Search results
Search for "tetrahydrofuran" in Full Text gives 291 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
One-pot synthesis of dicyclopenta-fused peropyrene via a fourfold alkyne annulation
- Ji Ma,
- Yubin Fu,
- Junzhi Liu and
- Xinliang Feng
Beilstein J. Org. Chem. 2020, 16, 791–797, doi:10.3762/bjoc.16.72
- solvents, such as dichloromethane, chloroform, tetrahydrofuran and toluene, allowing a full characterization by NMR analyses. Finally, the palladium-catalyzed cyclopentannulation of compound 5 with 1,2-diphenylethyne under microwave conditions using the catalyst system of [Pd2(dba)3] and P(o-tol)3 afforded

Graphical Abstract

Scheme 1: Chemical structures of dicyclopenta-fused pyrene derivatives i–iii, peropyrene and the dicyclopenta...

Scheme 2: Synthetic route towards compound 1. a) B2pin2, dtbpy, [Ir(OMe)cod]2, cyclohexane, 70 °C, 20 h, 67%;...

Figure 1: High-resolution MALDI-TOF mass spectrum of 1. Inset: isotopic distribution compared to mass spectru...

Figure 2: Single-crystal X-ray structure of 1. (a) Top view and (b) side view of the (P,P) isomer. c) Crystal...

Figure 3: (a) UV–vis absorption spectra of precursor 5 and 1 in CH2Cl2 solution (10−5 M). Inset: photograph o...

Figure 4: Molecular orbitals of peropyrene derivative 6 and the dicyclopenta-fused peropyrene 1.
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
- Valerian Dragutan,
- Ileana Dragutan,
- Albert Demonceau and
- Lionel Delaude
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- stereoselective formation of the tetrahydrofuran rings. Conclusion This review highlighted the most recent efforts regarding the development of enyne metathesis-based syntheses of complex bioactive, natural and nonnatural organic molecules. Both, intra- and intermolecular enyne metatheses have been valorized to

Graphical Abstract

Scheme 1: Intramolecular (A) and intermolecular (B) enyne metathesis reactions.

Scheme 2: Ene–yne and yne–ene mechanisms for intramolecular enyne metathesis reactions.

Scheme 3: Metallacarbene mechanism in intermolecular enyne metathesis.

Scheme 4: The Oguri strategy for accessing artemisinin analogs 1a–c through enyne metathesis.

Scheme 5: Access to the tetracyclic core of nanolobatolide (2) via tandem enyne metathesis followed by an Eu(...

Scheme 6: Synthesis of (−)-amphidinolide E (3) using an intermolecular enyne metathesis as the key step.

Scheme 7: Synthesis of amphidinolide K (4) by an enyne metathesis route.

Scheme 8: Trost synthesis of des-epoxy-amphidinolide N (5) [72].

Scheme 9: Enyne metathesis between the propargylic derivative and the allylic alcohol in the synthesis of the...

Scheme 10: Synthetic route to amphidinolide N (6a).

Scheme 11: Synthesis of the stereoisomeric precursors of amphidinolide V (7a and 7b) through alkyne ring-closi...

Scheme 12: Synthesis of the anthramycin precursor 8 from ʟ-methionine by a tandem enyne metathesis–cross metat...

Scheme 13: Synthesis of (−)‐clavukerin A (9) and (−)‐isoclavukerin A (10) by an enyne metathesis route startin...

Scheme 14: Synthesis of (−)-isoguaiene (11) through an enyne metathesis as the key step.

Scheme 15: Synthesis of erogorgiaene (12) by a tandem enyne metathesis/cross metathesis sequence using the sec...

Scheme 16: Synthesis of (−)-galanthamine (13) from isovanilin by an enyne metathesis.

Scheme 17: Application of enyne metathesis for the synthesis of kempene diterpenes 14a–c.

Scheme 18: Synthesis of the alkaloid (+)-lycoflexine (15) through enyne metathesis.

Scheme 19: Synthesis of the AB subunits of manzamine A (16a) and E (16b) by enyne metathesis.

Scheme 20: Jung's synthesis of rhodexin A (17) by enyne metathesis/cross metathesis reactions.

Scheme 21: Total synthesis of (−)-flueggine A (18) and (+)-virosaine B (19) from Weinreb amide by enyne metath...

Scheme 22: Access to virgidivarine (20) and virgiboidine (21) by an enyne metathesis route.

Scheme 23: Enyne metathesis approach to (−)-zenkequinone B (22).

Scheme 24: Access to C-aryl glycoside 23 by an intermolecular enyne metathesis/Diels–Alder cycloaddition.

Scheme 25: Synthesis of spiro-C-aryl glycoside 24 by a tandem intramolecular enyne metathesis/Diels–Alder reac...

Scheme 26: Pathways to (−)-exiguolide (25) by Trost’s Ru-catalyzed enyne cross-coupling and cross-metathesis [94].
Rhodium-catalyzed reductive carbonylation of aryl iodides to arylaldehydes with syngas
- Zhenghui Liu,
- Peng Wang,
- Zhenzhong Yan,
- Suqing Chen,
- Dongkun Yu,
- Xinhui Zhao and
- Tiancheng Mu
Beilstein J. Org. Chem. 2020, 16, 645–656, doi:10.3762/bjoc.16.61
- (PMP)), and solvents (N,N-dimethylacetamide (DMA), 1,3-dimethyl-2-imidazolidinone (DMI), N-methyl-2-pyrrolidinone (NMP), tetrahydrofuran (THF), 1,4-dioxane, ethanol, methanol, acetonitrile, and toluene) together with aryl iodides and other reagents were purchased from commercial sources (namely J&K

Graphical Abstract

Figure 1: Rhodium-catalyzed reductive carbonylation of iodobenzene with CO and H2 to afford benzaldehyde. a) ...

Scheme 1: Scaled-up experiment of the reductive carbonylation of iodobenzene to benzaldehyde under the optimi...

Scheme 2: Catalytic species participating in the catalytic process.

Scheme 3: Substrate scope for the Rh-catalyzed reductive carbonylation of aryl iodides using CO and H2. React...

Scheme 4: Isotope-labeling experiments.

Scheme 5: Proposed reaction mechanism for the Rh-catalyzed reductive carbonylation of aryl iodides using CO a...
Design and synthesis of diazine-based panobinostat analogues for HDAC8 inhibition
- Sivaraman Balasubramaniam,
- Sajith Vijayan,
- Liam V. Goldman,
- Xavier A. May,
- Kyra Dodson,
- Sweta Adhikari,
- Fatima Rivas,
- Davita L. Watkins and
- Shana V. Stoddard
Beilstein J. Org. Chem. 2020, 16, 628–637, doi:10.3762/bjoc.16.59

- designed analogues and evaluating their overall potential to inhibit neuroblastoma cell growth. Experimental General Reagents and solvents were purchased from commercial sources and used without further purification unless otherwise specified. Tetrahydrofuran (THF), ether, dichloromethane (DCM), and

Graphical Abstract

Figure 1: Chemical structures of the target diazine-based surrogates for the central core of panobinostat.

Figure 2: Docking pose for panobinostat and panobinostat derivatives in the HDAC8 receptor. (a) Overlay of al...

Figure 3: General building blocks for the visualized targets.

