Search results
Search for "lactones" in Full Text gives 153 result(s) in Beilstein Journal of Organic Chemistry.
Identification of volatiles from six marine Celeribacter strains
Beilstein J. Org. Chem. 2021, 17, 420–430, doi:10.3762/bjoc.17.38
- experiments also led to a suggestion for the mechanism for sulfur incorporation, but further research is required for a deep understanding of TDA biosynthesis. Besides its function as an antibiotic, TDA acts as a signaling molecule, similar to N-acylhomoserine lactones, at concentrations 100 times lower than
- some of the investigated strains. A series of aldehydes ranging from hexanal (7) to tetradecanal (13) was found in strain specific patterns, with all identified compounds present in the bouquet from C. manganoxidans. A similar series of γ-lactones spanning from pentan-4-olide (14) to dodecan-4-olide
- (20), in addition to 3-methylbutan-4-olide (21) and 4-methylhex-5-en-4-olide (22), was detected in strain-specific patterns, with almost all of these compounds present in C. marinus; only C. halophilus did not emit lactones. Furans included furan-2-ylmethanol (23), furfural (24), and 2-acetylfuran (25
3-Acetoxy-fatty acid isoprenyl esters from androconia of the ithomiine butterfly Ithomia salapia
Beilstein J. Org. Chem. 2020, 16, 2776–2787, doi:10.3762/bjoc.16.228
- (Nymphalidae: Danainae) have hairpencils on the forewings (i.e., androconia) that disseminate semiochemicals during courtship. While most ithomiines are known to contain derivatives of pyrrolizidine alkaloids, dihydropyrrolizines, or γ-lactones in these androconia, here we report on a new class of fatty acid
- ][12]. While dihydropyrrolizines are also used by other Lepidoptera, e.g., danaines [13][14][15] or arctiines [16][17], γ-lactones derived from necic acids are specific to ithomiines of more derived taxa [11]. Past studies of the androconia of Ithomia have reported the presence of 3 in Ithomia
Bifurcated synthesis of methylene-lactone- and methylene-lactam-fused spirolactams via electrophilic amide allylation of γ-phenylthio-functionalized γ-lactams
Beilstein J. Org. Chem. 2020, 16, 2769–2775, doi:10.3762/bjoc.16.227
- -butyrolactone; spirolactams; Introduction α-Methylene-γ-butyrolactone is a pharmaceutically important motif which is found in a wide variety of bioactive molecules including natural products (Scheme 1a) [1][2][3]. For example, sesquiterpene lactones represented by parthenolide and helenalin have attracted
- interest because of their useful biological activities, and many synthetic efforts have been made so far [4][5][6]. On the other hand, syntheses and biological evaluation of nonnatural methylene-lactones also have been reported [4][6][7][8]. For instance, Heindel and his co-worker synthesized methylene
- key reaction (Scheme 1b) [10][11]. More recently, an amide-functionalized allyl boronate was developed as an alternative reagent for the allylstannane [12][13][14][15][16], which led to the syntheses of not only cytotoxic methylene-lactones spiro-fused to a lactam ring (B) but also their analogous
Recent developments in enantioselective photocatalysis
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197

- photocycloaddition of coumarins 202 (Scheme 31) [89]. The proposed mechanism for this reaction is similar to that proposed by Bach and Krische, proceeding via a key hydrogen bonding complex 203. Interestingly, this catalyst allowed for reactivity with lactones, whereas Bach’s catalysts are limited to lactams. Yoon
Selective preparation of tetrasubstituted fluoroalkenes by fluorine-directed oxetane ring-opening reactions
Beilstein J. Org. Chem. 2020, 16, 1936–1946, doi:10.3762/bjoc.16.160
- oxetane 16. The latter was subjected to the HBr/AcOH ring-opening reaction conditions (Scheme 6). Remarkably, the cyclic products β-hydroxymethyl and β-bromomethyl-γ-lactones 17 and 18 were obtained in an 8:92 ratio [32]. This complete reversal of selectivity in comparison with fluoroalkylidene-oxetane 12
- alkenes E-1d and E-9, was studied either on the bromomethyl (CH2Br) or on the hydroxymethyl (CH2OH) arm, when applicable. First, from a mixture of lactones 15 and 19 substitution on the bromomethyl arm was performed using sodium azide (Scheme 7). The reaction proceeded smoothly in DMF but it proved
- formation of derivative 10. Mechanism for the formation of dihydrofuran 10. Mechanism for the formation of unsaturated lactones 14 and 15. Opening reaction of ethyl 2-(oxetanyl-3-idene)acetate (16). Functionalization of bromomethyllactone 15 and its analogues. Functionalization by substitution reaction of
