Search results
Search for "Suzuki–Miyaura cross coupling" in Full Text gives 80 result(s) in Beilstein Journal of Organic Chemistry.
One-pot and metal-free synthesis of 3-arylated-4-nitrophenols via polyfunctionalized cyclohexanones from β-nitrostyrenes
Beilstein J. Org. Chem. 2020, 16, 1830–1836, doi:10.3762/bjoc.16.150
- group hinder the electrophilic modification at the 3-position. Generally, the aryl group is introduced by a Suzuki–Miyaura cross-coupling reaction [4][5], for which 3-bromo-4-nitrophenol must be prepared by the nitration of 3-bromophenol [6][7]. An alternative approach is the nitration of 3-arylphenol
Suzuki–Miyaura cross coupling is not an informative reaction to demonstrate the performance of new solvents
Beilstein J. Org. Chem. 2020, 16, 1001–1005, doi:10.3762/bjoc.16.89
- , equilibria, solubility, and ultimately product yield. If there is an observable change in reaction performance correlating to one or more solvent properties (often polarity), then it is possible to identify and implement an optimum solvent. Suzuki–Miyaura cross coupling is the premier method of palladium
- applicable) of Suzuki–Miyaura cross couplings [8]. Despite this, the reaction is generally tolerant of a wide range of solvents (often an ether or amide solvent is used, and water is a common co-solvent). This calls into question the benefits of using Suzuki–Miyaura cross coupling as a test of new solvents
- , regardless of how vital the reaction is. Three variations of the Suzuki–Miyaura cross-coupling protocol were performed. Each case study is a transformation of phenylboronic acid (1.2 molar equivalents) under different conditions (see Scheme 1), but all using 1 part water to 3 parts organic solvent (by volume
Direct borylation of terrylene and quaterrylene
Beilstein J. Org. Chem. 2020, 16, 621–627, doi:10.3762/bjoc.16.58
- hinderance of Bpin moieties. Finally, to demonstrate the utility of the borylated oligorylenes, the Suzuki–Miyaura cross-coupling reaction of TB4 under the standard conditions was performed (Scheme 3). Coupling of TB4 and 2-bromomesitylene with Pd(PPh3)4, Cs2CO3 and CsF in a mixture of toluene/DMF furnished
- -dioxaborolan-2-yl. Synthesis of 2,5,12,15-tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quaterrylene (QB4): (a) (Bpin)2 (12 equiv), [Ir(OMe)cod]2 (20 mol %), di-tert-butylbipyridyl (40 mol %), 1,4-dioxane, at 105 °C, 38 h, yield 0.4%. Suzuki–Miyaura cross-coupling reaction of TB4 with 2-bromomesitylene
Efficient synthesis of 3,6,13,16-tetrasubstituted-tetrabenzo[a,d,j,m]coronenes by selective C–H/C–O arylations of anthraquinone derivatives
Beilstein J. Org. Chem. 2020, 16, 544–550, doi:10.3762/bjoc.16.51
- reaction, followed by dehydrogenative photocyclization, and FeCl3-mediated oxidative cyclization (Scheme 2a) [43]. Tao, Chao, and co-workers succeeded in the preparation of similar tetrasubstituted tetrabenzocoronenes by a Corey–Fuchs reaction, followed by a Suzuki–Miyaura cross-coupling, and a two-step
Synthesis of 4-(2-fluorophenyl)-7-methoxycoumarin: experimental and computational evidence for intramolecular and intermolecular C–F···H–C bonds
Beilstein J. Org. Chem. 2020, 16, 190–199, doi:10.3762/bjoc.16.22
- , numerous methods for the synthesis of these compounds have been developed, examples are the Pechmann condensation [10][11], Stille coupling reaction [12], Knoevenagel condensation [13], Heck coupling reaction [14], Kostanecki reaction, Baylis–Hillman reaction [15], Michael reaction [16], Suzuki–Miyaura
- cross-coupling reaction [17], Negishi cross-coupling reaction [18] and Wittig reaction [17]. The concept of the incorporation of fluorine into organic molecules has gained much interest since Fried and Sabo reported the improvement of the therapeutic index of cortisol by the incorporation of a fluorine
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein J. Org. Chem. 2019, 15, 2830–2839, doi:10.3762/bjoc.15.276
- , Québec, QC, G1V 4G2, Canada Leibniz-Institut für Katalyse an der Universität Rostock e.V., Albert Einstein Str. 29a, 18059 Rostock, Germany 10.3762/bjoc.15.276 Abstract A two-step palladium-catalyzed procedure based on Suzuki–Miyaura cross coupling, followed by a double Buchwald–Hartwig reaction, allows
An improved, scalable synthesis of Notum inhibitor LP-922056 using 1-chloro-1,2-benziodoxol-3-one as a superior electrophilic chlorinating agent
Beilstein J. Org. Chem. 2019, 15, 2790–2797, doi:10.3762/bjoc.15.271
