Search results
Search for "fluorescence" in Full Text gives 506 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Mono- and bithiophene-substituted diarylethene photoswitches with emissive open or closed forms
Beilstein J. Org. Chem. 2019, 15, 2344–2354, doi:10.3762/bjoc.15.227

- (470 nm, 530 nm). The on-switching of fluorescence due to the ring-closure reaction was observed also with visible light of 470 nm (to an extent of 10% for compound SyOTh1) and attributed to the Urbach tail effect. Due to a high degree of fluorescence modulation (>270), good fatigue resistance and
- large fluorescence quantum yield, compound SyOTh1 emerged as a candidate for single-molecule based super-resolution fluorescence microscopy. Keywords: diarylethenes; dyes; fluorescence; organic synthesis; photochromism; photoswitching; Introduction Reversibly photoswitchable diarylethenes (DAEs) with
- . Oligothiophenes are highly fluorescent [6][7], and therefore, we reasoned that their incorporation into DAEs with oxidized 2-alkylbenzo[b]thiophene units might produce fluorescent open forms in addition to the intrinsic fluorescence of the closed forms. In particular, we expected that the prolonged conjugation
Fluorescent phosphorus dendrimers excited by two photons: synthesis, two-photon absorption properties and biological uses
Beilstein J. Org. Chem. 2019, 15, 2287–2303, doi:10.3762/bjoc.15.221

- branches of the dendrimer structures, respectively. Also the functionalization in two compartments (core and surface, or branches and surface) was achieved. The consequences of the location of the fluorophores on the fluorescence and TPA properties have been studied. Several of these TPA fluorescent
- the mechanism of action of anticancer compounds, and for safer photodynamic therapy. Keywords: bioimaging; dendrimer; fluorescence; phosphorus; two-photon absorption; Introduction Natural luminescence phenomena such as the bioluminescence of fireflies or of certain marine microorganisms, or the
- excited state (S1). Depending on the type of molecules, two main ways can be taken to go back to the ground state: either a nonradiative decay of the energy absorbed, or the emission of fluorescence at a longer, less energetic wavelength. A third possibility (not shown in the Figure) concerns an
Aggregation-induced emission effect on turn-off fluorescent switching of a photochromic diarylethene
Beilstein J. Org. Chem. 2019, 15, 2204–2212, doi:10.3762/bjoc.15.217

- , diarylethene derivatives with photoswitchable fluorescent properties were prepared. They are applicable for fluorescence imaging including bio-imaging. On the other hand, a new system called “excited state intramolecular proton transfer (ESIPT)” is reported. In the system, absorption and emission bands are
- largely separated due to the proton transfer, hence it showed strong fluorescence even in the crystalline state. We aimed to construct the photochromic system incorporating the ESIPT mechanism. Results: A diarylethene incorporating a fluorescent moiety that exhibit ESIPT behavior was prepared. The ESIPT
- is one of the examples which express the mechanisms of aggregation-induced emission (AIE). This compound emits orange fluorescence with a large Stokes shift derived from ESIPT in aprotic solvents such as THF or hexane, while it exhibits only a photochromic reaction in protic solvents such as methanol
Isolation of fungi using the diffusion chamber device FIND technology
Beilstein J. Org. Chem. 2019, 15, 2191–2203, doi:10.3762/bjoc.15.216
- cells were seeded into a PDL-coated 96-well plate (25.000/well) and allowed to settle for 2 hours prior to addition of the compounds (solubilized in water). After incubating for 22 hours, CellTiter Blue reagent (Promega) has been added. After another 2 hours of incubation the fluorescence has been
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- fluorescence-based assay, using Z-Lys(acetyl)-AMC (ZMAL) as a substrate [38]. Compared to the lead structure 2a, all compounds except 2e–h show increased inhibitory activity against Sirt2 (Table 1). Compound 2c represents the most potent inhibitior with an IC50 value of about 7 µM. Moreover, a slight increase
