Search results
Search for "π-stacking" in Full Text gives 182 result(s) in Beilstein Journal of Organic Chemistry.
Synthesis and investigation of quadruplex-DNA-binding, 9-O-substituted berberine derivatives
Beilstein J. Org. Chem. 2020, 16, 2795–2806, doi:10.3762/bjoc.16.230
- terminated with an N-benzylamide functionality to establish the attractive hydrogen bonding and π stacking with the thymidine residues in the loops in G4-DNA, so that this ligand binds with very high selectivity to the particular quadruplex-forming oligonucleotide J19 [49]. Overall, the above-mentioned
- -established for berberines [33][34][35][36][37][38][39][40][41][42]. Based on these general similarities between the derivatives 4a–e and the established quadruplex-binding berberine derivatives it is deduced that the berberine unit in 4a–e binds like the latter ones to the G4-DNA by terminal π-stacking
- contributed significantly to the binding affinity depending on their spacing from the π-stacking unit. In the case of 4a–e, however, the position of the triazole and adenine unit relative to the berberine does not appear to be highly relevant for the overall binding affinity. It may be concluded that the
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- with a typical π-stacking distance of 3.58 Å and a tilt angle of 5.8°. The free electron pair of the tertiary amine points towards the inside of the crown ether. In contrast, single crystals of NDIC8 (Figure 2c), obtained by slow evaporation of a concentrated dimethylformamide (DMF) solution, exhibit a
NMR Spectroscopy of supramolecular chemistry on protein surfaces
Beilstein J. Org. Chem. 2020, 16, 2505–2522, doi:10.3762/bjoc.16.203

- best binding (KD = 0.6 mM) due to π-stacking between the phenyl side chain of the amino acid and the acylguanidinium unit of the GCP. Ac-Lys exhibits the least interaction (KD = 3 mM) due to electrostatic repulsion of the positively charged Lys side chain with the also positively charged GCP unit. For
Synthesis, docking study and biological evaluation of ᴅ-fructofuranosyl and ᴅ-tagatofuranosyl sulfones as potential inhibitors of the mycobacterial galactan synthesis targeting the galactofuranosyltransferase GlfT2
Beilstein J. Org. Chem. 2020, 16, 1853–1862, doi:10.3762/bjoc.16.152
- hydroxy group and the phenyl ring created a cation–π stacking interaction with the arginine R171 side chain (Figure 2). The observed theoretical binding energy in kcal/mol represented by the docking scores and the predicted binding affinities for the UDP-Galf, fructofuranose, and tagatofuranose compounds
Heterogeneous photocatalysis in flow chemical reactors
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125

- (PDI) aggregates have become a popular choice of heterogeneous photocatalyst. As PDI is a large, planar, polyaromatic hydrocarbon molecule, it spontaneously forms ordered 1D supramolecular assemblies through efficient π–π stacking and side chain interactions [192]. Despite the PDI units having only non
- -bonding interactions, the narrow π–π stacking provides a sufficient π orbital overlap to produce semiconductor-type electronic band structures and an efficient interplanar charge transport similar to g-C3N4 [192]. Duan and co-workers reported the synthesis of a zinc PDI assembly as a heterogeneous
- , first reported in 1834 by Liebig, which he named “melon” [119]. The material consists of two-dimensional sheets of hexatopic, hexagonal sp2-hybridised carbon and nitrogen atoms, linked by bridging tertiary amines (Figure 9) [120]. The material is crystalline as sheets are held together by strong π
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- 8b/7c and 8c/7c (Figure 4a,b and Supporting Information File 1, Appendix 6). All the observable π···π interactions were parallel-displaced (offset) π-stacking, and the distance values were within the range of the known values, whether determined from X-ray structures or calculated values (3.2–4.0 Å
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- [C11 to C16, centroid···centroid distance of 3.755(3) Å and shift distance of 1.593(5) Å] involved in a head-to-head slip plane π-stacking interaction with another triptycene molecule either side, while the third face ring iii of the triptycene exhibits C23–H23B···π interactions between the isopropyl
