine (223) by the RCM strategy. Reagents and condition: (i) Mg, THF, hexen...

Scheme 37: Synthesis of muscopyridine (73) via RCM. Reagents and conditions: (i) 225, NaH, THF, 0 °C to rt, 1....

Scheme 38: Synthesis of muscopyridine (73) via RCM strategy. Reagents and conditions: (i) NaH, n-BuLi, 5-bromo...

Scheme 39: Synthesis of pyridinophane derivatives 223 and 245. Reagents and conditions: (i) PhSO2Na, TBAB, CH3...

Scheme 40: Synthesis of metacyclophane derivatives 251 and 253. Reagents and conditions: (i) 240, NaH, THF, rt...

Scheme 41: Synthesis of normuscopyridine and its higher analogues. Reagents and conditions: (i) alkenyl bromid...

Scheme 42: Synthesis of fluorinated ferrocenophane 263 via a [2 + 2] cycloaddition. Reagents and conditions: (...

Scheme 43: Synthesis of [2.n]metacyclophanes 270 via a [2 + 2] cycloaddition. Reagents and conditions: (i) Ac2...

Scheme 44: Synthesis of metacyclophane 273 by a [2 + 2 + 2] co-trimerization. Reagents and conditions: (i) [Rh...

Scheme 45: Synthesis of paracyclophane 276 via a [2 + 2 + 2] cycloaddition reaction. Reagents and conditions: ...

Scheme 46: Synthesis of cyclophane 278 via a [2 + 2 + 2] cycloaddition reaction. Reagents and conditions: (i) ...

Scheme 47: Synthesis of cyclophane 280 via a [2 + 2 + 2] cycloaddition. Reagents and conditions: (i) [(Rh(cod)(...

Scheme 48: Synthesis of taxane framework by a [2 + 2 + 2] cycloaddition. Reagents and conditions: (i) Cp(CO)2 ...

Scheme 49: Synthesis of cyclophane 284 and 285 via a [2 + 2 + 2] cycloaddition reaction. Reagents and conditio...

Scheme 50: Synthesis of pyridinophanes 293a,b and 294a,b via a [2 + 2 + 2] cycloaddition. Reagents and conditi...

Scheme 51: Synthesis of pyridinophanes 296 and 297 via a [2 + 2 + 2] cycloaddition. Reagents and conditions: (...

Scheme 52: Synthesis of triazolophane by a 1,3-dipolar cycloaddition. Reagents and conditions: (i) propargyl b...

Scheme 53: Synthesis of glycotriazolophane 309 by a click reaction. Reagents and conditions: (i) LiOH, H2O, Me...

Figure 11: Cyclophanes 310 and 311 prepared via click chemistry.

Scheme 54: Synthesis of cyclophane via the Dötz benzannulation. Reagents and conditions: (i) THF, 100 °C, 12 h...

Scheme 55: Synthesis of [6,6]metacyclophane by a Dötz benzannulation. Reagents and conditions: (i) THF, 100 °C...

Scheme 56: Synthesis of cyclophanes by a Dötz benzannulation. Reagents and conditions: (i) THF, 65 °C, 3 h; (i...

Scheme 57: Synthesis of muscopyridine (73) via an intramolecular DA reaction of ketene. Reagents and condition...

Scheme 58: Synthesis of bis[10]paracyclophane 336 via Diels–Alder reaction. Reagents and conditions: (i) DMAD,...

Scheme 59: Synthesis of [8]paracyclophane via DA reaction. Reagents and conditions: (i) maleic anhydride, 3–5 ...

Scheme 60: Biomimetic synthesis of (−)-longithorone A. Reagents and conditions: (i) Me2AlCl, CH2Cl2, −20 °C, 7...

Scheme 61: Synthesis of sporolide B (349) via a [4 + 2] cycloaddition reaction. Reagents and conditions: (i) P...

Scheme 62: Synthesis of the framework of (+)-cavicularin (352) via a [4 + 2] cycloaddition. Reagents and condi...

Scheme 63: Synthesis of oxazole-containing cyclophane 354 via Beckmann rearrangement. Reagents and conditions:...

Scheme 64: Synthesis of cyclophanes 360a–c via benzidine rearrangement. Reagents and conditions: (i) 356a–d, K2...