- allows for isotype-specific interactions [46]. A recently developed fluorescence polarization (FP)-based assay enables mapping of ligand binding to this specific binding site [35]. For 2a an interaction with the selectivity pocket was already implied in the same work. Additionally performed docking
- : The inhibitory effect of compounds 2a–h, 4a/b, 8a and 11 on Sirt1–3 was detected via a previously reported fluorescence based assay [38]. The synthetic substrate Z-Lys(acetyl)-AMC (ZMAL) is deacetylated by sirtuins, followed by tryptic digestion and thereby release of 7-aminomethylcoumarin, leading to
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- -catalyzed RCM clipping mechanical bond forming methodology [48]. The 1H NMR spectroscopy in CDCl3 (293 K, 500 MHz) and the fluorescence titration experiments which were done in acetonitrile have demonstrated that the synthesized 1,2,3-triazolium macrocycle 6 was able to bind and sense several anions but the
- the smaller rings from the core tetrachloro substituted PDI motifs containing triazolium groups has been utilized for the selective colorimetric and fluorescence anion sensing in competitive protic organic and aqueous-organic solvent media. By addition of tetrabutylammonium (TBA) chloride to a
- solution of 7 in CHCl3/CH3OH (3:1, v/v), a remarkable naked-eye recognizable color change from red to orange was observed. According to fluorescence spectroscopy, the emission intensity of PDI increased considerably (quantum yield enhancement factor of 57%). Anions are recognizable by catenane 7 at low
Synthesis and properties of sulfur-functionalized triarylmethylium, acridinium and triangulenium dyes
Beilstein J. Org. Chem. 2019, 15, 2133–2141, doi:10.3762/bjoc.15.210
- of the ethylthiol substituents. For the triangulenium derivatives significant fluorescence was observed (Φf = 0.1 to Φf = 0.3). Keywords: acridinium dyes; aromatic nucleophilic substitution; fluorescent dyes; sulfur-functionalized dyes; triangulenium dyes; triarylmethylium; Introduction The design
- environments with high levels of autofluorescence, constantly challenges chemists to develop new dyes with improved or special properties. In the design of simple dyes parameters such as molar absorption coefficients (ε), absorption/emission wavelengths [8][9], fluorescence quantum yields (Φfl) [10][11], and
- fluorescence lifetime (τfl) [12][13] are key photophysical properties to consider and optimize for any given application. We have for quite some time been interested in the synthesis, properties and applications of dyes from the triangulenium family (Figure 1) [14][15]. The triangulenium dyes can be divided
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- observed upon attempts to sublime the brown byproduct about 200–210 °C affording colorless crystals. The NMR spectrum corresponded to 5 (described, sometimes, as light yellow solid [18][19]). It is worth of noting that sublimed 5 does not fluoresce so that previously observed fluorescence [20] and
Naphthalene diimides with improved solubility for visible light photoredox catalysis
Beilstein J. Org. Chem. 2019, 15, 2043–2051, doi:10.3762/bjoc.15.201
- fluorescence quantum yields are rather low and fluorescence lifetimes are rather short due to ISC into the triplet state [50][51]. NDIs are reversibly reducible and their stable radical anions absorb in the visible to NIR light range [37]. The aromatic core of NDIs can be easily modified by substituents in
- have only little influence on the optical properties but significantly modulate the solubility in DMF. This result agrees with other cNDIs in literature [47][48][58]. NDI 1 shows weak fluorescence in CH2Cl2 with a maximum at 384 nm and a quantum yield of 7%. The Stokes’ shift is small (413 cm−1). Based
- on these values, the excitation energy E00 for the singlet state which is an important prerequisite for photoredox catalysis with 1 can be estimated to be 3.25 eV. In DMF, the fluorescence of 1 is completely quenched. This is due to a photoinduced charge transfer between NDI 1 and DMF. DMF has an
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- . Upon complexation with a halide, the planar aromatic ring adopted a heavily pinched boat conformation, a result consistent with theoretical prediction [49]. We also examined the host–guest interaction in solution phase employing NMR and UV–vis spectroscopy and fluorescence technology. Unfortunately