Scheme 1: Reaction conditions: a) MeOH, H2SO4 (5 drops), MS 4 Å (2 pieces), 68 °C, 8 h, 81%; b) DIBAL-H (1.2 ...

Scheme 2: Reaction conditions: a) boronic acid 15 (1.3 equiv), PdCl2(PPh3)2 (0.1 equiv), dioxane/H2O (3:1), Na...

Scheme 3: Reaction conditions: a) 5-bromo-2-chloropyrimidine (1 equiv), ethyl formate (1.5 equiv), THF (20 mL...

Scheme 4: Reaction conditions: a) boronic acid 15 (1.3 equiv), PdCl2(PPh3)2 (0.1 equiv), dioxane/H2O (8:2, Na2...
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
- Pezhman Shiri and
- Jasem Aboonajmi
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52
- reacted with 4-aminomethylpiperidine (AMP, 21) or piperazine (PIP) to afford G1-AAA–SBA-15 (G1-AMP–SBA-15, 22, or G2-PIP–SBA-15, 23) materials (Scheme 3). The resulting solid product was then filtrated and washed with methanol, dichloromethane, and tetrahydrofuran. The organic part of the G1-AAA–SBA-15

Graphical Abstract

Scheme 1: Chemical structure of the catalysts 1a and 1b and their catalytic application in CuAAC reactions.

Scheme 2: Synthetic route to the catalyst 11 and its catalytic application in CuAAC reactions.

Scheme 3: Synthetic route of dendrons, illustrated using G2-AMP 23.

Scheme 4: The catalytic application of CuYAu–Gx-AAA–SBA-15 in a CuAAC reaction.

Scheme 5: Synthetic route to the catalyst 36.

Scheme 6: Application of the catalyst 36 in CuAAC reactions.

Scheme 7: The synthetic route to the catalyst 45 and catalytic application of 45 in “click” reactions.

Scheme 8: Synthetic route to the catalyst 48 and catalytic application of 48 in “click” reactions.

Scheme 9: Synthetic route to the catalyst 58 and catalytic application of 58 in “click” reactions.

Scheme 10: Synthetic route to the catalyst 64 and catalytic application of 64 in “click” reactions.

Scheme 11: Chemical structure of the catalyst 68 and catalytic application of 68 in “click” reactions.

Scheme 12: Chemical structure of the catalyst 69 and catalytic application of 69 in “click” reactions.

Scheme 13: Synthetic route to, and chemical structure of the catalyst 74.

Scheme 14: Application of the cayalyst 74 in “click” reactions.

Scheme 15: Synthetic route to, and chemical structure of the catalyst 78 and catalytic application of 78 in “c...

Scheme 16: Synthetic route to the catalyst 85.

Scheme 17: Application of the catalyst 85 in “click” reactions.

Scheme 18: Synthetic route to the catalyst 87 and catalytic application of 87 in “click” reactions.

Scheme 19: Chemical structure of the catalyst 88 and catalytic application of 88 in “click” reactions.

Scheme 20: Synthetic route to the catalyst 90 and catalytic application of 90 in “click” reactions.

Scheme 21: Synthetic route to the catalyst 96 and catalytic application of 96 in “click” reactions.

Scheme 22: Synthetic route to the catalyst 100 and catalytic application of 100 in “click” reactions.

Scheme 23: Synthetic route to the catalyst 102 and catalytic application of 23 in “click” reactions.

Scheme 24: Synthetic route to the catalysts 108–111.

Scheme 25: Catalytic application of 108–111 in “click” reactions.

Scheme 26: Synthetic route to the catalyst 121 and catalytic application of 121 in “click” reactions.

Scheme 27: Synthetic route to 125 and application of 125 in “click” reactions.

Scheme 28: Synthetic route to the catalyst 131 and catalytic application of 131 in “click” reactions.

Scheme 29: Synthetic route to the catalyst 136.

Scheme 30: Application of the catalyst 136 in “click” reactions.

Scheme 31: Synthetic route to the catalyst 141 and catalytic application of 141 in “click” reactions.

Scheme 32: Synthetic route to the catalyst 144 and catalytic application of 144 in “click” reactions.

Scheme 33: Synthetic route to the catalyst 149 and catalytic application of 149 in “click” reactions.

Scheme 34: Synthetic route to the catalyst 153 and catalytic application of 153 in “click” reactions.

Scheme 35: Synthetic route to the catalyst 155 and catalytic application of 155 in “click” reactions.

Scheme 36: Synthetic route to the catalyst 157 and catalytic application of 157 in “click” reactions.

Scheme 37: Synthetic route to the catalyst 162.

Scheme 38: Application of the catalyst 162 in “click” reactions.

Scheme 39: Synthetic route to the catalyst 167 and catalytic application of 167 in “click” reactions.

Scheme 40: Synthetic route to the catalyst 169 and catalytic application of 169 in “click” reactions.

Scheme 41: Synthetic route to the catalyst 172.

Scheme 42: Application of the catalyst 172 in “click” reactions.
Aerobic synthesis of N-sulfonylamidines mediated by N-heterocyclic carbene copper(I) catalysts
- Faïma Lazreg,
- Marie Vasseur,
- Alexandra M. Z. Slawin and
- Catherine S. J. Cazin
Beilstein J. Org. Chem. 2020, 16, 482–491, doi:10.3762/bjoc.16.43
- (see Supporting Information File 1 for details). Tetrahydrofuran (THF), 1,2-DCE, 1,4-dioxane and acetonitrile proved to be the most suitable solvents for this transformation (Table 1). Interestingly, similar results were obtained for complexes 1–5, while 6 displayed superior activity. Indeed, 71

Graphical Abstract

Scheme 1: Formation of sulfonyltriazoles and sulfonamidines.

Figure 1: Catalytic systems used in this study.

Scheme 2: Synthetic access to complexes 4–6 [30].

Scheme 3: Variation of sulfonylazides. Reaction conditions: phenylacetylene (0.5 mmol), sulfonyl azide (0.6 m...

Scheme 4: Variation of alkynes. Reaction conditions: alkyne (0.5 mmol), tosyl azide (0.6 mmol), diisopropylam...

Scheme 5: Variation of the amine substrate. Reaction conditions: phenylacetylene (0.5 mmol), tosyl azide (0.6...

Scheme 6: Reactivity of “non-sulfonyl” azide [33]. Reaction conditions: phenylacetylene (0.5 mmol), benzyl azide ...

Scheme 7: Reactivity of diphenylphosphoryl azide. Reaction conditions: phenylacetylene (0.5 mmol), diphenylph...

Scheme 8: Proposed mechanism for the formation of sulfonamidine.

Scheme 9: Stoichiometric reaction between 6 and 8.

Scheme 10: Synthesis of copper-acetylide intermediate A via [Cu(Cl)(Triaz)].