Scheme 65: Synthesis of cyclophanes 365a–c via benzidine rearrangement. Reagents and conditions: (i) BocNHNH2,...

Scheme 66: Synthesis of metacyclophane 367 via Ciamician–Dennstedt rearrangement. Reagents and conditions: (i)...

Scheme 67: Synthesis of cyclophane by tandem Claisen rearrangement and RCM as key steps. Reagents and conditio...

Scheme 68: Synthesis of cyclophane derivative 380. Reagents and conditions: (i) K2CO3, CH3CN, allyl bromide, r...

Scheme 69: Synthesis of metacyclophane via Cope rearrangement. Reagents and conditions: (i) MeOH, NaBH4, rt, 1...

Scheme 70: Synthesis of cyclopropanophane via Favorskii rearrangement. Reagents and conditions: (i) Br2, CH2Cl2...

Scheme 71: Cyclophane 389 synthesis via photo-Fries rearrangement. Reagents and conditions: (i) DMAP, EDCl/CHCl...

Scheme 72: Synthesis of normuscopyridine (223) via Schmidt rearrangement. Reagents and conditions: (i) ethyl s...

Scheme 73: Synthesis of crownophanes by tandem Claisen rearrangement. Reagents and conditions: (i) diamine, Et3...

Scheme 74: Attempted synthesis of cyclophanes via tandem Claisen rearrangement and RCM. Reagents and condition...

Scheme 75: Synthesis of muscopyridine via alkylation with 2,6-dimethylpyridine anion. Reagents and conditions:...

Scheme 76: Synthesis of cyclophane via Friedel–Craft acylation. Reagents and conditions: (i) CS2, AlCl3, 7 d, ...

Scheme 77: Pyridinophane 418 synthesis via Friedel–Craft acylation. Reagents and conditions: (i) 416, AlCl3, CH...

Scheme 78: Cyclophane synthesis involving the Kotha–Schölkopf reagent 421. Reagents and conditions: (i) NBS, A...

Scheme 79: Cyclophane synthesis involving the Kotha–Schölkopf reagent 421. Reagents and conditions: (i) BEMP, ...

Scheme 80: Cyclophane synthesis by coupling with TosMIC. Reagents and conditions: (i) (a) ClCH2OCH3, TiCl4, CS2...

Scheme 81: Synthesis of diaza[32]cyclophanes and triaza[33]cyclophanes. Reagents and conditions: (i) DMF, NaH,...

Scheme 82: Synthesis of cyclophane 439 via acyloin condensation. Reagents and conditions: (i) Na, xylene, 75%;...

Scheme 83: Synthesis of multibridged binuclear cyclophane 442 by aldol condensation. Reagents and conditions: ...

Scheme 84: Synthesis of various macrolactones. Reagents and conditions: (i) iPr2EtN, DMF, 77–83%; (ii) TBDMSCl...

Scheme 85: Synthesis of muscone and muscopyridine via Yamaguchi esterification. Reagents and conditions: (i) 4...

Scheme 86: Synthesis of [5]metacyclophane via a double elimination reaction. Reagents and conditions: (i) LiBr...

Figure 12: Cyclophanes 466–472 synthesized via Hofmann elimination.

Scheme 87: Synthesis of cryptophane via Baylis–Hillman reaction. Reagents and conditions: (i) methyl acrylate,...

Scheme 88: Synthesis of cyclophane 479 via double Chichibabin reaction. Reagents and conditions: (i) excess 478...

Scheme 89: Synthesis of cyclophane 483 via double Chichibabin reaction. Reagents and conditions: (i) 481, OH−;...

Scheme 90: Synthesis of cyclopeptide via an intramolecular SNAr reaction. Reagents and conditions: (i) TBAF, T...

Scheme 91: Synthesis of muscopyridine (73) via C-zip ring enlargement reaction. Reagents and conditions: (i) H...

Figure 13: Mechanism of the formation of compound 494.

Scheme 92: Synthesis of indolophanetetraynes 501a,b using the Nicholas reaction as a key step. Reagents and co...

Scheme 93: Synthesis of cyclophane via radical cyclization. Reagents and conditions: (i) cyclododecanone, phen...