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189

- File 1, Figure S19) cells. In both cell lines, compound 5 localized already after 30 min at 0.5 µM concentration, and the fluorescence intensity increased over a period of 2 h of incubation. In order to identify the intracellular localization of compound 5, co-localization experiments using endoplasmic
- partial co-localization also in mitochondria, in which SERCA is not present. Localization of a control (dansyl fluorophore) was not organelle-specific and the fluorescence intensity was very weak even at 2 µM concentration (Supporting Information File 1, Figure S18). Impact of compound 1 on cell viability
- , the endoplasmic reticulum was visualized by transfection with a plasmid DNA coding mCherry-ER (0.5 µg) using Fugene HD (Promega, USA; DNA/Fugene HD = 1:3). Fluorescence microscopy The intracellular localization of compound 5 was studied by real-time live-cell fluorescence microscopy using an inverse
Morphology-tunable and pH-responsive supramolecular self-assemblies based on AB2-type host–guest-conjugated amphiphilic molecules for controlled drug delivery
Beilstein J. Org. Chem. 2019, 15, 1925–1932, doi:10.3762/bjoc.15.188

- environment [47], so the host–guest interaction of β-CD and protonated BM decreased correspondingly, resulting in a self-assembly morphology transition from fan-shaped to spherical structures. Furthermore, 2D NOESY, UV–vis and fluorescence spectroscopy were employed to confirm the above proposed mechanism
- addition, the fluorescence spectra indicated that the maximum emission wavelength shifted from 297 nm at pH 7.4 to 370 nm at pH 5.0 (Figure 3d), indicating as well the protonation of BM inclusion complexes. These results further proved that the self-assembly morphology transitions of supramolecular self
- -loaded supramolecular self-assemblies Confocal laser scanning microscopy (CLSM) was further utilized to confirm the intracellular uptake of DOX-loaded FSSAs (Figure 6). The PC-3 cells treated with DOX-loaded FSSAs indicated Hoechst 33342 blue fluorescence in their nuclei and DOX red fluorescence in their
Identification of optimal fluorescent probes for G-quadruplex nucleic acids through systematic exploration of mono- and distyryl dye libraries
Beilstein J. Org. Chem. 2019, 15, 1872–1889, doi:10.3762/bjoc.15.183

- cationic dyes was synthesized and investigated with respect to their optical properties, propensity to aggregation in aqueous medium, and capacity to serve as fluorescence “light-up” probes for G-quadruplex (G4) DNA and RNA structures. Among the 61 compounds, 57 dyes showed preferential enhancement of
- fluorescence intensity in the presence of one or another G4-DNA or RNA structure, while no dye displayed preferential response to double-stranded DNA or single-stranded RNA analytes employed at equivalent nucleotide concentration. Thus, preferential fluorimetric response towards G4 structures appears to be a
- and quantum yield, low background fluorescence) are still poorly understood, mostly due to the lack of comparative studies. To explore this aspect, we report the synthesis and systematic study of a library of 61 di- and mono-styryl dyes, as potential “light-up” probes for G4 structures. The study aims
Functional panchromatic BODIPY dyes with near-infrared absorption: design, synthesis, characterization and use in dye-sensitized solar cells
Beilstein J. Org. Chem. 2019, 15, 1758–1768, doi:10.3762/bjoc.15.169

- phthalocyanines [10][11], organic push–pull compounds [12], and boron-dipyrromethene [13] (BODIPY®). BODIPY dyes are one of the most extensively studied class of fluorophores due to their unique properties, including high absorption coefficients in the visible and NIR ranges, high fluorescence quantum yields, and
Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–)2A fluorescent dyes
Beilstein J. Org. Chem. 2019, 15, 1712–1721, doi:10.3762/bjoc.15.167

- properties of the three dyes were investigated by photoabsorption and fluorescence spectroscopy, Lippert–Mataga plots, cyclic voltammetry and density functional theory calculations. The photoabsorption maximum (λmax,abs) and the fluorescence maximum (λmax,fl) for the intramolecular charge-transfer