Scheme 11: Catalytic reaction involving copper-acetylide complex A.
Architecture and synthesis of P,N-heterocyclic phosphine ligands
- Wisdom A. Munzeiwa,
- Bernard Omondi and
- Vincent O. Nyamori
Beilstein J. Org. Chem. 2020, 16, 362–383, doi:10.3762/bjoc.16.35

- ]. Thus, the diphosphine ligand 51 was obtained in good yield by reacting 2,6-bis(chloromethyl)pyridine (50) with phosphine lithiothiolate 49. The latter was obtained by treatment of diphenylphosphine (48) with n-BuLi and ethylene sulfide in tetrahydrofuran at very low temperatures. Metal–proton exchange
- protocol with free amine spiro-amino alcohol derivative 124 gave compounds 125 and 127 (R = H) in low yields. An optimized procedure was used where dichlorophenylphosphine and borane·dimethyl sulfide in tetrahydrofuran were premixed at −78 °C. The temperature was then raised to 25 °C before neutralizing

Graphical Abstract

Scheme 1: Synthesis of pyridylphosphine ligands.

Figure 1: Pyridylphosphine ligands.

Scheme 2: Synthesis of piperidyl- and oxazinylphosphine ligands.

Scheme 3: Synthesis of linear multi-chelate pyridylphosphine ligands.

Scheme 4: Synthesis of chiral acetal pyridylphosphine ligands.

Scheme 5: Synthesis of diphenylphosphine-substituted triazine ligands.

Scheme 6: Synthesis of (pyridine-2-ylmethyl)phosphine ligands.

Scheme 7: Synthesis of diphosphine pyrrole ligands.

Scheme 8: Synthesis of 4,5-diazafluorenylphosphine ligands.

Scheme 9: Synthesis of thioether-containing pyridyldiphosphine ligands starting from ethylene sulfide and dip...

Scheme 10: Synthesis of monoterpene-derived phosphine pyridine ligands.

Scheme 11: Synthesis of N-phenylphosphine-substituted imidazole ligands.

Scheme 12: Synthesis of triazol-4-ylphosphine ligands.

Scheme 13: Synthesis of phosphanyltriazolopyridines and product selectivity depending on the substituents’ eff...

Scheme 14: Synthesis of PTA-phosphine ligands.

Scheme 15: Synthesis of isomeric phosphine dipyrazole ligands by varying the reaction temperature.

Scheme 16: Synthesis of N-tethered phosphine imidazolium ligands (route A) and diphosphine imidazolium ligands...

Scheme 17: Synthesis of {1-[2-(pyridin-2-yl)- (R = CH) and {1-[2-(pyrazin-2-yl)quinazolin-4-yl]naphthalen-2-yl...

Scheme 18: Synthesis of oxazolylindolylphosphine ligands 102.

Scheme 19: Synthesis of pyrrolylphosphine ligands.

Scheme 20: Synthesis of phosphine guanidinium ligands.

Scheme 21: Synthesis of a polydentate aminophosphine ligand.

Scheme 22: Synthesis of quinolylphosphine ligands.

Scheme 23: Synthesis of N-(triazolylmethyl)phosphanamine ligands.

Figure 2: Triazolylphosphanamine ligands synthesized by Wassenaar’s method [22].

Scheme 24: Synthesis of oxazaphosphorines.

Scheme 25: Synthesis of paracyclophane pyridylphosphine ligands.

Scheme 26: Synthesis of triazolylphosphine ligands.

Figure 3: Click-phosphine ligands.

Scheme 27: Ferrocenyl pyridylphosphine imine ligands.

Scheme 28: Synthesis of phosphinooxazolines (PHOX).

Scheme 29: Synthesis of ferrocenylphosphine oxazoles.
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
- Panagiotis S. Gritzapis,
- Panayiotis C. Varras,
- Nikolaos-Panagiotis Andreou,
- Katerina R. Katsani,
- Konstantinos Dafnopoulos,
- George Psomas,
- Zisis V. Peitsinis,
- Alexandros E. Koumbis and
- Konstantina C. Fylaktakidou
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- + 12 + CR (200 μM) + UV. Synthesis of O-carbamoyl amidoximes (8–13), ethanone oximes (15–20) and aldoximes (22–27). Oxime 1 or 14 or 21, Et3N (1.1 equiv), R–NCO (1.1–1.8 equiv), dry chloroform or tetrahydrofuran, Ar, 0 °C → rt or reflux, 19–97%. Photodissociation reaction of the derivative 12 in the T1

Graphical Abstract

Figure 1: General structures of oxime derivatives with possible DNA photocleavage ability. Left: Oxime carbox...

Scheme 1: Synthesis of O-carbamoyl amidoximes (8–13), ethanone oximes (15–20) and aldoximes (22–27). Oxime 1 ...

Figure 2: UV–vis spectra of CT DNA ([DNA] = 1.1 × 10−4 M) in buffer solution in the absence or presence of in...

Figure 3: Relative viscosity (η/η0)1/3 of CT DNA (0.1 mM) in buffer solution in the presence of compounds 11 ...

Figure 4: Plot of EB-DNA relative fluorescence emission intensity at λ = 592 nm (I/I0, %) vs r (= [compound]/...

Figure 5: DNA photocleavage of amidoxime carbamates at a concentration of 500 μM and mechanistic studies of a...

Figure 6: Potential energy curve for the dissociation of 12 in the first excited triplet state, T1. For compo...

Scheme 2: Photodissociation reaction of the derivative 12 in the T1 state and the formation of ground state r...

Scheme 3: Decarboxylation reaction of the p-chlorophenylcarbamoyloxyl radical.

Figure 7: Proposed scheme showing a possible energy transfer from acetophenone sensitizer to oxime carbamate ...

Figure 8: DNA photocleavage of compounds 8–10 and 12–13 at concentration of 500 μM, at 365 nm, in the absence...

Figure 9: DNA photocleavage of compound 12 at a concentration of 500 μM, at 312 nm, in the absence and presen...
Halogen-bonding-induced diverse aggregation of 4,5-diiodo-1,2,3-triazolium salts with different anions
- Xingyu Xu,
- Shiqing Huang,
- Zengyu Zhang,
- Lei Cao and
- Xiaoyu Yan
Beilstein J. Org. Chem. 2020, 16, 78–87, doi:10.3762/bjoc.16.10
- good yield (Scheme 1). The product 2-I was characterized by 1H NMR, 13C NMR, and high-resolution mass spectrometry. 2-I has a poor solubility in most organic solvents such as dichloromethane, trichloromethane, tetrahydrofuran, and ethanol. A single crystal of 2-I was obtained by slow diffusion of ether

Graphical Abstract

Figure 1: 1,2,3-Triazole based XB donors: 1,2,3-triazole A, 1,2,3-triazolium B, 1,2,3-triazolylidene C and di...

Scheme 1: Synthesis of 4,5-diiodo-1,3-dimesityl-1,2,3-triazolium with iodide, Mes: 2,4,6-Me3C6H2.

Scheme 2: Synthesis of 4,5-diiodo-1,3-dimesityl-1,2,3-triazolium with different anion.

Figure 2: Packing structure of 2-I (top), 2-Br (middle) and 2-Cl (bottom). Hydrogen atoms have been omitted f...

Figure 3: Packing structure of 2-BF4. Hydrogen atoms have been omitted for clarity.

Figure 4: Packing structure of 2-OAc. Hydrogen atoms and solvent molecules have been omitted for clarity.

Figure 5: Packing structure of 2-TFA. Hydrogen atoms and disorder of fluorine atoms have been omitted for cla...

Figure 6: Packing structure of 2-I.1.5I2. Hydrogen atoms have been omitted for clarity.

Figure 7: Packing structure of 2-I.3.5I2. Hydrogen atoms have been omitted for clarity.

