Search results
Search for "cycloaddition" in Full Text gives 633 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Attempted synthesis of a meta-metalated calix[4]arene
Beilstein J. Org. Chem. 2019, 15, 1996–2002, doi:10.3762/bjoc.15.195
- Ullmann-type coupling to give aryl azide 2, which readily reacted with phenylacetylene in a copper-catalyzed Huisgen 1,3-dipolar cycloaddition to give 1,2,3-triazole 3 (Scheme 1). The formation of the ruthenacycle was then achieved using Albrecht’s method involving regioselective methylation of triazole 3
- cycloaddition between monoazidocalix[4]arene 7 and phenylacetylene, affording monotriazolocalix[4]arene 11 in 87% yield (Scheme 4). Methylation of the triazole was also achieved using excess iodomethane in a rather protracted reaction, to yield the calix[4]arene triazolium iodide salt 12 in effectively
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- reactions and a newly developed deoxygenative aromatization procedure. The first enantioselective Diels–Alder reaction, which is an intermolecular cycloaddition and lactonization between (Z)-3-iodo-4-methylpenta-2,4-dien-1-ol (29) and methyl acrylate (30) in the presence of Mikami’s (binol)TiCl2 catalyst to
Analysis of sesquiterpene hydrocarbons in grape berry exocarp (Vitis vinifera L.) using in vivo-labeling and comprehensive two-dimensional gas chromatography–mass spectrometry (GC×GC–MS)
Beilstein J. Org. Chem. 2019, 15, 1945–1961, doi:10.3762/bjoc.15.190
- photoinduced [2 + 2]-cycloaddition is very unlikely. The biosynthesis of β-bourbonene can be performed by cationic cyclization, as shown in Scheme 4. A total of 5 bicyclic, aromatic sesquiterpene hydrocarbons (21–25) with a cadinane skeleton were found in exocarp of the grape variety Lemberger, whose identity
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
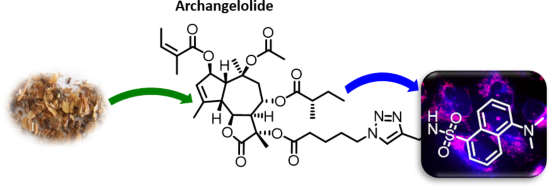
- studied by us and others, there are only scarce reports on the biological activity of archangelolide. Here we present the preparation of its fluorescent derivative based on a dansyl moiety using azide–alkyne Huisgen cycloaddition having obtained the two sesquiterpene lactones from the seeds of Laserpitium
- these compounds for further work including synthetic modifications using azide–alkyne Huisgen cycloaddition. We previously showed the preparation of fluorescent trilobolide conjugates [8] that retained the activity of the parental compounds and proved to be useful for live-cell imaging. In this article
Application of chiral 2-isoxazoline for the synthesis of syn-1,3-diol analogs
Beilstein J. Org. Chem. 2019, 15, 1840–1847, doi:10.3762/bjoc.15.179
- Juanjuan Feng Tianyu Li Jiaxin Zhang Peng Jiao Key Laboratory of Radiopharmaceuticals, College of Chemistry, Beijing Normal University, Beijing 100875, P.R. China 10.3762/bjoc.15.179 Abstract The asymmetric cycloaddition of TIPS nitronate catalyzed by “Cu(II)-bisoxazoline” gave the 2-isoxazoline
- product in 95% yield, which was converted into tert-butyl (3S,5R)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate in 14 steps through a β-hydroxy ketone. Keywords: β-hydroxy ketone; cycloaddition; 1,3-diol; isoxazoline; silyl nitronate; Introduction The chiral 1,3-diol structure is widespread in a
- our continuous efforts in asymmetric syntheses and applications of chiral 2-isoxazolines [49][50][51]. Results and Discussion Our synthesis commenced with a chiral 3,5-disubstituted-2-isoxazoline 3 or 4, which were prepared from silyl nitronate through an asymmetric 1,3-dipolar cycloaddition developed
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- azide–alkyne cycloaddtion (CBAAC) with the anthracene-derived propagylamine stopper 2. In CBAAC, the strong ammonium–CB[6] binding places the alkyne and azide in a close proximity inside the CB[6] cavity and facilitates the cycloaddition [40][41][42]. Since CB[6] binding is required for the covalent
Norbornadiene-functionalized triazatriangulenium and trioxatriangulenium platforms
Beilstein J. Org. Chem. 2019, 15, 1815–1821, doi:10.3762/bjoc.15.175

- (NBD) and quadricyclanes (QC) attached to TATA and TOTA platforms which can be used to check if these accelerated rates and the spin change mechanism also apply to [2 + 2] cycloreversions (QC→NBD). Keywords: [2 + 2] cycloaddition; [2 + 2] cycloreversion; norbornadiene; photochemical isomerization
