Search results
Search for "asymmetric reactions" in Full Text gives 62 result(s) in Beilstein Journal of Organic Chemistry.
Visible-light-driven NHC and organophotoredox dual catalysis for the synthesis of carbonyl compounds
Beilstein J. Org. Chem. 2025, 21, 2584–2603, doi:10.3762/bjoc.21.200
- asymmetric reactions and are functional transformations in synthetic organic chemistry. Especially, 1,2,3-triazole-based NHCs are generally more reactive and stronger σ-donors than imidazole or thiazole analogues. Triazolium NHC enhances their ability to stabilize reactive radical intermediates or acyl anion
Catalytic enantioselective synthesis of selenium-containing atropisomers via C–Se bond formations
Beilstein J. Org. Chem. 2025, 21, 2447–2455, doi:10.3762/bjoc.21.186
- participate in various types of asymmetric reactions, significantly improving the selectivity of reactions (Figure 1) [10][11][12][13][14]. Catalytic asymmetric synthesis is the main method to construct chiral organic selenium-containing compounds. Centrally chiral selenium-containing compounds can be
Measuring the stereogenic remoteness in non-central chirality: a stereocontrol connectivity index for asymmetric reactions
Beilstein J. Org. Chem. 2025, 21, 1995–2006, doi:10.3762/bjoc.21.155
- remained an intuitive and empirical practice, particularly for reactions that create non-central chirality. We put forward a stereocontrol connectivity index to parameterize asymmetric reactions according to the bond connectivity relationships between the prochiral stereogenic elements, the reactive sites
- chiral molecules. Keywords: asymmetric reactions; axial chirality; catalysis; planar chirality; stereocontrol; Introduction Chirality is a ubiquitous and fundamental phenomenon in nature and thus holds an irreplaceable position in organic synthesis. At its most rudimental definition, chirality in a
- parameterization of asymmetric reactions remains unavailable, and the remoteness of stereocontrol for reactions that establish non-central chirality is judged based on empirical chemical intuition. While developing catalytic methods to establish remote stereogenic elements, we became increasingly interested in
Enantioselective desymmetrization strategy of prochiral 1,3-diols in natural product synthesis
Beilstein J. Org. Chem. 2025, 21, 1932–1963, doi:10.3762/bjoc.21.151
- reactions, chemists have developed a series of catalysts composed of transition-metal cores and chiral ligands, which have been applied to various asymmetric reactions [50][51][52]. Compared to the enzymatic methods, the transition-metal-catalyzed approach may provide an advantage to access both enantiomers
Chiral phosphoric acid-catalyzed asymmetric synthesis of helically chiral, planarly chiral and inherently chiral molecules
Beilstein J. Org. Chem. 2025, 21, 1864–1889, doi:10.3762/bjoc.21.145

- effects of the CPAs, which establish a chiral microenvironment within the chiral scaffold that governs the stereoselectivity of asymmetric reactions. Chiral molecules, characterized as three-dimensional structures that are nonsuperimposable with their mirror image, have significant applications in
- potential applications in catalytic asymmetric reactions have also been showcased. Planar chirality Planarly chiral cyclophanes, a unique class of macrocyclic compounds featuring planar chirality, can be found in various natural products and are widely utilized in asymmetric catalysis, host–guest chemistry
- novel inherently chiral ligands have been explored. For example, they demonstrated excellent enantioselectivity control in some asymmetric reactions, such as the Rh/diphosphine ligand 66-catalyzed asymmetric addition reaction between cyclic enone and arylboronic acid. In 2024, our group reported the
Synthesis of chiral cyclohexane-linked bisimidazolines
Beilstein J. Org. Chem. 2025, 21, 1786–1790, doi:10.3762/bjoc.21.140
- bisoxazoline ligands, such as anthracene-1,8-linked bisoxazolines (AnBOX) [18][19][20] showed excellent enantioselectivities for certain substrates due to their ability to fix transition states in asymmetric reactions, realizing excellent stereoselectivities. However, they also presented some limitations to
- the substrate scope due to their complete rigidity. Cyclohexane-1,2-linked bisoxazolines (cHBOX) are a class of bisoxazoline ligands with the more flexible cyclohexane as linker [21][22]. Chiral cyclohexane-1,2-linked bisoxazolines fix transition states in catalytic asymmetric reactions, in whch the
- bisimidazoline ligands 5 on the stereocontrol in catalytic asymmetric reactions (Table 1). To improve the synthetic efficiency, a different strategy for the synthesis of a nonsulfonylated cyclohexane-linked bisimidazoline with subsequent sulfonylation with different sulfonyl chlorides was also considered and
Unique halogen–π association detected in single crystals of C–N atropisomeric N-(2-halophenyl)quinolin-2-one derivatives and the thione analogue