Figure 8: Packing structure of 2-BF4.0.5bpy. Hydrogen atoms and dichloromethane have been omitted for clarity....

Figure 9: 1,2,3-Triazole-based halogen model calculation: electrostatic potential surfaces mapped on total de...
Photoreversible stretching of a BAPTA chelator marshalling Ca2+-binding in aqueous media
- Aurélien Ducrot,
- Arnaud Tron,
- Robin Bofinger,
- Ingrid Sanz Beguer,
- Jean-Luc Pozzo and
- Nathan D. McClenaghan
Beilstein J. Org. Chem. 2019, 15, 2801–2811, doi:10.3762/bjoc.15.273

- MHz) or an Avance 300 (1H: 300 MHz, 13C: 75 MHz) spectrometer. Chemical shifts are reported in ppm (δ) and are referenced to the NMR solvent residual peaks. Abbreviations used are s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Reagent grade tetrahydrofuran (THF) was distilled under

Graphical Abstract

Figure 1: Azobenzene-BAPTA 1E and 1Z (a, b, c, d and e denote specific protons), showing idealized Ca2+ uptak...

Scheme 1: Synthesis of azobenzene-tethered BAPTA 1.

Figure 2: Energy-minimized molecular modelling structures of 1E•Ca2+ and 1Z•Ca2+ (PM6).

Figure 3: Electronic absorption spectra showing changes associated with photoisomerization of 1E (40 μM) to 1Z...

Figure 4: 1H NMR spectra (300 MHz) recorded at room temperature (298 K) in D2O of a) the thermodynamically st...

Figure 5: a) Multiple trans–cis cycles of 1E (40 μM) indicated by absorption changes at 362 nm in aqueous 0.0...

Figure 6: Electronic absorption spectra changes of 1E (42 μM) (a) and 1Z (43 μM) (b) in aqueous 0.03 M MOPS b...

Figure 7: a) Reversible Ca2+ exchange between photoregulated host 1 and turn-“on” fluorescent probe 3. b) Blu...
An improved, scalable synthesis of Notum inhibitor LP-922056 using 1-chloro-1,2-benziodoxol-3-one as a superior electrophilic chlorinating agent
- Nicky J. Willis,
- Elliott D. Bayle,
- George Papageorgiou,
- David Steadman,
- Benjamin N. Atkinson,
- William Mahy and
- Paul V. Fish
Beilstein J. Org. Chem. 2019, 15, 2790–2797, doi:10.3762/bjoc.15.271
- . Yields are the ranges obtained from repeated reactions. DMF, N,N-dimethylformamide; NBS, N-bromosuccinimide; THF, tetrahydrofuran. Chlorination of 6 with N-chlorosuccinimide (NCS). Reagents and conditions: (a) NCS (1.2 equiv), AcOH, 55 °C, 7 h, 15–32%. Improved synthesis of 5. Reagents and conditions: (a

Graphical Abstract

Figure 1: Chemical structure of Notum inhibitor LP-922056 (1).

Scheme 1: Synthesis of LP-922056 (1). Reagents and conditionsa: (a) (COCl)2 (3.3 equiv), DMF, CH2Cl2, 55 °C ,...

Scheme 2: Chlorination of 6 with N-chlorosuccinimide (NCS). Reagents and conditions: (a) NCS (1.2 equiv), AcO...

Scheme 3: Improved synthesis of 5. Reagents and conditions: (a) NaOMe (5 equiv), 1,4-dioxane, 0 °C then rt, 1...

Figure 2: Concentrations of 1 in mouse following oral administration (p.o.) at 10 mg/kg.

Scheme 4: Preparation of amides 17. Representative reagents and conditionsa: (a) HBTU (1.1 equiv), iPr2NEt (2...
Arylisoquinoline-derived organoboron dyes with a triaryl skeleton show dual fluorescence
- Vânia F. Pais,
- Tristan Neumann,
- Ignacio Vayá,
- M. Consuelo Jiménez,
- Abel Ros and
- Uwe Pischel
Beilstein J. Org. Chem. 2019, 15, 2612–2622, doi:10.3762/bjoc.15.254

- emission band with a maximum at 401, 442, and 420 nm, in acetonitrile, respectively. These are tentatively assigned to π–π* transitions of the variable aryl moiety. Interestingly, in tetrahydrofuran, containing oxygen as donor atom, only the SW emission band is seen, i.e., λfluo,max = 409 nm (16), 402 nm
- LW band is substituted by a strong blue-shifted emission. This leads to a clear ratiometric behavior and a large dynamic response. The blue-shifted emission for the fluoroboronate Lewis adduct is in accordance with the observations made for donor solvents such as tetrahydrofuran (see above). As for

Graphical Abstract

Figure 1: Structures of the dyes 16–19.

Scheme 1: Synthesis of the precursors 2, 3, and 5.

Scheme 2: Synthesis of the precursor triflates 8–11.

Scheme 3: Synthesis of 12–15 and the organoboron dyes 16–19.

Figure 2: UV–vis absorption (solid line) and fluorescence (dashed line) spectra of a) 16, b) 17, c) 18, and d...

Scheme 4: Jablonski diagram representing the photophysical processes in the dyes 16–19.

Figure 3: Transient absorption spectrum (600 ns delay) of dye 17 in nitrogen-purged acetonitrile on excitatio...

Figure 4: Fluorescence titrations of the dyes (ca. 4–11 μM) with Bu4NF in acetonitrile. a) 16 (up to 156 equi...
Synthetic terpenoids in the world of fragrances: Iso E Super® is the showcase
- Alexey Stepanyuk and
- Andreas Kirschning
Beilstein J. Org. Chem. 2019, 15, 2590–2602, doi:10.3762/bjoc.15.252
- yield a new cation, which in turn is nucleophilically trapped by the carbinol moiety. The resulting tetrahydrofuran 59 is chemically stable and this observation was used as rationale for the erosion of the isomeric ratio observed during prolonged reaction times. In the same piece of work Fráter et al
- Plus® (34). Products 58 (α double bond) and product 53 (β double bond) are not desired [26]. Iso E Super Plus® (34) can undergo a third cyclisation to tetrahydrofuran 59 through compound rac-53 [22]. New unnatural terpenoid 70 from unnatural farnesyl pyrophosphate derivative 69 and comparison with
- natural biotransformation (67→68) and olfactory property of tetrahydrofuran 70 [39]. Individual components of the complex Iso E Super® mixture. Top fragrances with regard to their volume percentage (listed down to about 20%; the large number of perfumes with lower percentages are not listed) of Iso E

Graphical Abstract

Figure 1: Terpene constituents 1–9 found in geranium and bergamot oils and specified odours of individual com...

Figure 2: Other selected mono- and sesquiterpenes (10–26) as fragrance materials [6].

Figure 3: Main constituents of natural iris oil: irone (27).

Scheme 1: First synthesis of ionone (30) [11].

Scheme 2: First synthesis of Ambrelux (32) [14].

Scheme 3: Industrial synthesis of myrcene (1) by pyrolysis of β-pinene (8).

Scheme 4: First synthesis of Iso E Super® (33), Iso E Super Plus® (34) and Georgywood® (35) as a mixture of i...

Figure 4: Iso E Super® region of GC spectra of Molecule 01 (left, 75 €–100 € per 100 mL; march 2019), a low-p...

