Search results
Search for "cytotoxic" in Full Text gives 267 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Plasma membrane imaging with a fluorescent benzothiadiazole derivative
Beilstein J. Org. Chem. 2019, 15, 2644–2654, doi:10.3762/bjoc.15.257

- effects and the concentrations to use the probe without causing any harm to the cells (Figure 5). Only at concentrations up to 100 μM cytotoxic effects were noted and at 10 μM no effect was observed for the designed BTD-4APTEG. For the subsequent bioimaging experiments, the fluorophore was tested at 1 μM
- , that is at a concentration 100-fold lower than that of the cytotoxic effect. The new compound was then tested as bioimaging probe in live and fixed cells (Figure 6). As can be seen, the green fluorescent dye BTD-4APTEG was found most concentrated at the plasma membranes of the MCF-7 cells in both, live
- MTT analysis after 24 h treatment with the developed dye BTD-4APTEG. No statistically significant cytotoxic effect was observed after 24 h incubation with the new dye BTD-4APTEG at 10 μM. However, the dye induced strong cytotoxic effects in all tested cell lines at 100 µM (p < 0.05). MCF-7 cells
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- activity observed for 2, 3, 6 and 8. Compounds 2, 5, 8, 9 and 10 exhibited low levels of cytotoxicity against the four mammalian cell lines tested, while 3, 6 and 7 were considerably more cytotoxic. Notably, 4 showed strong activity against the mouse myeloma NS-1 cell line, but no activity against the
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- affecting its growth, at very low concentrations, namely 10 μM, and were not cytotoxic to human fibroblasts at the same concentration. Due to the well-known relationship of the α-hemolysins expression with the accessory gene regulator (agr) of the S. aureus, we can suggest that these two peptidomimetics may
α,ß-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
Beilstein J. Org. Chem. 2019, 15, 2524–2533, doi:10.3762/bjoc.15.245
- 11b has been studied since it was the most cytotoxic among all. FITC (fluorescein-5-isothiocyanate)–annexin V/PI flow cytometry of HepG2 cells On HeLa cells, the efficacy of 11b was measured through the application of other apoptotic parameters like phosphatidylserine (PS) externalization, a known
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- family was widened by molecules isolated from other marine organisms, like sponges, sea whips and the brown algae of this genus Dictyota [70]. Two modifications would transform 3,7,18-dolabellatriene (12, Scheme 3) into a potent cytotoxic molecule with activity towards murine leukemia cells or human non
Mono- and bithiophene-substituted diarylethene photoswitches with emissive open or closed forms
Beilstein J. Org. Chem. 2019, 15, 2344–2354, doi:10.3762/bjoc.15.227

- ring-closure reaction was fully reversible by irradiation with longer wavelengths. This offers the possibility of reversible switching a DAE only with visible, avoiding harmful (cytotoxic) UV irradiation. In view of the forthcoming solubilizing strategies applicable to fluorescent DAEs and intended for
Isolation of fungi using the diffusion chamber device FIND technology
Beilstein J. Org. Chem. 2019, 15, 2191–2203, doi:10.3762/bjoc.15.216
- antibiotics (e.g., cephalosporins), immunosuppressants (e.g., mycophenolic acid) and immunomodulators (e.g., fingolimod), as well as cholesterol lowering agents (statins). Generally, fungal metabolites were shown to have antiviral, cytotoxic, antineoplastic, cardiovascular, anti-inflammatory, immune
Genome mining in Trichoderma viride J1-030: discovery and identification of novel sesquiterpene synthase and its products
Beilstein J. Org. Chem. 2019, 15, 2052–2058, doi:10.3762/bjoc.15.202