- characteristic band of the (D–π–)2A fluorescent dyes show bathochromic shifts in the order of OUY-2 < OUK-2 < OUJ-2. Moreover, the photoabsorption bands of the (D–π–)2A fluorescent dyes are nearly independent of solvent polarity, while the fluorescence bands showed bathochromic shifts with increasing solvent
- polarity (i.e., positive fluorescence solvatochromism). The Lippert–Mataga plots for OUY-2, OUK-2 and OUJ-2 indicate that the Δμ (= μe − μg) value, which is the difference in the dipole moment of the dye between the excited (μe) and the ground (μg) states, increases in the order of OUY-2 < OUK-2 < OUJ-2
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
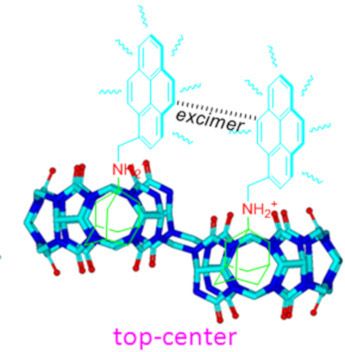
- Xiaodong Zhang Wei Wu Zhu Tao Xin-Long Ni Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.15.166 Abstract The unique monomer and excimer fluorescence emissions of pyrene were first exploited as distinctly
- the fluorescence emission spectra of G2 in the presence of host-1. As can be seen, free G2 produced typical monomer emissions at around 378 and 396 nm in aqueous solution (pH 2) upon excitation of the pyrene fluorophore at 340 nm. When we added host-1 at increasing concentrations to the G2 solution
- , the fluorescence intensity of the G2 monomer emissions gradually decreased, while the maximum emission intensity at around 485 nm (typical excimer emissions of pyrene) increased. The excimer emission band of G2 can be attributed to the interaction of two pyrene units resulting in intermolecular π–π
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- derivatives (P5A-DPA and P5A-Py) bearing bulky fluorophores were obtained in high yield by click reaction. The photophysical properties of both compounds were investigated in detail. P5A-DPA with two 9,10-diphenylanthracene (DPA) pigments grafted on the pillar[5]arene showed a high fluorescence quantum yield
- the chiral separation of the Sp and Rp enantiomers [25]. Also installing bulky substituents onto only one phenolic ring was found to be effective to inhibit the rotation of the units [27][28][29]. Pillar[5]arene itself shows only moderate absorption and weak fluorescence in the UV region, and the
- chemical introduction of chromophores onto the rims of pillar[5]arene has been applied to allow its use as receptors for molecular sensing or biological applications [30][31][32][33]. It occurred to us that connecting fluorophores with strong absorptions in the visible range and with a high fluorescence
A heteroditopic macrocycle as organocatalytic nanoreactor for pyrroloacridinone synthesis in water
Beilstein J. Org. Chem. 2019, 15, 1505–1514, doi:10.3762/bjoc.15.152
- ; pyrroloacridinones; sustainable chemistry; water; Introduction Acridines are a renowned heterocyclic entity not only for their biological activities but also for their fluorescence and chemiluminescence properties. Acridine dyes (e.g., acridine orange, acridine yellow, etc.), are predominantly used in staining of
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- maximum of 1 is located at 350 nm (Figure S22, Supporting Information File 1). It is known that the binding of metal ions could cause fluorescence quenching through photo-induced electron transfer [44]. Indeed, the fluorescence intensity of 1 is quenched gradually upon addition of TBA[AuCl4] (Figure 6a
- concentration range of 20–970 μM and a detection limit of 3.9 μM (Figure S23, Supporting Information File 1). For (TBA)2[PdCl4], the fluorescence intensity was enhanced at the beginning (Figure S24, Supporting Information File 1), and then quenched with increasing concentrations of (TBA)2[PdCl4] (Figure S25a
- −@1. Energy-minimized structure of a) AuCl4−@1 and b) AuCl4−@2 at the level of theory of PM3 by using Spartan ’14 (Wavefunction, Inc.). a) Fluorescence emission spectra of 1 (20 µM) upon addition of different amounts of TBA[AuCl4] (concentration range of 0.0−800 µM) and then recorded in
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- the β-position in 3a or 3b led to a ≈5 nm red shift when compared with the absorption spectrum of ZnTPP [48]. In addition, the fluorescence spectra of 3a and 3b were comparable with that of ZnTPP, and again shifted to short wavelength (≈14 nm) with respect to that of 2. Later the ESIMS spectrum