- biology of Notum and build target validation to underpin new drug discovery programs. Results: An improved, scalable synthesis of 1 is reported. Key modifications include: (1) the introduction of the C7-cyclopropyl group was most effectively achieved with a Suzuki–Miyaura cross-coupling reaction with MIDA
- group was most effectively achieved with a Suzuki–Miyaura cross-coupling reaction with MIDA-boronate 11 (5 → 6); and (2) C6 chlorination was performed with 1-chloro-1,2-benziodoxol-3-one (12) (6 → 7) as a mild selective electrophilic chlorination agent. 4-Chlorothieno[3,2-d]pyrimidine (3) was either
- efficient step in our sequence and justified further optimisation (vide infra). Suzuki–Miyaura cross coupling of bromide 5 with cyclopropylboronic acid (2.5 equiv) produced 6 in good yield (62–89%) but the product required extensive chromatographic purification. We reasoned that switching from the boronic
Progress in metathesis chemistry
Beilstein J. Org. Chem. 2019, 15, 2765–2766, doi:10.3762/bjoc.15.267

- catalysts work and decompose, how macrocycles are formed in ring-closing metathesis, etc. Representative examples of these directions have been the subject of the current, third thematic issue on Olefin Metathesis, including highly educative reviews on tandem olefin metathesis–Suzuki–Miyaura cross coupling
A new approach to silicon rhodamines by Suzuki–Miyaura coupling – scope and limitations
Beilstein J. Org. Chem. 2019, 15, 2569–2576, doi:10.3762/bjoc.15.250

- , we wanted to investigate if these dyes are also accessible by Suzuki–Miyaura coupling. Results and Discussion Optimization of reaction conditions At first we investigated the effects of different catalysts and boron compounds on the synthesis of silicon rhodamine 22 via Suzuki–Miyaura cross coupling
- - [27][31][32][33] substituted silicon rhodamines are already known, we investigated the synthesis of these dyes by Suzuki–Miyaura cross coupling. Firstly, pyridinylboronic acid 29a was used as a substrate after heating at 110 °C, but no conversion was observed presumably due to the formation of an
- reactions). Conclusion Since just three literature examples are known to date in which Suzuki–Miyaura cross-coupling reactions gave access to silicon rhodamines in poor to moderate yields (Scheme 2), we wanted to improve these first valuable experimental results. In general, the amount of re-isolated
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
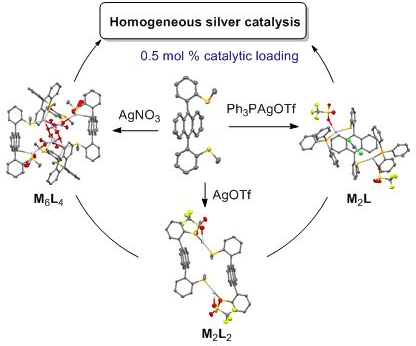
- reactions of alkynes. Results and Discussion Synthesis of silver(I) complexes Ligand 1 was synthesized in one step, from commercially available 9,10-dibromoanthracene and 2-(methylthio)phenylboronic acid, using a Suzuki–Miyaura cross-coupling reaction. Notably, the yield was low (26%) [55], and the X-ray
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- -Tyr motif has been devised. This approach comprises two key steps. The first one involves the cyclization of a linear peptidyl resin containing the corresponding halo- and boronoamino acids via a microwave-assisted Suzuki–Miyaura cross coupling. This step is followed by the macrolactamization of the
- macrolactamization and the intramolecular Suzuki–Miyaura cross coupling. Another crucial issue is the selection of the anchoring point to the solid support. The glutamine residue placed at the southern hemisphere of 1 was chosen for this purpose. Thus, the synthesis of 1 would involve the preparation of the linear
- peptidyl resin 4 bearing a 4-iodo- and a 4-boronophenylalanine residue. The latter would be incorporated at the N-terminus of the peptide sequence which would avoid the decomposition of the boronic ester during the coupling steps [26]. The intramolecular Suzuki–Miyaura cross coupling of 4 followed by
Calixazulenes: azulene-based calixarene analogues – an overview and recent supramolecular complexation studies
Beilstein J. Org. Chem. 2018, 14, 2488–2494, doi:10.3762/bjoc.14.225

- “OPC4A”, with several electron-deficient tetraalkyammonium salts. As a result of more recent methods developed by us and others employing Suzuki–Miyaura cross-coupling reactions to produce additional functionalized azulenes, the promise of further greater functionalized calixazulenes lies in store to be
Synergistic approach to polycycles through Suzuki–Miyaura cross coupling and metathesis as key steps
Beilstein J. Org. Chem. 2018, 14, 2468–2481, doi:10.3762/bjoc.14.223
- with Suzuki–Miyaura cross coupling in combination with metathesis (or vice-versa). Several cyclophanes, polycycles, macrocycles, spirocycles, stilbenes, biaryls, and heterocycles have been synthesized by employing a combination of Suzuki cross-coupling and metathesis. Various popular reactions such as