- structurally diverse (poly)cyclic core skeletons [3][12]. A set of post-modification enzymes can transform core sesquiterpene skeletons into different kinds of sesquiterpenoids with potential anticancer, cytotoxic and antibiotic functions [13]. More than 121 skeleton structures derived from the sesquiterpene
Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
Beilstein J. Org. Chem. 2019, 15, 2020–2028, doi:10.3762/bjoc.15.198
- ], helminthosporol (7) [15], bipolenin A (8) [3], secolongifolene diol (9) [15], dihydroprehelminthosporol (10) [1] and sorokinianin (11) [2], and the cytotoxic sesterterpenoid, terpestacin (12) [16][17] (Figure 1). Bipolenin K (1) was isolated as a colourless oil. Its molecular formula was determined as C15H22O3
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
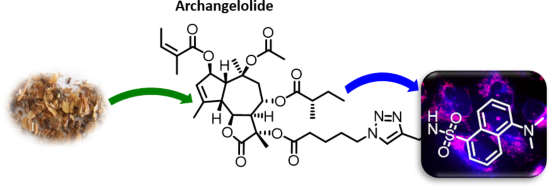
- . Interestingly, we found that neither archangelolide nor its dansyl conjugate did exhibit cytotoxic effects in contrast to the structurally closely related counterparts trilobolide and thapsigargin. We explain this observation by a molecular dynamics simulation, in which, in contrast to trilobolide
- ] and was also found to be cytotoxic with localization to the endoplasmic reticulum [8]. The cytotoxicity of compound 2 prompted the preparation and evaluation of compound 2 conjugates with porphyrins [9] and steroids [10] for targeted uptake of compound 2 into cancer cells, and the conjugates showed
- with compound 1 and its derivatives up to a final concentration of 50 µM for periods of 24, 48 and 72 h. Surprisingly, compound 1 exhibited almost no cytotoxic effect under these conditions (see Supporting Information File 1, Table S2 and Table S3, Figures S21–S23). The IC50 values were in the upper
Genomics-inspired discovery of massiliachelin, an agrochelin epimer from Massilia sp. NR 4-1
Beilstein J. Org. Chem. 2019, 15, 1298–1303, doi:10.3762/bjoc.15.128
- . A compound which was found to be predominantly produced under iron deficiency was subsequently isolated. Its structural characterization by spectroscopic and bioinformatic analyses revealed a previously not known diastereomer of the cytotoxic alkaloid agrochelin. The structure of this natural
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- substitution pattern at the phenyl moiety (Figure 2A). The small library of compounds enabled a better understanding of the structural factors determining the antiviral activity versus the cytotoxic one, with the major effect found for substituents at the para-position [23]. Eventually, this work allowed the
- design of new compounds with antiviral activity and reduced cytotoxic effects by changing the type and position of the substituent. Such small structural changes could be implemented in a rapid manner because of the easy setup of the MCR approach. To get a deeper insight into the biological effect of Ugi
- conjugation [62][63]. Cytotoxic spirostan saponins were chosen as model compounds for the preparation of Ugi-derived analogues, such as 55 and 56 suitable for biological evaluation [62]. Besides the glucose unit, other trisaccharidic moieties (e.g., β-chacotrioside) could also be conjugated to functionalized
New terpenoids from the fermentation broth of the edible mushroom Cyclocybe aegerita
Beilstein J. Org. Chem. 2019, 15, 1000–1007, doi:10.3762/bjoc.15.98
- synthesis, we tentatively conclude a 2S,3R,8S,9R absolute configuration for pasteurestin C (4). The systematic name for 4 is (4S,4aR,7aS,7bR)-4-hydroxy-6,6,7b-trimethyl-2,4,4a,5,6,7,7a,7b-octahydro-1H-cyclobuta[e]indene-3-carboxylic acid. Bovistol A (1) showed weak cytotoxic effects (IC50 for L929 = 15 µg
Heck- and Suzuki-coupling approaches to novel hydroquinone inhibitors of calcium ATPase
Beilstein J. Org. Chem. 2019, 15, 971–975, doi:10.3762/bjoc.15.94
- cytotoxic agent. The problem of concomitant toxicity to healthy cells has been circumvented by attaching a short peptide (His-Ser-Ser-Lys-Leu-Gln-Leu) to a tether at TG’s C-8 position (1b). This modification renders the inhibitor inactive [3]. Prostate cancer cells produce on their surface the serine
Synthesis of (macro)heterocycles by consecutive/repetitive isocyanide-based multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 906–930, doi:10.3762/bjoc.15.88
- successfully used in the synthesis of tubulysin analogues called tubugis (53–55) [27]. These molecules are N-substituted peptides, which possess a very high cytotoxic activity (on the picomolar range). They were prepared using three different IMCRs (Scheme 11): the Mep-Ileu-OH dipeptide fragment 47 was
Photochemical generation of the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical from caged nitroxides by near-infrared two-photon irradiation and its cytocidal effect on lung cancer cells
Beilstein J. Org. Chem. 2019, 15, 863–873, doi:10.3762/bjoc.15.84

