Search results
Search for "epoxide" in Full Text gives 238 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Syntheses of spliceostatins and thailanstatins: a review
Beilstein J. Org. Chem. 2020, 16, 1991–2006, doi:10.3762/bjoc.16.166
- oxidation stereoselectively generated the spirocyclic epoxide 76. A second-protecting group shuffle afforded the primary alcohol 77. A Mitsunobu substitution of 77 with 2-mercaptobenzothiazole, followed by an oxidation with a large excess of mCPBA afforded the sulfone 78. While not producing the identical
- removal of the dithiane protecting group and the cyclic-ketal formation gave 84. The oxidative hydrolysis of the PMB ether and the reaction with mCPBA afforded the epoxide 85. While relatively short (9 steps), the nonstereoselective formation of 81a/b led to a lower overall yield. Syntheses of the C-1–C-6
- exocyclic epoxide prior to the formation of the C-6–C-9 conjugated diene was necessary in order to avoid the unwanted epoxidation of the C-6–C-7 olefin. Koide employed a unique strategy in which the exocyclic epoxide was generated as the initial stereocenter (Scheme 15) [12][13]. The Sharpless asymmetric
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
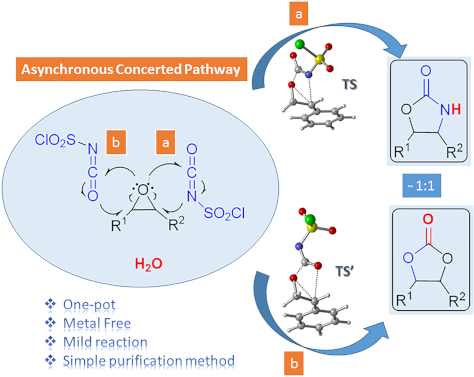
- natural products ranging from small molecules, such as sugars, lipids and amino acids to huge molecules [56]. Computational results A detailed mechanistic investigation of the synthesis of oxazolidinone and five-membered cyclic carbonate derivatives by the reaction between epoxide 7f and CSI has been
- performed. Formation of oxazolidinone 9f There are two possible channels for the cyclization reaction of epoxide 7f with CSI to form oxazolidinone intermediates 10 and 11 as shown in Figure 1. In both transition states it is found that the ring-opening reaction of the epoxide, a nucleophilic attack of N4
- N4 onto the less sterically encumbered C1 atom of the epoxide 7f forming intermediate 11. Optimized geometries of transition structures are depicted in Figure 1. Our calculated results for the reaction indicate 17.4 kcal/mol (gas phase) and 26.7 kcal/mol (in DCM) preference for the TS1 over the TS1
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- , cyclopentanone, and three carbon ring-expanded 1,3-diones from vinyl spiro epoxides [14]. The reaction is initiated by the addition of a thiyl radical to the vinyl epoxide 24, followed by the epoxide fragmentation to the alkoxy radical 25. Then, the β-cleavage to form the carbon-centered radical 26, the final
The charge-assisted hydrogen-bonded organic framework (CAHOF) self-assembled from the conjugated acid of tetrakis(4-aminophenyl)methane and 2,6-naphthalenedisulfonate as a new class of recyclable Brønsted acid catalysts
Beilstein J. Org. Chem. 2020, 16, 1124–1134, doi:10.3762/bjoc.16.99
- , providing some dissolved F-1 as the real catalyst. In all cases, the catalyst could easily be recovered and recycled. Keywords: Brønsted acid catalyst; charge-assisted hydrogen-bonded framework; Diels–Alder; epoxide ring opening; heterogeneous catalyst; Introduction Tremendous successes in homogeneous
- applications of the material. Thus, the catalytic properties of uncrystallized F-1 and F-1 with an F-1a phase were explored in a series of reactions typically promoted by Brønsted acids, such as epoxide ring openings with methanol and water (Scheme 2). The reactions were conducted at room temperature, and
- mixture was catalytically inactive, and after 24 hours, the reaction contained the epoxide 2 and traces of 3 (less than 1% yield, Table 1, run 6). This observation clearly showed that dissolved (leached) parts of F-1, even if present, could not be responsible for the catalytic performance. To investigate
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- cycloaddition, an epoxidation, and a biomimetic epoxide opening. Synthesis of (−)-amphidinolide E (3) using an intermolecular enyne metathesis as the key step. Synthesis of amphidinolide K (4) by an enyne metathesis route. Trost synthesis of des-epoxy-amphidinolide N (5) [72]. Enyne metathesis between the
Synthesis of disparlure and monachalure enantiomers from 2,3-butanediacetals
Beilstein J. Org. Chem. 2020, 16, 616–620, doi:10.3762/bjoc.16.57
- -5,6-dimethyl-1,4-dioxane-2-carboxylate as the starting material. Keywords: 2,3-butanediacetal; cis-epoxide; (−)-disparlure; (+)-disparlure; (−)-monachalure; (+)-monachalure; Introduction Compounds containing chiral epoxides display a wide range of biological activities and a number of them are
- , giving compounds 22 and 23. The butanediacetal groups were then removed with p-toluenesulfonic acid and diols 5 and 6 were obtained with 79% and 64% yield, respectively. They were then used in a well-established three-step, one-pot procedure for epoxide ring closure [26][30][40][41]. Pure (+)-disparlure
Photophysics and photochemistry of NIR absorbers derived from cyanines: key to new technologies based on chemistry 4.0
Beilstein J. Org. Chem. 2020, 16, 415–444, doi:10.3762/bjoc.16.40