Beilstein J. Org. Chem. 2025, 21, 1748–1756, doi:10.3762/bjoc.21.138

- ; single crystals; thiones; Introduction In the past several years, C–N atropisomers (C–N axially chiral compounds) owing to the rotational restriction around a C–N single bond have received great attention as new target molecules for catalytic asymmetric reactions. Highly enantioselective syntheses of
Catalytic asymmetric reactions of isocyanides for constructing non-central chirality
Beilstein J. Org. Chem. 2025, 21, 1648–1660, doi:10.3762/bjoc.21.129
- ], insertion reactions [16][17][18], cycloaddition reactions (e.g., [4 + 1], [3 + 2]) [19][20], and others [21][22][23]. Particularly, isocyanides have been widely exploited toward the preparation of centrally chiral structures through transition-metal-catalyzed or organocatalytic asymmetric reactions [24][25
Pd-Catalyzed asymmetric allylic amination with isatin using a P,olefin-type chiral ligand with C–N bond axial chirality
Beilstein J. Org. Chem. 2025, 21, 1018–1023, doi:10.3762/bjoc.21.83
- few studies have reported the use of isatin as a nucleophile in asymmetric reactions [9][10][11]. On the other hand, it has been revealed that compounds in which the carbon bonded to the nitrogen atom of newly constructed N-substituted isatin becomes a chiral center exhibit pharmacological properties
- activity against Huh7.5-FGR-JC1-Rluc2A cells, which carry HCV gt 2a [13]. Therefore, developing asymmetric reactions that simultaneously form a carbon–nitrogen bond and construct a chiral center is of great importance. Although a relatively large number of asymmetric allylic amination reactions using
Asymmetric synthesis of β-amino cyanoesters with contiguous tetrasubstituted carbon centers by halogen-bonding catalysis with chiral halonium salt
Beilstein J. Org. Chem. 2025, 21, 547–555, doi:10.3762/bjoc.21.43
- developed chiral halonium salts and applied them to asymmetric reactions such as vinylogous Mannich reactions of cyanomethylcoumarins 6 with isatin-derived ketimines 7 [33][35] and 1,2-addition reaction of thiols to ketimine [34], which formed the corresponding products 8 in high yields with high to
Vinylogous functionalization of 4-alkylidene-5-aminopyrazoles with methyl trifluoropyruvates
Beilstein J. Org. Chem. 2025, 21, 533–540, doi:10.3762/bjoc.21.41
- diastereoselectivity at 50 °C (Table 1, entry 16). Finally, the addition of molecular sieves was evaluated (Table 1, entries 17 and 18) affording in both cases lower yields for the reaction product. We also attempted asymmetric reactions using chiral organocatalysts to achieve an enantioselective outcome; however, we
Recent advances in electrochemical copper catalysis for modern organic synthesis
Beilstein J. Org. Chem. 2025, 21, 155–178, doi:10.3762/bjoc.21.9

- for synthesizing complex molecules from alkenes, a readily available feedstock [65]. Particularly, transition-metal-catalyzed difunctionalization has recently been extensively investigated, and asymmetric reactions have been developed [66]. Many approaches rely on the addition of a radical species to
Computational design for enantioselective CO2 capture: asymmetric frustrated Lewis pairs in epoxide transformations
Beilstein J. Org. Chem. 2024, 20, 2668–2681, doi:10.3762/bjoc.20.224

- . In orange, the hydrogen atom that illustrates the stereochemistry inversion. The free energies are given in kcal·mol−1 Schemes of the different asymmetric reactions observed. Hydrogen capable of rotation is marked in orange, influencing the stereochemistry at the TS. Reaction between propylene oxide
Chiral bifunctional sulfide-catalyzed enantioselective bromolactonizations of α- and β-substituted 5-hexenoic acids
Beilstein J. Org. Chem. 2024, 20, 1794–1799, doi:10.3762/bjoc.20.158
- -hexenoic acids 2, β-substituted substrates 2j–n were submitted to the present catalytic system. As a result of these asymmetric reactions, β,β-dialkyl-δ-valerolactones 3j–k and β-spiro-δ-lactones 3l–n were obtained in good levels of enantioselectivity. The reaction of 2-allylbenzoic acid 2o as a related
Primary amine-catalyzed enantioselective 1,4-Michael addition reaction of pyrazolin-5-ones to α,β-unsaturated ketones
Beilstein J. Org. Chem. 2024, 20, 1518–1526, doi:10.3762/bjoc.20.136
- [10][11][12][13][14][15][16][17][18][19][20][21]. Among the developed organocatalyzed enantioselective 1,4-addition reactions of pyrazolin-5-ones, the catalytic asymmetric reactions of pyrazolin-5-ones with α,β-unsaturated ketones are comparatively less studied. In 2009, Zhao’s group were the first
Evaluation of the enantioselectivity of new chiral ligands based on imidazolidin-4-one derivatives
Beilstein J. Org. Chem. 2024, 20, 684–691, doi:10.3762/bjoc.20.62
- chiral metal complex catalyst but also as an enantioselective organocatalyst [17]. Accordingly, its application in enantioselective organocatalysis, particularly in asymmetric reactions through “enamine activation”, warrants further investigation. Results and Discussion The corresponding copper(II