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- four stereoisomers with low (ca. 3:1) diastereoselectivity and in a non-enantioselective manner (Scheme 46) [21]. Undoubtedly, it was the 1,3-dipolar cycloaddition of the azomethine ylide 180 with an electron-rich alkene. Fortunately, cycloadducts (2R,3R,4S)-, (2S,3S,4R)-, (2R,3S,4R)- and (2S,3R,4S
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- , inexpensive, water-tolerant Lewis acid catalyst in the formation of both carbon–carbon and carbon–heteroatom bonds, and thereby the formation of various biologically promising organic compounds [68]. Important advances in scandium-catalyzed chemistry include [4 + 2] and [2 + 2] cycloaddition reactions, Baeyer
- alcohols and asymmetric sulfide oxidation [91]. Diverse reactivity, cost efficiency and variable oxidation state [Ni(0)–Ni(IV)] associated with nickel led to remarkable developments in the field of catalytic applications [68]. Nickel catalysis involved cycloaddition, cyclization, C–H bond functionalization
Transient and intermediate carbocations in ruthenium tetroxide oxidation of saturated rings
Beilstein J. Org. Chem. 2019, 15, 1552–1562, doi:10.3762/bjoc.15.158

- , the rate-limiting step of the reaction takes place through a highly asynchronous (3 + 2) concerted cycloaddition through a single transition structure (one kinetic step). The ELF analysis identifies the reaction as a typical one-step-two-stages process and corroborates the existence of a transient
Synthesis of pyrrolo[1,2-a]quinolines by formal 1,3-dipolar cycloaddition reactions of quinolinium salts
Beilstein J. Org. Chem. 2019, 15, 1480–1484, doi:10.3762/bjoc.15.149
- with triethylamine promoted the reaction of the resulting quinolinium ylides (formally azomethine ylides) with electron-poor alkenes by conjugate addition followed by cyclization or by [3 + 2] dipolar cycloaddition. The pyrroloquinoline products were formed as single regio- and stereoisomers. These
- could be converted to other derivatives by Suzuki–Miyaura coupling, reduction or oxidation reactions. Keywords: azomethine ylide; cycloaddition; heterocycle; pyrrolidine; stereoselective; Introduction Cycloaddition reactions of azomethine ylides are an important class of pericyclic reactions that give
- rise to pyrrolidine rings, prevalent in a large number of natural products and bioactive compounds. Many methods have been used to prepare azomethine ylides that undergo cycloaddition with π-systems, especially electron-poor alkenes to give pyrrolidine products [1][2][3][4]. Azomethine ylides can be
Cyclobutane dication, (CH2)42+: a model for a two-electron four-center (2e-4c) Woodward–Hoffmann frozen transition state
Beilstein J. Org. Chem. 2019, 15, 1475–1479, doi:10.3762/bjoc.15.148
- the allowed cycloaddition of ethylene to ethylene dication. Electron delocalizations take place in the plane of the conjugated systems unlike cyclobutadiene dication (vi), where delocalization takes place through conventional p-type orbitals. GIAO-CCSD(T) derived 13C NMR chemical shifts of the
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- Collaborative Innovation Center of Chemical Science and Engineering, Tianjin 300072, China 10.3762/bjoc.15.143 Abstract Isoxazoline-linked porphyrins have been synthesized by a regioselective 1,3-dipolar cycloaddition reaction between vinylporphyrin 2 and nitrile oxides. The steric interaction directed the
- confirmed the basic features of the NMR-derived structure. Furthermore, a pair of enantiomers of 3a presented in the crystal, which formed a dimeric complex through intermolecular coordination between the Zn2+ center and the carbonyl group of the second molecule. Keywords: dipolar cycloaddition
- [15][16][17][18][19][20][21][22][23][24]. Among them, the 1,3-dipolar cycloaddition reaction [25] is an efficient method to fuse five-membered rings on the periphery of the porphyrin framework. Because the periphery double bonds of the porphyrin macrocycle are nice dipolarophiles, and can trap 1,3
Formation of an unexpected 3,3-diphenyl-3H-indazole through a facile intramolecular [2 + 3] cycloaddition of the diazo intermediate