- new caged nitroxides (nitroxide donors) 2a and 2b having the TP-responsive NPBF chromophore and the NIR TP-triggered generation of the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical under atmospheric conditions using these species (Scheme 1). Because free radicals are cytotoxic due to their
New sesquiterpenoids from the South China Sea soft corals Clavularia viridis and Lemnalia flava
Beilstein J. Org. Chem. 2019, 15, 695–702, doi:10.3762/bjoc.15.64

- , cytotoxic, to anti-inflammatory properties [10]. In the framework of our ongoing research for the bioactive metabolites from South China Sea soft corals [11][12], we made the collection of the title samples Clavularia viridis and Lemnalia flava off the Xisha Islands, Hainan Province, China. The chemical
Cyclopropene derivatives of aminosugars for metabolic glycoengineering
Beilstein J. Org. Chem. 2019, 15, 584–601, doi:10.3762/bjoc.15.54
- cytotoxic when applied in higher concentrations. Thus, novel glucosamine derivatives with improved properties would be beneficial. Based on the findings described above, especially the excellent incorporation efficiency of Ac4ManNCp(H2), we hypothesized, that also the corresponding glucosamine derivative
Synthesis of the polyketide section of seragamide A and related cyclodepsipeptides via Negishi cross coupling
Beilstein J. Org. Chem. 2019, 15, 577–583, doi:10.3762/bjoc.15.53
- Jan Hendrik Lang Thomas Lindel TU Braunschweig, Institute of Organic Chemistry, Hagenring 30, 38106 Braunschweig, Germany 10.3762/bjoc.15.53 Abstract The synthesis of the polyketide section present in the potently cytotoxic marine cyclodepsipeptide jasplakinolide and related natural products
- cytotoxicity [3]. A photoreactive derivative of the cytotoxic jasplakinolides, geodiamolides [4][5], or seragamides (2–6, seragamides A–E) [6] could also enable the search for additional targets in the cell, including proteins involved in transport or even membrane components. For this purpose, the 2
- most of the jasplakinolides, geodiamolides, and seragamides, potently cytotoxic peptide-polyketide cyclodepsipeptides stabilizing filamentous actin. The key step is a Negishi cross coupling, and the carbon atoms stem from (R)-propylene oxide (C7–C9), tert-butyl propionate (9, C5–C6, 6-Me), CBr4 (C4
Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines
Beilstein J. Org. Chem. 2019, 15, 474–489, doi:10.3762/bjoc.15.41

- biocompatible, cell-permeable, not cytotoxic and do not influence cell proliferation. Thus, one can predict that the developed purine and 7-deazapurine derivatives possessing the novel substitution pattern may find their application also in cell labeling in the future besides the potential use as materials in
Metal-free C–H mercaptalization of benzothiazoles and benzoxazoles using 1,3-propanedithiol as thiol source
Beilstein J. Org. Chem. 2019, 15, 279–284, doi:10.3762/bjoc.15.24
- -mercaptobenzoxazoles are not only fundamental building blocks in organic synthesis, but also possess various biological activities (Figure 1) [1][2]. A complex of a transition metal (such as Ru, Pt, Bi, etc.) with either a 2-mercaptobenzoxazole or a 2-mercaptobenzothiazole often provides cytotoxic activity against
Synthesis of a tubugi-1-toxin conjugate by a modulizable disulfide linker system with a neuropeptide Y analogue showing selectivity for hY1R-overexpressing tumor cells
Beilstein J. Org. Chem. 2019, 15, 96–105, doi:10.3762/bjoc.15.11