Scheme 5: First synthetic route to (−)-Georgywood® (35) by Corey and Hong [33].

Scheme 6: First synthetic route to the odour-active (+)-enantiomer of Iso E Super Plus® (+)-34 [33].

Scheme 7: Analysis of the isomerisation process and formation of products. Most importantly, Iso E Super® (33...

Scheme 8: Isomerisation using additives such as alcohols or carboxylic acids. The product with the γ-position...

Scheme 9: Iso E Super Plus® (34) can undergo a third cyclisation to tetrahydrofuran 59 through compound rac-53...

Figure 5: (Adapted from ref. [8]) Ionone (30, 1893, odour threshold: 0.8 ng L−1), koavone (1982, odour threshold...

Figure 6: Branched, terpene-like cyclohexene derivatives, that are synthetic fragrance components: 60: Iso da...

Scheme 10: New unnatural terpenoid 70 from unnatural farnesyl pyrophosphate derivative 69 and comparison with ...
α,ß-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
- Shital K. Chattopadhyay,
- Subhankar Ghosh,
- Sarita Sarkar and
- Kakali Bhadra
Beilstein J. Org. Chem. 2019, 15, 2524–2533, doi:10.3762/bjoc.15.245
- , removal of the O-benzyl group keeping the double bond intact proved to be problematic under a range of conditions. Pleasingly, use of BCl3 in tetrahydrofuran solvent effected the desired transformation but in a modest yield of 51%. Changing the solvent to dichloromethane and using a lower temperature

Graphical Abstract

Figure 1: Some hydroxamic acid-based anti-tumor drugs.

Scheme 1: Synthesis of SAHA and DDSAHA.

Figure 2: Cell viability from MTT assay for SAHA, 11b, 11f and 11g on HeLa after 24 h treatment.

Figure 3: Percent of cell death by LDH assay at a GI50 dose of SAHA, 11b, 11f and 11g after 24 h incubation a...

Figure 4: ROS generation by DCFDA.

Figure 5: The quantitative results of bivariate FITC-Annexin V/PI FCM of HeLa cells after treatment with 11b ...

Figure 6: Fluorescence microscopic images of 11b at different concentrations (8.9, and 14.2 µM, respectively)...

Figure 7: DNA Ladder formation in a gel electrophoresis study of 11b at different concentrations (at 8.9, and...
Sugar-derived oxazolone pseudotetrapeptide as γ-turn inducer and anion-selective transporter
- Sachin S. Burade,
- Sushil V. Pawar,
- Tanmoy Saha,
- Navanath Kumbhar,
- Amol S. Kotmale,
- Manzoor Ahmad,
- Pinaki Talukdar and
- Dilip D. Dhavale
Beilstein J. Org. Chem. 2019, 15, 2419–2427, doi:10.3762/bjoc.15.234

- stereochemically stable quaternary carbon center [1]. For example, TAA-derived peptides containing a cyclopropane ring and ʟ/ᴅ-dimethyl tartrate showed an α-turn and form 310-helical conformations in higher oligomers [2][3][4]. While, TAA-derived peptides having a tetrahydrofuran ring demonstrated a β-turn type

Graphical Abstract

Figure 1: Oxazolone pseudodipeptide 1 and tetrapeptide 2a.

Scheme 1: Synthesis of linear azido ester dipeptide 5 and tetrapeptide 7.

Scheme 2: Synthesis of oxazolone pseudopeptides 1, 2a and 2b.

Figure 2: Characteristic NOEs of 2a.

Figure 3: DMSO titration study of 2a.

Figure 4: 1H NMR temperature study of 2a.

Figure 5: Optimized helical conformations of (A) 2a, (B) 2b and (C) 9.

Figure 6: Ion transport activity (A) for 1, (B) for 2a, across EYPC-LUVs HPTS.

Figure 7: Cation (A) and anion (B) transport activity of 2a.

Figure 8:
Comparison of the ion transport activity of 2a and 2b at 20 µM across EYPC-LUVslucigenin (A). Conce...
Effect of ring size on photoisomerization properties of stiff stilbene macrocycles
- Sandra Olsson,
- Óscar Benito Pérez,
- Magnus Blom and
- Adolf Gogoll
Beilstein J. Org. Chem. 2019, 15, 2408–2418, doi:10.3762/bjoc.15.233

- dichloromethane (DCM), ethyl acetate, pentane, tetrahydrofuran (THF) and toluene that were distilled before use. N,N-Dimethylformamide (DMF) was used as supplied (biotech. grade, ≥99.9%). Unless stated differently, all reactions were carried out under atmospheric pressure and with argon atmosphere. Microwave (MW

Graphical Abstract

Scheme 1: The stiff stilbene photoisomerization from Z to E and vice versa by irradiation at 300 nm and 360 n...

Figure 1: The investigated SS-macrocycles (Z)-1a–d.

Scheme 2: Synthetic route to SS-macrocycles. i. (1) Triflic acid (3 equiv), DCM (dry), Ar atmosphere, MW (110...

Scheme 3: The photoisomerization of the stiff stilbene macrocycles, showing the stretching of the linker (gre...

Scheme 4: Noncyclic stiff stilbene diester 7 used as reference in the photoisomerization study.

Figure 2: The photoisomerization of the SS-macrocycles shows a clear correlation between the Z/E ratio in the...

Figure 3: Gibbs free energy differences (ΔG) between Z- and E-isomers of 1a–d and of the reference compound 7...

Figure 4: Ring strain for E and Z-isomers of 1a–d expressed as the Gibbs free energy difference to an acyclic...

Figure 5: The differences in ring strain between the E- and Z-isomers show an exponential correlation to the ...

Figure 6: Conformer ensembles for the macrocyclic stiff stilbene diethers 1a–d. Dihedral angles between the t...

Figure 7: Distances derived from NOE buildup experiments. Distances between pairs of protons or groups of pro...

Figure 8: Numbering of carbons in compounds 6a–d, showing 6d as an example.

Figure 9: Numbering of carbons in compounds (Z)-1a–d, showing (Z)-1d as an example.
Synthesis of acremines A, B and F and studies on the bisacremines
- Nils Winter and
- Dirk Trauner
Beilstein J. Org. Chem. 2019, 15, 2271–2276, doi:10.3762/bjoc.15.219
- should then close the tetrahydrofuran ring of the natural product. Upon irradiation of 5 using a Hg lamp, however, the only productive pathway which could be observed was isomerization of the disubstituted double bond (Table 1, entries 17 and 18). Again, we attempted to promote the reaction by tethering

Graphical Abstract

Figure 1: Selected members of the acremine family [3-5].

Scheme 1: Retrosynthetic analysis of acremine F (5).

Scheme 2: Total synthesis of acremine F (5).

Scheme 3: Synthesis of acremines A and B through selective oxidation of acremine F.

Scheme 4: Proposed biomimetic dimerization of 5.

Scheme 5: Attempted intramolecular cyclization of 23.