- photopolymerization with cyanine as sensitizers combined with 88 as PF6−-salt. Exposure with a high power NIR LED emitting at 805 nm initiated cationic photopolymerization of the epoxide Epikote 357, Figure 3. Decomposition of the oxidized sensitizer/oxidized photoactive compound PA+• (Equation 7) provided the
Bacterial terpene biosynthesis: challenges and opportunities for pathway engineering
Beilstein J. Org. Chem. 2019, 15, 2889–2906, doi:10.3762/bjoc.15.283

- sesquiterpenes (type I TCs), diterpenes can either be generated by type I TCs or type II TCs [11][55]. Type II TCs initiate carbocation formation by Brønsted acid catalysis to protonate a terminal isoprene double bond or an epoxide ring (Figure 5b) [56]. Thus, cyclization mediated by type II TCs leads to an
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- -small cell lung cancer with ED50 values of 6.5 and 16.7 µg mL−1, respectively [71]. To obtain this interesting compound isolated from Dictyota dichotoma, C14 has to be modified with a keto function and the double bond between C3 and C4 has to be converted into an epoxide. Additionally, 3,7,18
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- singlet state oxygen to form similar peroxide, epoxide or epidioxide [16] derivatives, which can be isolated at room temperature (Scheme 4). An interesting physical characteristic of pentafulvene derivatives is their bright colour, which results from their cross conjugation, and varies with substitution
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- C19H24O5 for the first compound, which was supported by the 13C spectrum (Table 1). By comparing the spectrum to NMR data of other withanolides, we quickly identified the Michael system in ring A based on two olefinic protons (δH 6.94 and 6.22 ppm), a secondary alcohol at C-4 (δH 3.79 ppm), a 5,6-epoxide
- formula of C19H24O3 based on HRESIMS and 13C NMR (Table 1). In contrast to the first compound, no epoxide and no secondary alcohol at C-4 was present, in agreement with the different elemental composition. Instead, 13C NMR indicated a double bond at C5–C6 (δC 135.7 and 124.3 ppm). Otherwise, all spin
- -12α. The 5,6-epoxide was determined as β by a correlation from H-6 to H-3. These assignments are in complete agreement with the relative stereochemistry of 4ß-hydroxywithanolide E (1). In irinan B, the configuration of OH-14 could not be unambiguously inferred from NOE data due to the signal overlap
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- isomer 43. Treatment of 43 with methoxide ions in methanol at room temperature for 15 min gave the desired C-5 trans-butenolide 8 (40%) along with its C-5 cis-epimer 44 (60%), which is the sole product from the base-catalyzed isomerization of 43. Epoxidation of 43 gave a C-4,5-epoxide intermediate, which
- isomerization of olefin 43, the benzylic oxidation of 8, the use of m-CPBA to introduce the C-9,11 epoxide and the non-stereoselective reduction of the C-14 carbonyl group using sodium borohydride, caused an unacceptable overall yield (1.6%). This pioneering work undoubtedly established the basis for the future
- epoxide as a single diastereomer via in situ-generated methyl(trifluoromethyl)dioxirane and the reduction of the C-14 ketone in the presence of Eu(fod)3 to give triptolide (47%) together with its C-14 α-hydroxy epimer epi-triptolide (47%). In 2014, Li’s group reported a divergent synthesis for triptolide
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- acids and their cyclic forms (pyrrolidin-2-ones) takes advantage of the stereoselective epoxidation of the aziridine acrylaldehyde 161 to predominantly (98:2) form the aziridine epoxide 162 when (S)-[diphenyl(trimethylsilyloxy)methyl]pyrrolidine was used as a catalyst (Scheme 42) [94]. A key β
- -hydroxyester 163 was produced from the epoxide 162 employing N-heterocyclic carbene catalysis. Openings of the aziridine ring in 163a with azide or in 163b with in acetic acid provided enantiomerically pure methyl (3S,4S)-4,5-di-N-Boc-amino-3-hydroxypentanoate 164 or 4-N-Boc-amino-3,5-dihydroxypentanoate 165
- as components of a variety of plants and exhibit a wide range of biological properties including inhibition of glucosidases [102]. When the aziridine epoxide 162 was treated with acetic acid the aziridine ring cleavage was observed to give the epoxyaldehyde 178 (Scheme 45) [94]. Catalytic
Reaction of oxiranes with cyclodextrins under high-energy ball-milling conditions
Beilstein J. Org. Chem. 2019, 15, 1448–1459, doi:10.3762/bjoc.15.145