Beilstein J. Org. Chem. 2019, 15, 1347–1354, doi:10.3762/bjoc.15.134
- a = 9.2107 (4), b = 10.0413 (5), c = 14.4363 (6) Å, α = 78.183 (2), β = 87.625 (2), γ = 71.975 (2)°. The formation of 8 proceeded through a facile intramolecular [2 + 3] cycloaddition of the diazo intermediate 9. The molecules of 8 are organised by edge–face Ar–H···π, face–face π···π, and bifurcated
- OCH2–H···N interactions. In addition to these, there are Ar–H···H–Ar close contacts, (edge–edge and surrounding inversion centres) arranged as infinite tapes along the a direction. Keywords: Ar-H...H-Ar contact; [2 + 3] cycloaddition; diazo; 3H-indazole; X-ray structure; Introduction The use of
- ]. More recently, the formation of cyclic 3,3-disubstituted 3H-indazoles was reported to form mainly through the [2 + 3] cycloaddition of diazo compounds with arynes under mild reaction conditions [29][30][31]. However, none of these contained a 2-diphenylmethyl (benzhydryl) aniline system, as found in
Synthesis of non-racemic 4-nitro-2-sulfonylbutan-1-ones via Ni(II)-catalyzed asymmetric Michael reaction of β-ketosulfones
Beilstein J. Org. Chem. 2019, 15, 1289–1297, doi:10.3762/bjoc.15.127
- cytotoxicity [9]. However, it is of great importance to obtain all stereoisomers for the study of biological activity. Therefore, the development of methods for the asymmetric synthesis of polyfunctional sulfones is valuable. The most notable of them are Ag- and Cu-catalyzed 1,3-dipolar cycloaddition reactions
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- stepwise Pd(II)-catalyzed cascade process or a [4 + 2] cycloaddition reaction, both providing the cis stereochemistry at C-2 and C-3. When the reaction was carried out with the epimer of substrate 33 (having the 5β-hydroxy group), the cis stereochemistry was also achieved at the newly formed C-2 and C-3
Alkylation of lithiated dimethyl tartrate acetonide with unactivated alkyl halides and application to an asymmetric synthesis of the 2,8-dioxabicyclo[3.2.1]octane core of squalestatins/zaragozic acids
Beilstein J. Org. Chem. 2019, 15, 1194–1202, doi:10.3762/bjoc.15.116
- –Stevens-derived diazoesters 23 and 27, respectively. Only triethylsilyl-protected diazoester 27 proved viable to deliver a diazoketone 28. The latter underwent stereoselective carbonyl ylide formation–cycloaddition with methyl glyoxylate and acid-catalysed rearrangement of the resulting cycloadduct 29, to
- ,S-configuration found in several monoalkylated tartaric acid motif-containing natural products. Keywords: alkylation; cycloaddition; diazoester; epimerisation; tartaric acid; Introduction Since their isolation was reported in the early 1990s [1][2], the squalestatins/zaragozic acids (e.g
- area have recently culminated in two communicated syntheses of 6,7-dideoxysqualestatin H5 (DDSQ (2), Figure 1) [12][13]. The centrepiece of both of these strategies is a rhodium(II)-catalysed tandem carbon ylide formation from a diazoketone 3 (Scheme 1) and stereoselective [3 + 2] cycloaddition with a
Synthesis of aryl cyclopropyl sulfides through copper-promoted S-cyclopropylation of thiophenols using cyclopropylboronic acid
Beilstein J. Org. Chem. 2019, 15, 1162–1171, doi:10.3762/bjoc.15.113
- diastereoselective [3 + 2] cycloaddition reaction [14][15]. The ring expansion sequence 1 → 2 → 6 → 8 has been used as a key step in the synthesis of (±)-fragranol [16], (±)-grandisol [16], (±)-α-cuparenone [17] and (±)-herbertene [17]. Aryl cycloropyl sulfides 1 are most frequently prepared by cyclopropylation of
Switchable selectivity in Pd-catalyzed [3 + 2] annulations of γ-oxy-2-cycloalkenones with 3-oxoglutarates: C–C/C–C vs C–C/O–C bond formation
Beilstein J. Org. Chem. 2019, 15, 1107–1115, doi:10.3762/bjoc.15.107
- cycloaddition to form dimer 6 [45]. We next turned our attention to the [3 + 2] C–C/C–C annulation by using the conditions B and C in DMSO at 130 °C (Scheme 4). The 2-cyclohexenone 4-benzoate (2a) afforded the expected bicyclo[4.3.0]nonane-3,8-dione (5a) in 69% yield (64% from 1.0 mmol of 1a) under either
Multicomponent reactions (MCRs): a useful access to the synthesis of benzo-fused γ-lactams
Beilstein J. Org. Chem. 2019, 15, 1065–1085, doi:10.3762/bjoc.15.104