- molecule. In this structure, it should be noted that there is a high degree of π-stacking between the porphyrin macrocycle. This is highlighted in Figure 4; however, there is a clear preference for the homo-metallic selective interactions between the porphyrin rings. As such, in the crystal packing the Ni
- Supporting Information File 1). While this feature is an interesting take on the selective homo interactions, the explanation of this motif formation is potentially more mundane in this case. Considering that during the crystallization process, the spontaneous formation of the homo or hetero π-stacking of
Direct borylation of terrylene and quaterrylene
Beilstein J. Org. Chem. 2020, 16, 621–627, doi:10.3762/bjoc.16.58
- their intrinsic physical properties as a function of molecular length (Figure 1e) [19][20], while the low solubility of tert-butylpentarylene indicates that even such bulky group had reached its limit to interrupt π-stacking. The synthesis of higher oligorylenes would require more robust solubilizing
Synthesis of triphenylene-fused phosphole oxides via C–H functionalizations
Beilstein J. Org. Chem. 2020, 16, 524–529, doi:10.3762/bjoc.16.48
- packing of 8a did not involve columnar π–π stacking of the PAH moiety (Figure S1, Supporting Information File 1). This is likely due to the fact that such π-stacking is inhibited by the steric bulk of the phosphole substituents (i.e., the butyl groups, the phenyl group, and the oxygen atom). Upon the
Synthesis and circularly polarized luminescence properties of BINOL-derived bisbenzofuro[2,3-b:3’,2’-e]pyridines (BBZFPys)
Beilstein J. Org. Chem. 2020, 16, 325–336, doi:10.3762/bjoc.16.32
- . The crystal of 4b is classified into a space group P4322 (tetragonal) with a biaryl torsion angle of 74.4° (Figure 4b). A considerable intermolecular π–π stacking interaction was observed in between its polyaromatic fragments whose distance is approximately 3.44 Å. The polycyclic subunits overlap each
- other, being line-symmetrically aligned (Figure 5a). Meanwhile, the isomer 4c has two independent molecules in the unit cell, and the torsion angles are 67.1° and 106.8°, respectively (Figure 4d and 4f). As displayed in Figure 4b, the aromatic fragments are point-symmetrically overlapped with the π–π
- stacking distance of around 3.45 Å. It is noteworthy that both 4b and 4c pile up while minimizing the steric repulsion between the tert-butyl groups which occupy “staggered” orientations in their crystal structures (Figure 5c and 5d). Unfortunately, the crystal structure of 4a was not determined after
Synthesis and herbicidal activities of aryloxyacetic acid derivatives as HPPD inhibitors
Beilstein J. Org. Chem. 2020, 16, 233–247, doi:10.3762/bjoc.16.25
- -dicarbonyl bidentate chelation with the active center metal, and 2) through favorable sandwich π–π stacking interactions between aromatic rings and the Phe360, Phe403 residues of the active site. Thus, 1,3-dicarbonyl and aromatic moieties are indispensable pharmacophores for potent HPPD-inhibiting compounds
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20

- , intensity, resolution and peaks of some phase transitions are lost. Considering that molecules self-organize upon cooling, first by hydrogen bonds followed by π-stacking and van der Waals forces, DSC scanning at 10 °C/min is too fast to allow the molecules to self-assemble in a perfect 3D matrix. Even with
Reversible photoswitching of the DNA-binding properties of styrylquinolizinium derivatives through photochromic [2 + 2] cycloaddition and cycloreversion
Beilstein J. Org. Chem. 2020, 16, 111–124, doi:10.3762/bjoc.16.13
- aggregates in the solid state and even in solution through dipole–dipole interactions and directional π stacking (Scheme 2), as observed, for example, with donor-substituted benzoquinolizinium derivatives [79] or donor-substituted styrylpyridinium derivatives [80][81][82]. Hence, an ideal overlap of the π
Influence of the cis/trans configuration on the supramolecular aggregation of aryltriazoles
Beilstein J. Org. Chem. 2019, 15, 2881–2888, doi:10.3762/bjoc.15.282
- as between the triazole rings, and therefore the presence of multiple intermolecular π–π stacking and π–bromine [19] interactions. This compound precipitated in the presence of any amount of water and gelled only in DMSO at a low temperature. Compound 10 produced pseudo-crystals in DMSO/H2O (1:1, v/v