- derivatisation method that uses low-boiling epoxide reagents in a high-energy ball mill (HEBM). The simplified preparation and purification of low substitution-degree common (2-hydroxy)propylated β- and γ-cyclodextrins (β/γ-CDs) has been realised. The intelligent use of propylene oxide has also facilitated the
- is that the first step produces CD-propyl chlorohydrin, which is immediately transformed into an epoxide that can either react with other CDs, to form CDP, or with a hydroxy ion, to produce a (2,3-dihydroxy)propyl side chain. The hydrolytic reaction is unavoidable because of the aqueous solution and
- reagent, which can occur either via hydrolysis by the residual water or escape via evaporation, the jar was cooled with liquid N2 below −30 °C after the sodium salt formation of the CDs and before the epoxide was added to the light, electrostatic powder. Liquid N2 not only worked as a cooling medium, but
Robust perfluorophenylboronic acid-catalyzed stereoselective synthesis of 2,3-unsaturated O-, C-, N- and S-linked glycosides
Beilstein J. Org. Chem. 2019, 15, 1275–1280, doi:10.3762/bjoc.15.125
- activation of alcohols [24], epoxide opening [25][26], Friedel–Crafts alkylations [27], dehydrative glycosylation [28] and many other reactions [29][30][31]. The robustness and mildness of organoboronic acid catalysts in comparison to traditional strong Lewis and Brønsted acid catalysts inspired us to
Phylogenomic analyses and distribution of terpene synthases among Streptomyces
Beilstein J. Org. Chem. 2019, 15, 1181–1193, doi:10.3762/bjoc.15.115

- enantiomers of the corresponding alcohols (R)- and (S)-albaflavenol (16ab) and the epoxide 4β,5β-epoxy-2-epi-zizaan-6β-ol (18) are known oxidation products that are all made by a cytochrome P450 monooxygenase [10][29] that is genetically clustered with the epi-isozizaene synthase for the cyclisation of FPP to
Synthesis of acylglycerol derivatives by mechanochemistry
Beilstein J. Org. Chem. 2019, 15, 811–817, doi:10.3762/bjoc.15.78

- by epoxide ring-opening and esterification reactions with fatty acids 3 (Scheme 1). If successful, developing a multistep approach to prepare DAGs would contribute to the expansion of synthetic mechanochemical methodologies in ball mills [28][29][30][31], which are often limited to single-step
- hands, a selective epoxide ring-opening reaction with fatty acids 3 leading to the formation of the corresponding sn-1,3-protected monoacylglycerols (MAGs 4) was attempted (Scheme 3). Initially, commonly used solution-based protocols were tested in the ball mill [33]. For example, 2 was reacted with
- catalysts such as iron(III) chloride or bismuth(III) triflate was reported. However, the implementation of these protocols in the ball mill only led to trace amounts (less than 5% yield) of the protected monoacylglycerol 4a. Finally, one of the well-established Jacobsen catalysts for the epoxide ring
New sesquiterpenoids from the South China Sea soft corals Clavularia viridis and Lemnalia flava
Beilstein J. Org. Chem. 2019, 15, 695–702, doi:10.3762/bjoc.15.64