NHC-catalyzed enantioselective synthesis of β-trifluoromethyl-β-hydroxyamides
Beilstein J. Org. Chem. 2020, 16, 1572–1578, doi:10.3762/bjoc.16.129
- with amine nucleophiles allowed access to β-trifluoromethyl-β-hydroxyamides in moderate to good yield as single diastereoisomers in excellent er following purification [61]. Organocatalytic enantioselective aldol approaches using trifluoroacetophenone derivatives. NHC-catalyzed approaches to β-lactones
The McKenna reaction – avoiding side reactions in phosphonate deprotection
Beilstein J. Org. Chem. 2020, 16, 1436–1446, doi:10.3762/bjoc.16.119
- ester [9], which is cleaved in the second step, upon solvolysis, forming the final product (Scheme 1). Bromotrimethylsilane (BTMS) is the main reagent in this reaction and is also known for its ability to cleave lactones, epoxides, acetals, and ethers [10]. BTMS also acted as a brominating agent and
Oxime radicals: generation, properties and application in organic synthesis
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- (products 46a,b, 46m,n), 1,3-diketones (product 46r), as well as lactones (products 46o–q) were used in the oxidative C–O coupling reaction. The coupling of oximes with 1,3-diketones proceeded in lower yields than with 1,3-ketoesters (products 46a and 46r). Despite the presence of t-BuOOH in the system, a
Anthelmintic drug discovery: target identification, screening methods and the role of open science
Beilstein J. Org. Chem. 2020, 16, 1203–1224, doi:10.3762/bjoc.16.105
- cattle treated annually is hundreds of millions [1]. Treatment of horses, other equids, and companion animals is also a major use of anthelmintics. Anthelmintic drug discovery has been a continued emphasis in the animal health industry, driven by the spread of resistance to the macrocyclic lactones [2
- action of anthelmintic compounds, since it has been recognised that we do not fully understand how many anthelmintics work – for example the concentrations of macrocyclic lactones that paralyse worms in vitro are much greater than the concentration achieved by effective doses in vivo [147]. Several
Activated carbon as catalyst support: precursors, preparation, modification and characterization
Beilstein J. Org. Chem. 2020, 16, 1188–1202, doi:10.3762/bjoc.16.104

- forming phosphate esters or polymerization byproducts that bind on the solid carbon matrix [30]. Boehm titrations: Boehm titrations are used for the determination of acidic or basic surface oxygen functional groups of solid materials. The acidic character is caused by carboxyl groups (R–COOH), lactones (R
- gases were determined by mass spectrometry. In general, each type of surface group decomposes to a defined product such as CO2 from carboxylic acid or lactones and CO from carbonyl, hydroxide, phenol, ether or quinone groups and thus, information on the amounts of oxygen-containing surface groups are
Copper-catalysed alkylation of heterocyclic acceptors with organometallic reagents
Beilstein J. Org. Chem. 2020, 16, 1006–1021, doi:10.3762/bjoc.16.90
- alkenylaluminium and alkylaluminium reagents to the β-substituted unsaturated conjugated lactams 25 was utilized (Scheme 7B) [32]. Chiral lactones are yet another interesting class of heterocyclic substrates that have attracted great attention because of their usefulness in both organic synthesis and medicinal
- chemistry. The copper-catalysed ACA of organozinc reagents to 5,6-dihydro-2-pyranone is one of the best methods to obtain chiral lactones. During the past two decades, the research groups of Chan, Hoveyda (who applied an approach depending on the trapping by an aldehyde), Mauduit, and Wang reported a
- variety of methods employing the chiral ligands L9–L13 that, in combination with copper, efficiently catalysed the ACA of diethylzinc to 5,6-dihydro-2-pyranone (Scheme 8), providing access to chiral β-substituted lactones with high enantioselectivity and conversion [33][34][35][36][37]. Although the
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- scope of this silicon addition to unsaturated lactones using copper catalysis together with NHC ligand L16. The ligand used previously to great advantage by the Hoveyda and Oestreich groups for conjugate additions did not yield high ees for these substrates. The authors designed a new NHC possessing
A green, economical synthesis of β-ketonitriles and trifunctionalized building blocks from esters and lactones