- -tetrazole, which was successfully used in many asymmetric reactions via “enamine activation”, especially in asymmetric aldol reactions [20][21][22][23]. Moreover, compound IV was previously included in a study dealing with asymmetric cascade reactions (based on aldol reactions) of aldehydes with α-keto
Trifluoromethylated hydrazones and acylhydrazones as potent nitrogen-containing fluorinated building blocks
Beilstein J. Org. Chem. 2023, 19, 1741–1754, doi:10.3762/bjoc.19.127
- Brønsted acid-assisted Lewis base catalysis. Synthesis of CF3-pyrazoles and CF3-1,6-dihydropyridazines. Asymmetric reactions of trifluoromethylimines with organometallic reagents. Mannich-type reaction of trifluoroacetaldehyde hydrazones. Synthesis of trifluoromethylated hydrazonoyl halides. Early work of
One-pot double annulations to confer diastereoselective spirooxindolepyrrolothiazoles
Beilstein J. Org. Chem. 2022, 18, 1607–1616, doi:10.3762/bjoc.18.171
- ]. Pyrrolothiazole and spirooxindole moieties occupy exclusive positions as valuable source of natural products and therapeutic agents in organic synthesis and drug discovery [60][61][62][63][64][65][66][67][68]. We have developed a number of asymmetric reactions to construct spirooxindole-based scaffolds through
Visible-light-mediated copper photocatalysis for organic syntheses
Beilstein J. Org. Chem. 2021, 17, 2520–2542, doi:10.3762/bjoc.17.169
- with diverse chiral ligands to provide a chiral environment for asymmetric control. Despite the remarkable achievements in this field, copper-based catalytic asymmetric reactions still remain a challenging task because of the difficulty of stereocontrol of the highly reactive radical intermediates
Asymmetric organocatalyzed synthesis of coumarin derivatives
Beilstein J. Org. Chem. 2021, 17, 1952–1980, doi:10.3762/bjoc.17.128
- recently, the strategy via introducing secondary interactions for the design of the bifunctional catalysts achieved wide application in asymmetric reactions [74]. Wu et al. described a Mannich asymmetric addition of cyanocoumarins 39 to isatin imines 112 catalyzed by an amide-phosphonium salt 114. This
Stereoselective Biginelli-like reaction catalyzed by a chiral phosphoric acid bearing two hydroxy groups
Beilstein J. Org. Chem. 2020, 16, 1875–1880, doi:10.3762/bjoc.16.155
- expand the applicability of this kind of catalysts to other types of asymmetric reactions, which is underway in our laboratory. Experimental Materials and general methods: 1H and 13C NMR spectra were performed on a Varian Mercury VS 300 or Bruker Avance III 400. Optical rotations were measured on a PE
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- electrochemistry with photo-, organo-, bio- and metal catalysts, many new and unexpected asymmetric reactions will be developed resulting in the further development of catalysis and synthesis in general. General classification of asymmetric electroorganic reactions. Asymmetric reduction of 4-acetylpyridine using a
Chiral terpene auxiliaries V: Synthesis of new chiral γ-hydroxyphosphine oxides derived from α-pinene
Beilstein J. Org. Chem. 2019, 15, 2493–2499, doi:10.3762/bjoc.15.242
- research groups [1]. Compounds with a phosphorus atom attached to a stereogenic carbon center in acyclic and cyclic structures play an important role as chiral ligands in transition metal complexes [2]. They were applied to various catalytic asymmetric reactions [3][4], such as hydrogenations [3][4][5][6
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
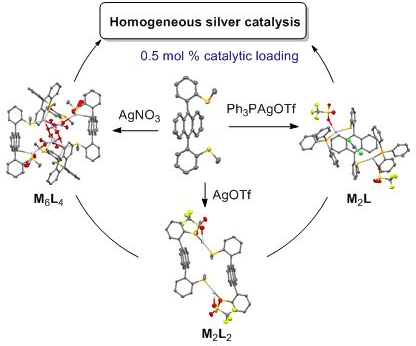
- catalysis has proved its effectiveness for numerous transformations involving unsaturated bond activation (allene, alkyne, alkene) [11][12][13][14][15][16][17], radical-based reactions [18][19][20] and several applications in asymmetric reactions [21][22]. This successful chemistry was usually conducted in
Ugi reaction-derived prolyl peptide catalysts grafted on the renewable polymer polyfurfuryl alcohol for applications in heterogeneous enamine catalysis
Beilstein J. Org. Chem. 2019, 15, 1210–1216, doi:10.3762/bjoc.15.118
- ) – derived from a renewable resource like sugar cane biomass – for the incorporation of chiral pyrrolidine-based motifs capable to catalyze relevant asymmetric reactions. The incorporation of an organocatalyst into a polymer support requires either conjugation to the polymer or functionalization with a




























































































































































































































































































































































