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- representative candidate of luminescent transition-metal complexes. We determined the association constants of the GC5A–dye complexes by fluorescence titration and discuss the complexation-induced photophysical changes. In addition, a comparison of the complexation behavior of GC5A with that of other macrocycles
- and potential applications according to the diverse photophysical responses are provided. Keywords: calixarene; host–guest complexation; luminescent dyes; macrocycles; photophysical properties; Introduction Fluorescence sensing represents a powerful detection methodology due to its low cost, ease of
- -consuming syntheses. Alternatively, supramolecular chemistry provides a non-covalent approach to achieve fluorescence sensing [2]. An elegant supramolecular strategy, named indicator displacement assay (IDA), was established and popularized by Anslyn and co-workers (Scheme 1a) [3][4]. The complexation of a
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- properties The optical properties of the four heterotriacenes were investigated by UV–vis and fluorescence spectroscopy in dichloromethane solution (Figure 6, left and Table 4). The absorption spectra in the series of DTT 1 to DSS 4 showed one main absorption band exhibiting vibronic fine structure according
- 3/DSS 4 (28,400 to 20,830 L mol−1 cm−1). No fluorescence was observed for each of the four heteroacenes DTT 1 to DSS 4 neither in DCM nor in THF. Electrochemical properties and electropolymerization The redox properties of the heterotriacenes 1–4 were investigated by means of cyclic voltammetry in
Selective detection of DABCO using a supramolecular interconversion as fluorescence reporter
Beilstein J. Org. Chem. 2019, 15, 1371–1378, doi:10.3762/bjoc.15.137
- -component rectangle [Cu4(1)2(2)2]4+ and the four-component sandwich complex [Cu2(1)(2)(4)]2+ is triggered by inclusion and release of DABCO (4). The fully reversible and clean switching between two multicomponent supramolecular architectures can be monitored by fluorescence changes at the zinc porphyrin
- compounds. Keywords: copper; detection; fluorescence; interconversion; macrocycles; self-assembly; self-sorting; zinc porphyrin; Introduction Since dynamic multicomponent supramolecular structures are nowadays abundant [1][2], the weak intercomponent binding [3][4][5][6][7][8][9] is often instrumentalized
- 1H DOSY confirms the successful rearrangement. To probe the selectivity of the guest-induced transformation of 5, the structure interconversion was tested with other potential guests, using fluorescence and 1H NMR spectroscopy. In the absence of any external ligand, the rectangle 5 shows its typical
Synthesis of dipolar molecular rotors as linkers for metal-organic frameworks
Beilstein J. Org. Chem. 2019, 15, 1331–1338, doi:10.3762/bjoc.15.132
- linkers in MOFs with potential applications as ferroelectric materials and for optical signal processing. Keywords: benzothiadiazole; dipolar rotor; fluorescence; large dipole moment; metal organic framework linker; Introduction Rotors are among the fundamental functional units in engineering in our
- is provided by ethynyl units as rotary joints. Carboxylate groups are terminating the axes on both sides because they are known to form the typical paddle wheel structures in MOFs [36]. Benzo-annelated 2,1,3-thiadiazoles were introduced (2 and 3) to shift absorption and fluorescence into the visible
- temperatures. Supporting Information Supporting Information File 454: Experimental procedures, 1H and 13C NMR spectra of new compounds as well as UV–vis and fluorescence spectra of compounds 1–5 and crystallographic data for compound 12b. Supporting Information File 455: cif file for compound 12b. Supporting
Mechanochemical Friedel–Crafts acylations
Beilstein J. Org. Chem. 2019, 15, 1313–1320, doi:10.3762/bjoc.15.130
- is rapid, and within 3 minutes of milling all anhydride is consumed (Figure 1). After 3 minutes of milling, high fluorescence prevents further following of the reaction progress. These spectra indicate that rapid complexation of anhydride with AlCl3 takes place, whereas the formation of the acylium




















































































































































































































