Beilstein J. Org. Chem. 2019, 15, 2930–2935, doi:10.3762/bjoc.15.287
- Daniel P. Pienaar Kamogelo R. Butsi Amanda L. Rousseau Dean Brady Molecular Sciences Institute, School of Chemistry, University of the Witwatersrand, PO Wits 2050, Johannesburg, South Africa 10.3762/bjoc.15.287 Abstract The acylation of the acetonitrile anion with lactones and esters in ethereal
- solvents was successfully exploited using inexpensive KOt-Bu to obtain a variety of β-ketonitriles and trifunctionalized building blocks, including useful O-unprotected diols. It was discovered that lactones react to produce the corresponding derivatized cyclic hemiketals. Furthermore, the addition of a
- preparation of trifunctionalized building blocks (hydroxylated β-ketonitriles) and valuable β-ketonitriles (including enolizable compounds) in modest to good yields from lactones and esters. We are currently investigating novel applications of diols 1 and 8, as well as the application of this methodology for
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- lactonization of diols 51. However, in this method instead of a chiral base, they used 1-azaspiro[5.5]undecane N-oxyl 52 as a radical mediator for modifying the electrodes which resulted in optically active lactones 53 with enantiopurity of up to 99% (conditions B, Scheme 20) [52]. Chiral medium In this section
- 66 (Scheme 26). Galvanostatic electrolysis of 66 in an undivided cell using a catalytic amount of 67 resulted in the corresponding lactones 68 in moderate yields and good stereoselectivities. The stereoselectivity was proposed to be controlled through the preferential formation of transition state A
- over B after reaction of the enolates of 66 with chiral iodoarene 67. Steric factors restrict the formation of transition state B, and the reaction preferentially proceeds via transition state A to stereoselectively form the lactones [62]. Chiral catalysts Achieving enantiocontrol in electrochemical
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- File 1) yielded ten drimane metabolites shown in Figure 1: one trihydroxylated drimane sesquiterpenoid lactone, nanangenine A (1), four drimane lactones bearing C6/C8 acyl chains, nanangenines B, C, D and E (2, 4, 5 and 6), two acylated drimanes bearing isomeric lactones, isonanangenines B and D (3 and
- Our investigations into unique Australian microbial biodiversity have led to discovery of a family of drimane sesquiterpenoids, the nanangenines, which serve as key chemotaxonomic markers for the novel fungal species, A. nanangensis. Although related drimane sesquiterpenoid lactones have been found in
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- the formation of several different products, although predominantly enol lactones [47][50][51][52] (Scheme 3). Highly reactive intermediates formed during these reactions (Scheme 3) have only been observed spectroscopically at low temperatures (−55 °C) [52]. Heptafulvenes also undergo reactions with
Characterization of two new degradation products of atorvastatin calcium formed upon treatment with strong acids
Beilstein J. Org. Chem. 2019, 15, 2085–2091, doi:10.3762/bjoc.15.206

- dehydration of the δ-hydroxy group and some epimers resulting from acid-catalyzed isomerization reactions. In contrast, Vukkum et al. [13] describe, besides lactones 2 and 3, an α,β-unsaturated carboxylic acid 4. Treatment under more drastic conditions (6 M HCl, reflux, 3 h) was reported to result mainly in
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- Withanolides are steroidal lactones widespread in Nightshade plants with often potent antiproliferative activities. Additionally, the structural diversity of this compound class holds much potential for the discovery of novel biological activity. Here, we report two newly characterised withanolides, named
- research. An important example is Withania somnifera, also known as ashwaghanda or Indian ginseng, which has been used in Ayurvedic medicine to treat a large variety of ailments [1]. Extensive studies revealed withanolides, a class of steroidal lactones, to be primarily responsible for the medicinal
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- -catalyzed lactonization, and iii) construction of the epoxides by a newly developed methodology. The synthesis commenced from the alkylation of tetralone 22 with 3-(2-iodoethyl)dihydrofuran-2(3H)-one (23) to give diastereomeric lactones that subsequently reacted with dimethylamine to afford a 1:1 mixture of
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