Scheme 6: Attempted photochemical cyclization of 25.
Multiple threading of a triple-calix[6]arene host
- Veronica Iuliano,
- Roberta Ciao,
- Emanuele Vignola,
- Carmen Talotta,
- Patrizia Iannece,
- Margherita De Rosa,
- Annunziata Soriente,
- Carmine Gaeta and
- Placido Neri
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- mass spectra were calibrated externally, and a linear calibration was applied. All chemicals were reagent grade and were used without further purification. Tetrahydrofuran was dried by heating under reflux over sodium wire in the presence of benzophenone as indicator while dimethylformamide was dried

Graphical Abstract

Figure 1: Sketch of the currently known prototypical examples of handcuff-derived architectures.

Figure 2: Chemical drawing of the known bis-calix[6]arene 1 and its handcuff pseudorotaxane architectures 32+...

Scheme 1: Synthesis of triple-calix[6]arene host 6.

Scheme 2:
Formation of the 7+6, (7+)2
6, (7+)3
6 pseudorotaxane architectures by multiple-threading of 6 with 7+...

Figure 3:
(Bottom) Portion of the ESI-FT-ICR mass spectrum of 7+6. (Top a–c) Significant portions of: (a) 1H ...

Figure 4: 1H NMR titration of 6 with 7+·TFPB– (CDCl3 , 298 K, 600 MHz). Significant portions of the 1H NMR sp...

Figure 5:
ESI-FT-ICR-MS and HR-ESI-FT-ICR-CID mass spectrum of (7+)26.

Figure 6:
Different views of the minimized structures of (7+)36 obtained by molecular mechanics calculations.

Scheme 3:
Formation of the 4+6, (4+)2
6, (4+)3
6 pseudorotaxane architectures by multiple-threading of 6 with 4+...

Figure 7: (a–d) 1H NMR titration of 6 with 4+·TFPB− (CDCl3, 298 K, 600 MHz). Significant portions of the 1H N...

Figure 8:
Different views of the minimized structures of (4+)36 obtained by molecular mechanics calculations.

Figure 9: (Top) Possible endo-benzyl and endo-alkyl stereoisomers obtainable by directional threading of cali...

Figure 10:
(Left) HR-ESI-FT-ICR mass spectrum of 8+6. (Right) HR-ESI-FT-ICR-CID mass spectrum of (8+)2
6.

Figure 11: (a–d) 1H NMR titration of 6 with 8+·TFPB− (CDCl3 , 298 K, 600 MHz). Significant portions of the 1H ...
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
- Silvie Rimpelová,
- Michal Jurášek,
- Lucie Peterková,
- Jiří Bejček,
- Vojtěch Spiwok,
- Miloš Majdl,
- Michal Jirásko,
- Miloš Buděšínský,
- Juraj Harmatha,
- Eva Kmoníčková,
- Pavel Drašar and
- Tomáš Ruml
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
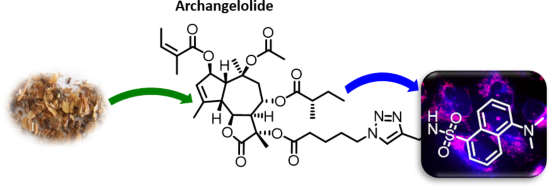
- , dicyclohexylurea; Dns, dansyl amide; DMAP, 4-dimethylaminopyridine; DTB, 8-O-(dodecanoyl-8-O-debutanoyltrilobolid); IL-6, interleukin 6; IL-1β, interleukin 1β; INF-γ, interferon gamma; TBTA, tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; TEA, triethylamine; THF, tetrahydrofuran; TLC, thin-layer chromatography

Graphical Abstract

Figure 1: The structure of the sesquiterpene lactones archangelolide (1) and trilobolide (2).

Scheme 1: Reagents and conditions: a) MeOH, TEA, 48 h, yield 32%; b) (i) 5-azidopentanoic acid, DCC, DCM, 90 ...

Figure 2: Intracellular localization of archangelolide-dansyl (5) in human cells from osteosarcoma (U-2 OS). ...

Figure 3: Co-localization of dansylarchangelolide 5 with a marker of endoplasmic reticulum (top row) and with...

Figure 4: Cartoon representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A, C) archang...

Figure 5: Molecular surface representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A) ...

Figure 6: Structural formulae of (i) thapsigargin, (ii) trilobolide (2), and (iii) archangelolide (1). Red pa...

Figure 7: Viability of rat peritoneal cells treated with archangelolide (1), dansylarchangelolide 5 and dansy...

Figure 8: NO production in primary rat macrophages. The cells were treated with archangelolide (1) and dansyl...

Figure 9: Evaluation of cytokine TNF-α secretion in rat peritoneal cells. Stimulation of primary cells was in...

Figure 10: Structure of laserolide.
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
- Jing Li,
- Qiang Shi,
- Ying Han and
- Chuan-Feng Chen
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- methyl-substituted derivative H2 were prepared according to previously reported methods [30]. Starting from helic[6]arene H1, helic[6]arene derivatives H3 and H4 were conveniently synthesized by etherification of H1 with bromobutane or 2-bromoethyl methyl ether, respectively, in tetrahydrofuran in the

Graphical Abstract

Figure 1: Structures and proton designations of hosts H1–5 and guests G1–4.

Scheme 1: Synthesis of hosts H3–5.

Figure 2: Partial 1H NMR spectra (400 MHz CDCl3/acetone-d6 1:2 (v/v), 298 K) of (a) free H1, (b) H1 with 1.0 ...

Figure 3: Partial 1H NMR spectra (400 MHz, CD2Cl2, 298 K) of (a) free H1, (b) H1 with 1.0 equiv G4, (c) free ...

Figure 4: Crystal structure of complex H1·G1. (a) Top view, (b) side view, and (c) packing viewed along c-axi...

Figure 5: Crystal structure of complex H5·G1. (a) Top view, (b) side view, and (c) packing viewed along the a-...

Figure 6: Calculated structures of the complexes at the B3LYP/6-31G level of theory. (a) Top view and (b) sid...

Figure 7: Schematic representation of the acid–base controlled complexation process and partial 1H NMR spectr...
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
- Iwona E. Głowacka,
- Aleksandra Trocha,
- Andrzej E. Wróblewski and
- Dorota G. Piotrowska
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- both pairs of ethyl esters [26]. When a mixture of tert-butyl esters (2R,1'S)-5d and (2S,1'S)-5d was subjected to kinetic resolution in the presence of potassium tert-butoxide in tetrahydrofuran (2R,1'S)-5d was produced with low 40% de [27]. The absolute configuration at C2 in esters 5 was established

Graphical Abstract

Figure 1: Examples of three-carbon chirons.

Figure 2: Structures of derivatives of N-(1-phenylethyl)aziridine-2-carboxylic acid 5–8.

Figure 3: Synthetic equivalency of aziridine aldehydes 6.

Scheme 1: Synthesis of N-(1-phenylethyl)aziridine-2-carboxylates 5. Reagents and conditions: a) TEA, toluene,...

Scheme 2: Absolute configuration at C2 in (2S,1'S)-5a. Reagents and conditions: a) 20% HClO4, 80 °C, 30 h the...

Scheme 3: Major synthetic strategies for a 2-ketoaziridine scaffold [R* = (R)- or (S)-1-phenylethyl; R′ = Alk...

Scheme 4: Synthesis of cyanide (2S,1'S)-13. Reagents and conditions: a) NH3, EtOH/H2O, rt, 72 h; b) Ph3P, CCl4...

Scheme 5: Synthesis of key intermediates (R)-16 and (R)-17 for (R,R)-formoterol (14) and (R)-tamsulosin (15)....