- be isolated. Finally, cyclopropyl ketone 91 would first rearrange by copper catalysis and the so-obtained furane derivative 96 would add to the carbocation in 95, followed by Friedel–Crafts cyclization, thus generating the polycyclic isoindolinones 92 in a formal hetero [4 + 2] cycloaddition process
- . This is a good partner for a cycloaddition with an imine such as 99 that would give rise to the spiro β- and γ-lactam derivative 101 in a diastereoselective manner. Indeed, a seminal contribution also made use of a similar Pd-catalysed carbonylation followed by amide formation and cyclization in a
Stereo- and regioselective hydroboration of 1-exo-methylene pyranoses: discovery of aryltriazolylmethyl C-galactopyranosides as selective galectin-1 inhibitors
Beilstein J. Org. Chem. 2019, 15, 1046–1060, doi:10.3762/bjoc.15.102
- (aryltriazolyl)methyl galactopyranosyls 10a–n in fair to good yields (42–78% yields) via a copper-catalyzed Huisgen cycloaddition [17][32]. The resulting (aryltriazolyl)methyl galactopyranosyls 10a–n were debenzylated using palladium hydroxide on carbon in a 2:1 cyclohexene and ethanol mixture to give 1a–n in
New terpenoids from the fermentation broth of the edible mushroom Cyclocybe aegerita
Beilstein J. Org. Chem. 2019, 15, 1000–1007, doi:10.3762/bjoc.15.98
- which indicates that this gene is presumably involved in the bovistol synthesis pathway. In addition, the deduced protein sequence of the adjacent gene with the ID AAE3_04121 shows similarities to the Diels-Alderase Sol5 from Alternaria solani, which is involved in the cycloaddition of prosolanapyrone
- II into solanapyrone [18]. A similar cycloaddition is needed to form bovistol out of two illudine precursors. Although the respective illudine has not been detected in this study, it is known that C. aegerita is able to produce several illudanes [11]. As proposed in recent studies on sesquiterpenes
- production of bovistol to the putative Δ6-protoilludene synthase AAE3_04120 whose gene is located adjacent to a Diels-Alderase needed for cycloaddition of two illudine monomers. With this information, we made a biosynthesis proposal for these metabolites. Further studies will address this assumption to prove
SO2F2-mediated transformation of 2'-hydroxyacetophenones to benzo-oxetes
Beilstein J. Org. Chem. 2019, 15, 976–980, doi:10.3762/bjoc.15.95
- + 2] cycloaddition that furnishes substituted oxetanes rapidly, for example Paterno–Büchi reaction (Scheme 1b) [21]. However, the recognition of facial selectivity control in [2 + 2] cycloaddition is not an easy task. The availability of various methods for the synthesis of oxetanes and their strained
Catalytic asymmetric oxo-Diels–Alder reactions with chiral atropisomeric biphenyl diols
Beilstein J. Org. Chem. 2019, 15, 955–962, doi:10.3762/bjoc.15.92
- stereogenic centers in a one-step [4 + 2] cycloaddition or cyclization reaction [6][7][8] and it has become hugely popular in preparing vital intermediates for the syntheses of key structural subunits of natural products with biological activities (e.g., carbohydrates, antibiotics, toxins etc.) [9][10
An anomalous addition of chlorosulfonyl isocyanate to a carbonyl group: the synthesis of ((3aS,7aR,E)-2-ethyl-3-oxo-2,3,3a,4,7,7a-hexahydro-1H-isoindol-1-ylidene)sulfamoyl chloride
Beilstein J. Org. Chem. 2019, 15, 931–936, doi:10.3762/bjoc.15.89
- material was 2-ethyl-3a,4,7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione (9), which we have synthesized in previous studies [16][17]. Imide 9 was synthesized via the cycloaddition of 3-sulfone to maleic anhydride. The reaction of ethylamine with anhydride 8 in the presence of a toluene/triethylamine mixture (3





































































































































































































































































































































































































































































































