- Pharmaceuticals GmbH, Leberstr. 20, A-1110 Vienna, Austria 10.3762/bjoc.15.11 Abstract Tubugi-1 is a small cytotoxic peptide with picomolar cytotoxicity. To improve its cancer cell targeting, it was conjugated using a universal, modular disulfide derivative. This allowed conjugation to a neuropeptide-Y (NPY
- )-inspired peptide [K4(C-βA-),F7,L17,P34]-hNPY, acting as NPY Y1 receptor (hY1R)-targeting peptide, to form a tubugi-1–SS–NPY disulfide-linked conjugate. The cytotoxic impacts of the novel tubugi-1–NPY peptide–toxin conjugate, as well as of free tubugi-1, and tubugi-1 bearing the thiol spacer (liberated from
- (Ewing`s sarcoma), MDA-MB-468, MDA-MB-231 (both breast cancer) and 184B5 (normal breast; chemically transformed) were investigated. As hoped, the toxicity of tubugi-1 was masked, with IC50 values decreased by ca. 1,000-fold compared to the free toxin. Due to intracellular linker cleavage, the cytotoxic
Ring-closing-metathesis-based synthesis of annellated coumarins from 8-allylcoumarins
Beilstein J. Org. Chem. 2018, 14, 2991–2998, doi:10.3762/bjoc.14.278
- ). Angelicin (3a, also named isopsoralen), for instance, is an angular furanocoumarin from Psoralea corylifolia [11][12] that is moderately cytotoxic [13] and exhibits anti-oxidative activity [14], but is significantly less phototoxic than the linear isomer psoralen, due to its inability to cross link DNA [15
N-Acylated amino acid methyl esters from marine Roseobacter group bacteria
Beilstein J. Org. Chem. 2018, 14, 2964–2973, doi:10.3762/bjoc.14.276
- on the LuxR type receptors for AHLs in bacteria [23][24]. Other potential functions are antimicrobial or cytotoxic activity of the tested derivatives. Roseovarius sp. D12_1.68 showed antimicrobial activity against a Rhodobacteraceae sp. TL and antialgal activity against Skeletonema costatum while
- Loktanella sp. D3 showed no antagonistic activity [29]. This observation fits well with the detection of antimicrobial or cytotoxic NAVME 9 and NABMEs 7 and 8 only in Roseovarius sp. D12_1.68. Bacteria are known to produce acylated amino acids, although the number reported so far is small, including tyrosine
Novel solid-phase strategy for the synthesis of ligand-targeted fluorescent-labelled chelating peptide conjugates as a theranostic tool for cancer
Beilstein J. Org. Chem. 2018, 14, 2665–2679, doi:10.3762/bjoc.14.244

- successfully designed and demonstrated a novel continuous process for assembling targeting ligands, peptidic spacers, fluorescent tags and a chelating core for the attachment of cytotoxic molecules, radiotracers, nanomaterials in a standard Fmoc solid-phase peptide synthesis in high yield and purity. The
- non-specific uptake. The specificity of bioconjugates is indispensable to prevent collateral damage and toxicity to healthy cells when the chelating core is tethered to deliver radionuclides or cytotoxic drugs. The confocal microscopic images in Figure 3, panel (ii) shows the uptake of chelating DUPA
- can be employed as potential theranostic tools by attaching macromolecules, cytotoxic warheads, radioactive tracers, nanomaterials etc., via the chelating core. Experimental Materials and methods H-Cys-2-ClTrt resin, Fmoc amino acids and amide coupling agents, reagents and solvents used in solid-phase














































































































































































































