Scheme 6: Synthesis of mitotic kinesin inhibitors (2R/S,1'R)-23. Reagents and conditions: a) H2, Pd(OH)2, EtO...

Scheme 7: Synthesis of (R)-mexiletine ((R)-24). Reagents and conditions: a) TsCl, TEA, DMAP, CH2Cl2, rt, 1 h;...

Scheme 8: Synthesis of (−)-cathinone ((S)-27). Reagents and conditions: a) PhMgBr, ether, 0 °C; b) H2, 10% Pd...

Scheme 9: Synthesis of N-Boc-norpseudoephedrine ((1S,2S)-(+)-29) and N-Boc-norephedrine ((1R,2S)-29). Reagent...

Scheme 10: Synthesis of (−)-ephedrine ((1R,2S)-31). Reagents and conditions: a) TfOMe, MeCN then NaBH3CN, rt; ...

Scheme 11: Synthesis of xestoaminol C ((2S,3R)-35), 3-epi-xestoaminol C ((2S,3S)-35) and N-Boc-spisulosine ((2S...

Scheme 12: Synthesis of ʟ-tryptophanol ((S)-41). Reagents and conditions: a) CDI, MeCN, rt, 1 h then TMSI, MeC...

Scheme 13: Synthesis of ʟ-homophenylalaninol ((S)-42). Reagents and conditions: a) NaH, THF, 0 °C to −78 °C, 1...

Scheme 14: Synthesis of ᴅ-homo(4-octylphenyl)alaninol ((R)-47) and a sphingolipid analogue (R)-48. Reagents an...

Scheme 15: Synthesis of florfenicol ((1R,2S)-49). Reagents and conditions: a) (S)-1-phenylethylamine, TEA, MeO...

Scheme 16: Synthesis of natural tyroscherin ((2S,3R,6E,8R,10R)-55). Reagents and conditions: a) I(CH2)3OTIPS, t...

Scheme 17: Syntheses of (−)-hygrine (S)-61, (−)-hygroline (2S,2'S)-62 and (−)-pseudohygroline (2S,2'R)-62. Rea...

Scheme 18: Synthesis of pyrrolidine (3S,3'R)-68, a fragment of the fluoroquinolone antibiotic PF-00951966. Rea...

Scheme 19: Synthesis of sphingolipid analogues (R)-76. Reagents and conditions: a) BnBr, Mg, THF, reflux, 6 h;...

Scheme 20: Synthesis of ᴅ-threo-PDMP (1R,2R)-81. Reagents and conditions: a) TMSCl, NaI, MeCN, rt, 1 h 50 min,...

Scheme 21: Synthesis of the sphingolipid analogue SG-14 (2S,3S)-84. Reagents and conditions: a) LiAlH4, THF, 0...

Scheme 22: Synthesis of the sphingolipid analogue SG-12 (2S,3R)-88. Reagents and conditions: a) 1-(bromomethyl...

Scheme 23: Synthesis of sphingosine-1-phosphate analogues DS-SG-44 and DS-SG-45 (2S,3R)-89a and (2S,3R)-89a. R...

Scheme 24: Synthesis of N-Boc-safingol ((2S,3S)-95) and N-Boc-ᴅ-erythro-sphinganine ((2S,3R)-95). Reagents and...

Scheme 25: Synthesis of ceramide analogues (2S,3R)-96. Reagents and conditions: a) NaBH4, ZnCl2, MeOH, −78 °C,...

Scheme 26: Synthesis of orthogonally protected serinols, (S)-101 and (R)-102. Reagents and conditions: a) BnBr...

Scheme 27: Synthesis of N-acetyl-3-phenylserinol ((1R,2R)-105). Reagents and conditions: a) AcOH, CH2Cl2, refl...

Scheme 28: Synthesis of (S)-linezolid (S)-107. Reagents and conditions: a) LiAlH4, THF, 0 °C to reflux; b) Boc2...

Scheme 29: Synthesis of (2S,3S,4R)-2-aminooctadecane-1,3,4-triol (ᴅ-ribo-phytosphingosine) (2S,3S,4R)-110. Rea...

Scheme 30: Syntheses of ᴅ-phenylalanine (R)-116. Reagents and conditions: a) AcOH, CH2Cl2, reflux, 4 h; b) MsC...

Scheme 31: Synthesis of N-Boc-ᴅ-3,3-diphenylalanine ((R)-122). Reagents and conditions: a) PhMgBr, THF, −78 °C...

Scheme 32: Synthesis of ethyl N,N’-di-Boc-ʟ-2,3-diaminopropanoate ((S)-125). Reagents and conditions: a) NaN3,...

Scheme 33: Synthesis of the bicyclic amino acid (S)-(+)-127. Reagents and conditions: a) BF3·OEt2, THF, 60 °C,...

Scheme 34: Synthesis of lacosamide, (R)-2-acetamido-N-benzyl-3-methoxypropanamide (R)-130. Reagents and condit...

Scheme 35: Synthesis of N-Boc-norfuranomycin ((2S,2'R)-133). Reagents and conditions: a) H2C=CHCH2I, NaH, THF,...

Scheme 36: Synthesis of MeBmt (2S,3R,4R,6E)-139. Reagents and conditions: a) diisopropyl (S,S)-tartrate (E)-cr...

Scheme 37: Synthesis of (+)-polyoxamic acid (2S,3S,4S)-144. Reagents and conditions: a) AD-mix-α, MeSO2NH2, t-...

Scheme 38: Synthesis of the protected 3-hydroxy-ʟ-glutamic acid (2S,3R)-148. Reagents and conditions: a) LiHMD...

Scheme 39: Synthesis of (+)-isoserine (R)-152. Reagents and conditions: a) AcCl, MeCN, rt, 0.5 h then Na2CO3, ...

Scheme 40: Synthesis of (3R,4S)-N3-Boc-3,4-diaminopentanoic acid (3R,4S)-155. Reagents and conditions: a) Ph3P...

Scheme 41: Synthesis of methyl (2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoate (2S,3S,4S)-159. ...

Scheme 42: Syntheses of methyl (3S,4S) 4,5-di-N-Boc-amino-3-hydroxypentanoate ((3S,4S)-164), methyl (3S,4S)-4-N...

Scheme 43: Syntheses of (3R,5S)-5-(aminomethyl)-3-(4-methoxyphenyl)dihydrofuran-2(3H)-one ((3R,5S)-168). Reage...

Scheme 44: Syntheses of a series of imidazolin-2-one dipeptides 175–177 (for R' and R'' see text). Reagents an...

Scheme 45: Syntheses of (2S,3S)-N-Boc-3-hydroxy-2-hydroxymethylpyrrolidine ((2S,3S)-179). Reagents and conditi...

Scheme 46: Syntheses of enantiomers of 1,4-dideoxy-1,4-imino-ʟ- and -ᴅ-lyxitols (2S,3R,4S)-182 and (2R,3S,4R)-...

Scheme 47: Synthesis of 1,4-dideoxy-1,4-imino-ʟ-ribitol (2S,3S,4R)-182. Reagents and conditions: a) AcOH, CH2Cl...

Scheme 48: Syntheses of 1,4-dideoxy-1,4-imino-ᴅ-arabinitol (2R,3R,4R)-182 and 1,4-dideoxy-1,4-imino-ᴅ-xylitol ...