- ) and its X-ray analysis was not accurate enough to resolve its structure. However, its crystal packing (Figure 5) deserves discussion. As with compound 12, this packing revealed a number of π–bromine interactions [19], together with a high degree of π–π stacking interactions between the aromatic rings
- configuration through X-ray diffraction studies can be considered good approximations to the supramolecular structure of aryltriazoles in gels. Thus, these compounds showed a high degree of parallelism between the phenyltriazolyl rings, therefore revealing the presence of π–π stacking and π–bromine interactions
Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series
Beilstein J. Org. Chem. 2019, 15, 2655–2663, doi:10.3762/bjoc.15.258
- ) are narrowly clustered in a range of ca. 2 kcal/mol, with the lowest energy isomer predicted to be o,o'-quaterphenyl (17). This low ranking for the most congested isomer may be attributed to intramolecular π stacking. Very similar results were obtained with M06-2X/6-311+G(d,p) theory, which also
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- encapsulated two molecules of E-1 (in agreement with the NMR spectra), arranged in an antiparallel fashion (Figure 3). The binding of E-1 within 2 is driven by a combination of π–π stacking, van der Waals forces, and C–H···π interactions (vide infra); in addition, the encapsulation is likely facilitated by the
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- interactions such as hydrogen bonding, π–π stacking, electrostatic interactions, van der Waals forces, hydrophobic/solvatophobic effects and coordination bonds [2][3]. Advances in supramolecules from molecular to macroscopic size with pre-structured or functionalized receptors and multivalent binding positions
- macrocycle has been reported by Nakamura and his co-workers containing three 1,2,3-triazolium and carbazole moieties 12 (Figure 10). π–π stacking interactions and the self-association ability of the synthesized macrocycle are increased in comparison with its neutral analogue due to the decreased electron
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197

- , connected by C–H···F contacts. The azobenzenes A2 interact by lamellar 2D π-stacking, anthracene U1 interact predominantly by C–H···π interactions as both the solubilizing mesitylene group and the two perpendicular lutidine acceptors effectively prevent stacking of the anthracenes body (Supporting
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- broadened peaks, which indicated that π–π stacking interactions between the bipyridinium unit of G4 and the benzene ring of the hosts might exist. The signals for protons 2 and 13 of H4 showed a downfield shift with broadened signals due to deshielding effect, while the signals for protons 11 and 12 showed
- protons of G1 and the aromatic rings of H1 with distances of 2.683 for A, 2.845 for B, 2.788 for C, 2.802 for D, and 2.868 Å for E, respectively. There also exist π–π stacking interactions between the pyridinium of G1 and the aromatic ring of H1 with the distance of 3.854 Å for F, a CH···O hydrogen bond
Functional panchromatic BODIPY dyes with near-infrared absorption: design, synthesis, characterization and use in dye-sensitized solar cells
Beilstein J. Org. Chem. 2019, 15, 1758–1768, doi:10.3762/bjoc.15.169

- BOD-TTPA is observed from diluted solution to the anchored dye (see Figure 5a). Despite the same amount of CDCA in the dyeing solution (10 molar equivalents), this result suggests a higher tendency for π stacking interactions. Once anchored, BOD-TTPA exhibits consequently a broader absorption profile
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
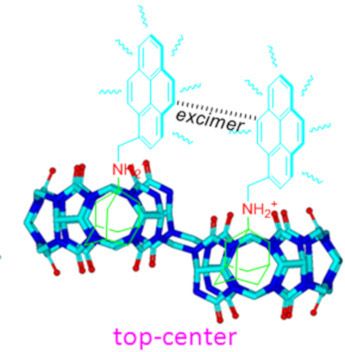
- encapsulation of this moiety. Pyrene group, that are too large for the individual CB[6]–CB[7] sized cavities of ns-CB[10] [23], it is thus believed that the upfield shift of the pyrene protons is attributed the intermolecular π–π stacking between the two pyrenyl moieties as proposed in the plausible inclusion