- ; metathesis; polycycles; Suzuki–Miyaura cross coupling; Introduction Transition-metal catalysts are used in metathesis and cross-coupling reactions. Such advances have opened the door for efficient construction of C–C bonds in organic synthesis. These catalysts tolerate diverse functional groups and the
Some mechanistic aspects regarding the Suzuki–Miyaura reaction between selected ortho-substituted phenylboronic acids and 3,4,5-tribromo-2,6-dimethylpyridine
Beilstein J. Org. Chem. 2018, 14, 2384–2393, doi:10.3762/bjoc.14.214
- atropselective Suzuki–Miyaura cross-coupling reaction on 3,4,5-tribromo-2,6-dimethylpyridine was studied. Results: Reactions with various amounts of ortho-substituted phenylboronic acids with 3,4,5-tribromo-2,6-dimethylpyridine gave a series of mono- di- and triarylpyridine derivatives which allowed to draw
- presence of a chiral solvating agent. Conclusion: This regio- and atropselectivity may be generally applicable to other arylpyridine systems. A regio- and atropselective Suzuki–Miyaura cross-coupling process has been observed, giving an efficient access to a class of atropisomeric compounds. An opposite
- [10][11][12][13]. In recent years, many successful attempts to regioselective [14][15][16][17], chemoselective [18][19][20][21] or atropselective [1][22][23][24][25][26] synthesis of biaryls were presented, often taking advantage of the popular and useful Suzuki–Miyaura cross-coupling reaction
Water-soluble SNS cationic palladium(II) complexes and their Suzuki–Miyaura cross-coupling reactions in aqueous medium
Beilstein J. Org. Chem. 2018, 14, 1859–1870, doi:10.3762/bjoc.14.160
- investigation of their catalytic activity in the aqueous Suzuki–Miyaura coupling reaction. Herein, we report the synthesis of the SNS Pd(II) pincer complexes and their interesting catalytic activities in the Suzuki–Miyaura cross coupling reactions in neat water. Results and Discussion Our study commenced with
- -donating substituents, in the coupling reaction. Examples of reported SCS palladium(II) pincer complexes 1–13. a) Reported SNS palladium(II) pincer complexes 14–16 as catalysts for Suzuki–Miyaura cross coupling [34]; b) Proposed SNS palladium(II) pincer complexes 17. Molecular structure of 17d. Selected
- reaction involving 4-bromoanisole and Pd(II) catalyst precursor 17d. The change in energy for 17d was calculated by computing it in kcal/mol as a cation at 298.15 K and 1 atm. Reusability of pincer complex 17d as a catalyst for the Suzuki–Miyaura cross coupling reaction. Synthesis of pincer ligands 19a–d
Recent applications of chiral calixarenes in asymmetric catalysis
Beilstein J. Org. Chem. 2018, 14, 1389–1412, doi:10.3762/bjoc.14.117

- reaction in the following order: phase-transfer catalysis, Henry reaction, Suzuki–Miyaura cross-coupling and Tsuji–Trost allylic substitution, hydrogenation, Michael addition, aldol and multicomponent Biginelli reactions, epoxidation, Meerwein−Ponndorf−Verley reduction, aza-Diels−Alder and epoxide ring
- . The low enantioselectivities obtained were mainly attributed to the high flexibility of catalytic amino groups of N,O-type enantiomers. Suzuki–Miyaura cross–coupling and Tsuji–Trost allylic substitution reaction Manoury et al. described the synthesis of ferrocene-bearing enantiomerically pure
- were tested in the palladium-catalyzed asymmetric Suzuki–Miyaura cross-coupling reaction for the first time in this study (Scheme 8). In order to see whether the calixarene backbone can effect the the coupling reaction between 1-naphthaleneboronic acid (30) and 1-bromo-2-methylnaphthalene (31), their
[3 + 2]-Cycloaddition reaction of sydnones with alkynes
Beilstein J. Org. Chem. 2018, 14, 1317–1348, doi:10.3762/bjoc.14.113
- group can be easily substituted by an aryl group using a Suzuki–Miyaura cross-coupling reaction. In those cases when a trimethylsilyl group (R4) is also present, it can be removed by TBAF-mediated protodesilylation to give a 1,4,5-trisubstituted pyrazole. It is worth noting that the parent 4,4,5,5
Heterogeneous Pd catalysts as emulsifiers in Pickering emulsions for integrated multistep synthesis in flow chemistry
Beilstein J. Org. Chem. 2018, 14, 648–658, doi:10.3762/bjoc.14.52

- development of heterogeneous Pd catalysts that are ready to be used in combination with biocatalysts for catalytic cascade synthesis of active pharmaceutical ingredients (APIs). In particular, we focus on the application of the catalytic systems for Suzuki–Miyaura cross-coupling reactions, which is the key