Scheme 49: Syntheses of natural 2,5-imino-2,5,6-trideoxy-ʟ-gulo-heptitol ((2S,3R,4R,5R)-184) and its C4 epimer...

Scheme 50: Syntheses of (−)-dihydropinidine ((2S,6R)-187a) (R = C3H7) and (2S,6R)-isosolenopsins (2S,6R)-187b ...

Scheme 51: Syntheses of (+)-deoxocassine ((2S,3S,6R)-190a, R = C12H25) and (+)-spectaline ((2S,3S,6R)-190b, R ...

Scheme 52: Synthesis of (−)-microgrewiapine A ((2S,3R,6S)-194a) and (+)-microcosamine A ((2S,3R,6S)-194b). Rea...

Scheme 53: Syntheses of ʟ-1-deoxynojirimycin ((2S,3S,4S,5R)-200), ʟ-1-deoxymannojirimycin ((2S,3S,4S,5S)-200) ...

Scheme 54: Syntheses of 1-deoxy-ᴅ-galacto-homonojirimycin (2R,3S,4R,5S)-211. Reagents and conditions: a) MeONH...

Scheme 55: Syntheses of 7a-epi-hyacinthacine A1 (1S,2R,3R,7aS)-220. Reagents and conditions: a) TfOTBDMS, 2,6-...

Scheme 56: Syntheses of 8-deoxyhyacinthacine A1 ((1S,2R,3R,7aR)-221). Reagents and conditions: a) H2, Pd/C, PT...

Scheme 57: Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, L...

Scheme 58: Syntheses of 8-epi-swainsonine (1S,2R,8S,8aR)-231. Reagents and conditions: a) Ph3P=CHCOOMe, MeOH, ...

Scheme 59: Synthesis of a protected vinylpiperidine (2S,3R)-237, a key intermediate in the synthesis of (−)-sw...

Scheme 60: Synthesis of a modified carbapenem 245. Reagents and conditions: a) AcOEt, LiHMDS, THF, −78 °C, 1.5...
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165


Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
- Guojuan Li,
- Chunying Fan,
- Guo Cheng,
- Wanhua Wu and
- Cheng Yang
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- toluene (40 mL), tetrahydrofuran (60 mL) and water (10 mL). After bubbling argon through the mixture for 15 min, Pd(PPh3)4 (300 mg, 0.3 mmol) was added, and the mixture was stirred and refluxed for 8 h. Then, the reaction mixture was extracted with dichloromethane, and the organic layer was dried over

Graphical Abstract

Scheme 1: Preparation of A1/A2-difunctionalized pillar[5]arenes (P5A-DPA and P5A-Py) by click reactions. Reag...

Figure 1: UV–vis absorption (a) and fluorescence emission spectra (b) of Py-6, P5A-Py (λex = 420 nm) and DPA-6...

Figure 2: (a) Fluorescence decay curves of Py-6 and P5A-Py at 450 nm and (b) fluorescence decay curves of DPA...

Figure 3: (a) Chiral HPLC traces of P5A-DPA, (b), (c) the first and second fractions of P5A-DPA, detected by ...

Figure 4: (a) CD, (b) UV–vis and (c) fluorescence spectra of the RP5A-DPA (20 μM) in THF and THF/H2O solvent ...
Transient and intermediate carbocations in ruthenium tetroxide oxidation of saturated rings
- Manuel Pedrón,
- Laura Legnani,
- Maria-Assunta Chiacchio,
- Pierluigi Caramella,
- Tomás Tejero and
- Pedro Merino
Beilstein J. Org. Chem. 2019, 15, 1552–1562, doi:10.3762/bjoc.15.158

- tetroxide-mediated oxidation of cyclopentane, tetrahydrofuran, tetrahydrothiophene and N-substituted pyrrolidines has been studied computationally by DFT and topological (analysis of the electron localization function, ELF) methods. In agreement with experimental observations and previous DFT calculations
- oxidation of cyclopentane, tetrahydrofuran, tetrahydrothiophene, and N-methyl- and N-benzylpyrrolidine to evaluate the extension in which transient carbocations can be formed (and whether they can become energy minima) during the rate-limiting step (Scheme 3). The RuO4 oxidation of cyclopentane [44] and
- tetrahydrofuran [45] have been experimentally reported as well as the oxidation of N-acylpyrrolidines to the corresponding lactams [46]. Admittedly, the oxidation of tetrahydrothiophene has been approached only computationally since in that case the sulfur atom would be more easily oxidized. Since the general

Graphical Abstract

Scheme 1: Oxidation of alkanes with RuO4.

Scheme 2: Mechanisms for RuO4 oxidation of alkanes.

Scheme 3: Oxidation of saturated five-membered (hetero)cyclic compounds.

Scheme 4: Rate-limiting step for the oxidation of cyclopentane (R1), tetrahydrofuran (R2) and tetrahydrothiop...

Figure 1: Optimized (B3LYP-d3bj/Def2SVP/cpcm=MeCN) geometries of transition structures corresponding to the o...

Figure 2: ELF analysis for the oxidation of cyclopentane (R1). Left: evolution of the electron population alo...

Figure 3: ELF analysis for the oxidation of tetrahydrofuran (R2, A) and tetrahydrothiophene (R3, B). Left: ev...

Figure 4: ELF assignment of electrons to the Ru environment. C(Ru) corresponds to a monosynaptic core basin a...

Scheme 5: Rate-limiting step for the oxidation of N-methyl- and N-benzylpyrrolidines R4 and R5, respectively.

Figure 5: Energy profile for the oxidation of R4 and R5. Relative energies, calculated at the B3LYP-d3bj/Def2...

Figure 6: Optimized (B3LYP-d3bj/Def2SVP/cpcm=water) transition structures for the oxidation of R4 and R5.
A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: a mild, catalyst-free approach towards O-thiocarbamates and dithiocarbamates
- András György Németh,
- György Miklós Keserű and
- Péter Ábrányi-Balogh
Beilstein J. Org. Chem. 2019, 15, 1523–1533, doi:10.3762/bjoc.15.155
- and HPLC–MS. Based on preliminary experiments in our laboratory, the reaction was performed in tetrahydrofuran (THF) at 40 °C for 1 h using a 1.5 equiv excess of sodium hydride as the base, S8 and the alcohol component (Table 1, entry 1) resulting in the desired thiocarbamate 3a in 58% yield. During

Graphical Abstract

Scheme 1: Synthetic routes to O-thiocarbamates and dithiocarbamates.

Scheme 2: Substrate scope of isocyanides. aReaction conditions: 1 (1 mmol), S8 (2 mmol), 2a (2mmol), NaH (2 m...

Scheme 3: Substrate scope of alcohols. Reaction conditions: 1a (1 mmol), S8 (2 mmol), 2 (2mmol), NaH (2 mmol)...

Scheme 4: Substrate scope of thiols. Reaction conditions: 1a (1 mmol), S8 (1.2 mmol), 4 (2 mmol), NaOH (2 mmo...

Scheme 5: Scaled-up synthesis for 3a.

Scheme 6: Multicomponent domino synthesis of quinazolinone 7.

Scheme 7: Control experiments.

Scheme 8: Proposed mechanism.