Scheme 94: Synthesis of (−)-cylindrocyclophanes A (156) and (−)-cylindrocyclophanes F (155). Reagents and cond...

Scheme 95: Cyclophane synthesis via Wittig reaction. Reagents and conditions: (i) LiOEt (2.1 equiv), THF, −78 ...

Figure 14: Representative examples of cyclophanes synthesized via Wittig reaction.

Scheme 96: Synthesis of the [6]paracyclophane via isomerization of Dewar benzene. Reagents and conditions: (i)...
Synthesis of a sucrose dimer with enone tether; a study on its functionalization
- Zbigniew Pakulski,
- Norbert Gajda,
- Magdalena Jawiczuk,
- Jadwiga Frelek,
- Piotr Cmoch and
- Sławomir Jarosz
Beilstein J. Org. Chem. 2014, 10, 1246–1254, doi:10.3762/bjoc.10.124
- evidenced by the steadily increasing number of reports in the literature about its successful application in the determination of the AC of 1,2-diols [30][31][32]. Therefore, in assignment of the AC of compounds under the present study (13, 14 and 16), we decided just to take advantage of the in situ

Graphical Abstract

Figure 1: Examples of sucrose-based macrocycles.

Figure 2: Synthesis of higher sugar precursors by a Wittig-type methodology.

Scheme 1: Synthesis of higher sugar enone 10.

Scheme 2: Synthesis of the diol 13 containing two sucrose units.

Figure 3: CD spectra of in situ formed chiral complexes of 13 (green line), 14 (purple line) and 16 (blue lin...

Scheme 3: Synthesis of model sucrose diols.
Homogeneous and heterogeneous photoredox-catalyzed hydroxymethylation of ketones and keto esters: catalyst screening, chemoselectivity and dilution effects
- Axel G. Griesbeck and
- Melissa Reckenthäler
Beilstein J. Org. Chem. 2014, 10, 1143–1150, doi:10.3762/bjoc.10.114
- contrast to that, photochemical redox activation is possible in the presence of titanium(IV) catalysts [19][20][21][22]. As shown in a series of papers by Sato and coworkers, carbonyl compounds 1 as well as imines couple with methanol to give the 1,2-diols or 1,2-amino alcohols, respectively, when

Graphical Abstract

Scheme 1: Photohydroxymethylation of carbonyl compounds and imines.

Scheme 2: Model process: photocatalyzed acetophenone/methanol reaction.

Scheme 3: Photocatalyzed acetophenone/methanol reaction: types I–III.

Scheme 4: Photohydroxymethylation and subsequent lactonization of keto esters.

Scheme 5: Model reaction for heterogeneous and dye-sensitized catalysis.

Scheme 6: Product forming routes I to III for photoredox catalysis of methanol/carbonyl compounds.

Scheme 7: Photoredox initiated steps on semiconductor particle surfaces, CB, VB = conduction and valence band....
Synthesis and crystallographic analysis of meso-2,3-difluoro-1,4-butanediol and meso-1,4-dibenzyloxy-2,3-difluorobutane
- Bruno Linclau,
- Leo Leung,
- Jean Nonnenmacher and
- Graham Tizzard
Beilstein J. Org. Chem. 2010, 6, No. 62, doi:10.3762/bjoc.6.62
- hypervalent iodine species [21]. Such approaches often display poor stereoselectivity or result in rearrangement products. Treatment of 1,2-diols with SF4 [22][23], DAST [24], or deoxofluor [25] also leads to vicinal difluorides. Reaction with vicinal triflates has also been successful in some cases [7][26

Graphical Abstract

Figure 1: Vicinal difluoride containing building blocks.

Scheme 1: Synthesis of meso-2,3-difluoro-1,4-butanediol.

Scheme 2: Monoprotection of 3, and activation of the remaining alcohol.

Scheme 3: Reaction of 12 leading to defluorinated products.

Figure 2: Molecular overlay of both conformers of 7.

Figure 3: Crystal packing of 7 viewed along the b axis. Short contacts (see text) are shown in light blue.

Figure 4: Crystal structure of 3.

Figure 5: Crystal packing of 3 viewed along the c axis. H-bonds are shown in light blue.