- minimal leaching behaviour is demonstrated with various Suzuki–Miyaura cross-coupling reactions in batch as well as in continuous flow employing the so-called “plug & play reactor”. Finally, we demonstrate the use of these particles as the sole emulsifier of oil–water emulsions for a range of oils
- ) [31][32][33][34][35]. A preliminary scheme of the planned synthetic route is shown in Figure 1. As can be seen, the key step of our processes is the formation of the biaryl unit via a Suzuki–Miyaura cross-coupling reaction. To provide solid Pd catalysts with a high potential for the planned approaches
One-pot sequential synthesis of tetrasubstituted thiophenes via sulfur ylide-like intermediates
Beilstein J. Org. Chem. 2018, 14, 243–252, doi:10.3762/bjoc.14.16
- methods [44][45][46][47][48][49]. In general, Pd-catalyzed Suzuki–Miyaura cross-coupling reactions are the most popular synthetic strategy for aryl–aryl bond-forming reactions [50][51][52]. However, it has been reported that the Suzuki cross-coupling of nitrogen- and sulfur-containing heterocycles is more
Synthesis and spectroscopic properties of β-meso directly linked porphyrin–corrole hybrid compounds
Beilstein J. Org. Chem. 2018, 14, 187–193, doi:10.3762/bjoc.14.13
- -porphyrin, β-corrole-linked hybrid structure described a Suzuki–Miyaura cross-coupling reaction between a β-borylated corrole and meso-bromoporphyrins (Scheme 1D) [37]. Recently, we have successfully synthesized meso–meso and β-meso-linked imine-bridged porphyrin–corrole conjugates and investigated
The chemistry and biology of mycolactones
Beilstein J. Org. Chem. 2017, 13, 1596–1660, doi:10.3762/bjoc.13.159
Synthesis of the heterocyclic core of the D-series GE2270
Beilstein J. Org. Chem. 2017, 13, 1407–1412, doi:10.3762/bjoc.13.137
- heterocyclic core of the D-series thiopeptide antibiotic GE2270 was prepared. The synthetic strategy that combines direct C–H arylation, Borylation Suzuki–Miyaura cross-coupling (BSC) and Hantzsch thiazole synthesis methods proved to be highly effective regarding the fair 22% yield over 7 synthetic steps from
Nitration of 5,11-dihydroindolo[3,2-b]carbazoles and synthetic applications of their nitro-substituted derivatives
Beilstein J. Org. Chem. 2017, 13, 1396–1406, doi:10.3762/bjoc.13.136
- performed by using the Suzuki–Miyaura cross-coupling reaction with phenylboronic acid under Pd catalysis (Scheme 5). The location of the formyl group and bromine atoms in ICZ derivatives 12 and 13 has been established by X-ray crystallography analysis, performed for single crystals of 12b and 13b (Figure 5
Total synthesis of elansolids B1 and B2
Beilstein J. Org. Chem. 2017, 13, 1280–1287, doi:10.3762/bjoc.13.124
- . Results and Discussion The improved synthesis utilizes the Suzuki–Miyaura cross-coupling reaction to merge the western fragment derived from ketone 9 with the newly designed eastern building block 13. This fragment was obtained in very good yield from vinyl iodide 12 [9] by a Stille protocol using doubly
- elansolid B1 (2). The improvements are mainly associated with the preparation of the triene unit at C10–C15 by utilizing the Stille and the Suzuki–Miyaura cross-coupling reactions as well as the highly versatile difunctionalized building block 14. In principal, the synthesis sheds light on how such (Z,E,Z
- )-configured triene units are ideally be constructed, clearly demonstrating that enediynes are less preferred precursors for such structural elements. It has to be noted that there is precedence in the literature for the use of the Suzuki–Miyaura cross-coupling reaction as key step to assemble differently
Automating multistep flow synthesis: approach and challenges in integrating chemistry, machines and logic
Beilstein J. Org. Chem. 2017, 13, 960–987, doi:10.3762/bjoc.13.97

- kinetics parameters of a series-parallel substitution reaction [38]. Reizman et al. have studied Suzuki–Miyaura cross-coupling optimization using a DoE-based algorithm and feedback system [45]. The authors studied both continuous and discrete variables for optimization. Recently Fitzpatrick and Ley have


























































































































































































































































































