- , the fluorescence intensity of the G2 monomer emissions gradually decreased, while the maximum emission intensity at around 485 nm (typical excimer emissions of pyrene) increased. The excimer emission band of G2 can be attributed to the interaction of two pyrene units resulting in intermolecular π–π
- stacking, which was due to the two identical cavities of host-1. Consequently, the top–center isomerism of the G2·host-1 ternary complexes was conveniently confirmed from the optical signal after the changes in monomer/excimer fluorescence emissions of the pyrene groups on G2. Surprisingly, the
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- quantum yield was significantly decreased to 46.4% compared with 78.2% for Py-6. We ascribed the decreased fluorescence of P5A-Py to the π–π stacking of the Py units caused by the high local concentration of perylene. For P5A-DPA, which also bears two DPA units in one macrocyclic host, the fluorescent
- quantum yield was only slightly decreased. This should be mainly due to the steric hindrance of the 9- and 10-phenyl groups, which inhibited the π–π stacking of the anthracene core in DPA. The fluorescent lifetimes of P5A-Py and P5A-DPA were compared with Py-6 and DPA-6. As shown in Figure 2, the lifetime
- of P5A-Py is 3.4 ns which is shorter than that of Py-6 (4.4 ns), demonstrating that grafting two Py units in close proximity in one host, opened a new route for nonradiative decay. This phenomenon further verified the occurrence of π–π stacking of the Py fragments in P5A-Py. The lifetime of P5A-DPA
A heteroditopic macrocycle as organocatalytic nanoreactor for pyrroloacridinone synthesis in water
Beilstein J. Org. Chem. 2019, 15, 1505–1514, doi:10.3762/bjoc.15.152
- of macrocycles with multiple functional groups has been employed as alternative templates to induce the organic environment for catalysis via multiple weak interactions viz. π–π stacking, hydrogen bonding, etc. [25][26][27][28][29]. At the same time, several efforts have been made to develop
- aggregation tendency. Herein we have extensively explored a multifunctional macrocycle (BATA-MC), comprising bis-amide and tris-amine functionalities as H-bond donor/acceptor moieties, and parallel benzene moieties for aromatic π–π stacking interactions as an organocatalytic nanoreactor for organic
- observed an ESIMS peak at m/z 679.32 corresponding to [BATA-MC + 1a + H]+ (Supporting Information File 1, Figure S2) which supported the association of the macrocycle with isatin. Hence, further condensation between enaminoketone and isatin provides the intermediate B. The isatin moiety also has π-stacking
Selective detection of DABCO using a supramolecular interconversion as fluorescence reporter
Beilstein J. Org. Chem. 2019, 15, 1371–1378, doi:10.3762/bjoc.15.137
- repulsion. For instance, pyrazine creates notable repulsive interactions of the α-H towards the zinc porphyrin ring in a sandwich complex. Apparently, stability gains through π–π stacking in the sandwich with pyrene, coronene, etc. are not strong enough to compensate for the strain in [Cu2(1)(2)(guest)]2
Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium
Beilstein J. Org. Chem. 2019, 15, 1203–1209, doi:10.3762/bjoc.15.117

- through hydrogen bonding and metal ion coordination in nonpolar solvents [66][67][68]. Compared with NDI, PDI has more aromatic rings to generate stronger intermolecular π−π stacking, leading to molecular aggregates more easily, and these aggregates or supramolecular assemblies can give rise to desirable
- in the above-tested five solvent systems were also recorded. Because of the relatively weaker π–π stacking effect of the NDI units comparing with PDI units, 2 has a better solubility in the organic solvents. Consequently, the UV–vis absorption bands of 2 in all the organic solvents can be clearly
- were investigated. As shown in Figure 2a, micelle-like assemblies of 1 were observed, while, because of the higher polarity of MeCN, the aggregation of 1 through π–π stacking was considerably enhanced forming linear assemblies (Figure 2b). Interestingly, when imposed to an aqueous medium through adding



















































































































































































































































