- known compounds were readily identified as cupalaurenol (5) [13], 1-hydroxyalloaromadendrene (7) [14], and humulene epoxide II (8) [15] by comparing their NMR spectroscopic data and optical rotation with those reported in the literature. Isobromolaurenisol (1) was obtained as an optically active
Oxidative radical ring-opening/cyclization of cyclopropane derivatives
Beilstein J. Org. Chem. 2019, 15, 256–278, doi:10.3762/bjoc.15.23
- naphthaldehyde 33 was obtained in 61% yield (Scheme 9, reaction b). Furthermore, the product 31a could also be transformed to the CF3-substituted epoxide 34 in the presence of 2 equiv m-CPBA (m-chloroperbenzoic acid) (Scheme 9, reaction c). A radical-trapping experiment was conducted with the addition of TEMPO
- aerobic oxidation of easily available cyclopropanols 91 via intermediate formation of peroxyketone intermediates 145, followed by enantioselective epoxide formation in the presence of a poly-L-leucine catalyst and DBU (Scheme 40) [120]. In 2014, a practical method for the conversion of 1,2-disubstituted
Synthesis of nonracemic hydroxyglutamic acids
Beilstein J. Org. Chem. 2019, 15, 236–255, doi:10.3762/bjoc.15.22

- acid Cleavage of the 5-membered ring in the protected epoxide 88 obtained from (S)-pyroglutamic acid [93][94][95] gave the methyl ester 89 which, when adsorbed on silica gel, smoothly underwent stereospecific epoxide ring opening to give the oxazolidinone 90 (Scheme 23) [96]. Before installation of the
Organometallic vs organic photoredox catalysts for photocuring reactions in the visible region
Beilstein J. Org. Chem. 2018, 14, 3025–3046, doi:10.3762/bjoc.14.282

- photoreticulation processes of multifunctional radical (acrylate, methacrylate…) or cationic (epoxide) monomers. Undoubtedly, the advantage of the photoredox catalysis approach is the high efficiency of the system to initiate the polymerization upon mild light irradiation conditions (as the catalyst is regenerated
Copolymerization of epoxides with cyclic anhydrides catalyzed by dinuclear cobalt complexes
Beilstein J. Org. Chem. 2018, 14, 2779–2788, doi:10.3762/bjoc.14.255
- epoxide/CA copolymerization was constricted until recently because of low catalytic activity and poor control over the main chain sequence (formation of ether linkages through consecutive epoxide enchainment) and molecular weight. In 2007, Coates and co-workers reported that β-diiminate zinc complexes
- exhibited a high catalytic activity for the epoxide/CA copolymerization [14]. The resulting polyesters were found to possess completely alternating structures with high molecular weight and relatively narrow polydispersity. Following this report, a range of highly active and/or selective catalysts has been
- methods [22][23][24][25][26]. Cooperative dinuclear metal catalysts have been considered as a promising design for high activity and/or selectivity in organic transformations including polymerizations [27][28][29][30]. This was found to be true for the epoxide/CA copolymerization. In 2013, Lu and co
Pathoblockers or antivirulence drugs as a new option for the treatment of bacterial infections
Beilstein J. Org. Chem. 2018, 14, 2607–2617, doi:10.3762/bjoc.14.239
- of LecA with its electrophilic epoxide warhead [40]. It could be demonstrated that 11 is a covalent lectin inhibitor, which provided the first proof-of-concept for this new approach to lectin inhibition. To date, the most potent LecA inhibitor 12 has been designed by the Pieters group, where two
Synergistic approach to polycycles through Suzuki–Miyaura cross coupling and metathesis as key steps
Beilstein J. Org. Chem. 2018, 14, 2468–2481, doi:10.3762/bjoc.14.223
- the corresponding epoxide 110. Unfortunately, generation of epoxide was not realized (Scheme 16). Macrocycles To develop new synthetic strategies to various cyclophanes, we conceived a sequential usage of the SM coupling and RCM as key steps [48][49]. In this context, the required dialdehyde 113 (80
































































































































































































































































































































































































































































































































































