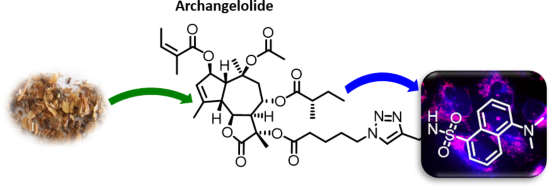
- Sciences of the Czech Republic, Flemingovo náměstí 2, 166 10 Prague 6, Czech Republic Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, v.v.i., 14220 Prague 4, Czech Republic 10.3762/bjoc.15.189 Abstract Sesquiterpene lactones are secondary plant metabolites with sundry
- studied by us and others, there are only scarce reports on the biological activity of archangelolide. Here we present the preparation of its fluorescent derivative based on a dansyl moiety using azide–alkyne Huisgen cycloaddition having obtained the two sesquiterpene lactones from the seeds of Laserpitium
- fluorescent conjugate; sarco/endoplasmic reticulum calcium ATPase; sesquiterpene lactone; trilobolide analogue; Introduction Sesquiterpene lactones (SLs) have been attracting interest already for some time due to the plethora of biological effects they elicit. Various SLs show anticancer, antimicrobial
Vicinal difunctionalization of alkenes by four-component radical cascade reaction of xanthogenates, alkenes, CO, and sulfonyl oxime ethers
Beilstein J. Org. Chem. 2019, 15, 1822–1828, doi:10.3762/bjoc.15.176
- reaction using xanthogenates, alkenes, and sulfonyl oxime ethers (Scheme 1, reaction 1) [21][22]. The reaction proceeds efficiently to provide good yields of α-alkoxyimino esters, potential precursors of lactams, lactones and β-keto esters. Since the three-component radical reaction involving alkyl halides
Mechanochemical synthesis of poly(trimethylene carbonate)s: an example of rate acceleration
Beilstein J. Org. Chem. 2019, 15, 963–970, doi:10.3762/bjoc.15.93
- biomedical applications [24]. The amidine base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) is one of the best studied and most popular organocatalysts for ring-opening polymerizations of cyclic carbonates and lactones [25][26][27]. In contrast to the high activity of lactone polymerization, cyclic carbonate
Synthesis of 2H-furo[2,3-c]pyrazole ring systems through silver(I) ion-mediated ring-closure reaction
Beilstein J. Org. Chem. 2019, 15, 679–684, doi:10.3762/bjoc.15.62
- AgOTf efficiently catalyzed the intramolecular cyclization of phenoxyethynyl diols into 2,3-unsaturated lactones [38]. In our case, the addition of 10 mol % chloro(triphenylphosphine)gold(I) improved the yield of product 4a to 69% (Table 1, entry 4). However the best results were obtained when AgOTf was
Reusable and highly enantioselective water-soluble Ru(II)-Amm-Pheox catalyst for intramolecular cyclopropanation of diazo compounds
Beilstein J. Org. Chem. 2019, 15, 357–363, doi:10.3762/bjoc.15.31
- proceeded smoothly at room temperature, affording the corresponding bicyclic cyclopropane ring-fused lactones and lactams in high yields (up to 99%) with excellent enantioselectivities (up to 99% ee). After screening of various catalysts, the Ru(II)-Amm-Pheox complex having an ammonium group proved to be
- intramolecular cyclopropanation of a variety of diazo compounds such as diazoacetates and diazoacetamides in a biphasic medium. Diazoacetates were tested in our catalytic system because they are widely used for intramolecular cyclopropanation reactions and also the resulted lactones are widely distributed in
Regioselective addition of Grignard reagents to N-acylpyrazinium salts: synthesis of substituted 1,2-dihydropyrazines and Δ5-2-oxopiperazines
Beilstein J. Org. Chem. 2019, 15, 72–78, doi:10.3762/bjoc.15.8
- nucleophilic addition of bis(trimethylsilyl)ketene acetals to pyrazines activated with methyl chloroformate was found to afford polycyclic γ-lactones in moderate yields [3][10][11]. The work by Garduño-Alva and co-workers demonstrated that these TMS-ketene acetals can be regioselectively added to substituted N
- -triflate pyrazinium salts to also generate γ-lactones [12]. Grignard reagents have been used as nucleophiles on a variety of N-acyl-activated pyridines in the production of natural products and biologically active small molecules [13][14][15][16][17][18][19][20]. To our surprise, there are no reports on

















































































































































































































































































































































































































































































