Search results
Search for "host–guest" in Full Text gives 208 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- also discussed. Keywords: host–guest chemistry; macrocycles; noncovalent interactions; supramolecular photocatalysis; Introduction Enzyme-catalyzed reactions are often carried out fantastically in nature via noncovalent interactions of a substrate [1][2]. Inspired by these natural processes, chemists
- have begun to develop artificial supramolecular systems that provide a similar environment to perform various catalytic reactions where substrates are captured via host–guest interactions [3]. In addition, these systems aim to control the product selectivity and the rate of catalytic reactions by
- materials that have been exploited for photocatalytic applications, including photocatalytic dye degradations and hydrogen evolution. To successfully perform supramolecular photocatalytic reactions, various photophysical and photochemical properties of the host–guest system need to be considered [4]: i) the
Construction of pillar[4]arene[1]quinone–1,10-dibromodecane pseudorotaxanes in solution and in the solid state
Beilstein J. Org. Chem. 2020, 16, 2954–2959, doi:10.3762/bjoc.16.245

- 450001, P. R. China 10.3762/bjoc.16.245 Abstract We report novel pseudorotaxanes based on the complexation between pillar[4]arene[1]quinone and 1,10-dibromodecane. The complexation is found to have a 1:1 host–guest complexation stoichiometry in chloroform but a 2:1 host–guest complexation stoichiometry
- the further capping of the pseudorotaxanes to construct rotaxanes. Keywords: host–guest chemistry; pillar[4]arene[1]quinones; pillararenes; pseudorotaxanes; supramolecular chemistry; Introduction Relying on the research of basic science, supramolecular chemistry has become an important mean for
- reports [44][45]. We found that H and G can be used to build a [3]pseudorotaxane in the solid state but a [2]pseudorotaxane in solution. Results and Discussion Host–guest complexation in the solid state Cocrystals of H and G were obtained by slow evaporation of the solution in methanol. The X-ray
Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- ), cucurbit[7]uril (3), and the two chalcones 1 and 2 in aqueous solution [48]. When the hosts 3 and 4 were mixed with the trans-chalcones 1 and 2 as guests in a 1:1:1:1 ratio, instantly a statistical mixture formed displaying all four possible host–guest complexes. After the exposure to an acid, the ensuing
- specific release of the guest (PF6−) with the consequent disassembly of the cage [Zn4(65')4]8+ (SelfSORT-II). In SelfSORT-II, the host–guest complex [Zn4(64')4]8+•(C6H12) remained intact, while ≈17% decrease in [Zn4(66')4]8+•(NO3−) was observed. The addition of another aliquot of 4-methoxyaniline to
- SelfSORT-II disassembled the host–guest complex [Zn4(64')4]8+•(C6H12) with the concomitant release of the encapsulated cyclohexane thus generating the state SelfSORT-III. In this process, the 1H NMR signals corresponding to [Zn4(66')4]8+•(NO3−) remained unchanged. At last, heating the mixture to 70 °C for
A heterobimetallic tetrahedron from a linear platinum(II)-bis(acetylide) metalloligand
Beilstein J. Org. Chem. 2020, 16, 2701–2708, doi:10.3762/bjoc.16.220

- obtain analogues cages in future work to explore the properties of these system in terms of their host–guest chemistry or their magnetic behavior. Experimental General All reagents and solvents were purchased from commercial sources and used as received without any further purification. NMR spectra were
Water-soluble host–guest complexes between fullerenes and a sugar-functionalized tribenzotriquinacene assembling to microspheres
Beilstein J. Org. Chem. 2020, 16, 2551–2561, doi:10.3762/bjoc.16.207

- hydrophobic effect and to host–guest π–π interactions. Hydrophobic surface simulations showed that TBTQ-(OG)6 and C60 forms an amphiphilic supramolecular host–guest complex, which further assembles to microspheres with diameters of 0.3–3.5 μm, as determined by scanning electron microscopy. Keywords
- : fullerenes; host–guest systems; microspheres; supramolecular chemistry; tribenzotriquinacene; Introduction In the field of supramolecular chemistry, host–guest association through noncovalent interactions is an interesting and exciting topic, especially for the encapsulation of various fullerenes, such as
- attention for potential applications in host–guest recognition, such as gas storage and cationic complexation [31][32][33]. In the past years, the recognition of the biological and pharmaceutical relevance of fullerenes, such as their photodynamic activity, phototoxicity, HIV-1 protease inhibitor ability
Host–guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A
Beilstein J. Org. Chem. 2020, 16, 2332–2337, doi:10.3762/bjoc.16.194

- the inclusion compound with Q[8]. Keywords: cucurbit[8]uril; host–guest interaction; inclusion complex; oroxin A; properties; Introduction Cucurbit[n]urils (Q[n]s) are a family of macrocyclic cage compounds synthesized by the condensation of glycoluril and formaldehyde in a strong acidic solution [1
- ][2][3]. As a consequence of the specific structural features of Q[n]s, which have two hydrophilic “portals” decorated with partially negatively charged carbonyl groups and a hydrophobic cavity [4], cucurbit[n]urils are able to form host–guest complexes with a range of drugs [5][6][7]. These complexes
- constants [14] have shown that Q[n]s are ideal drug carriers [15][16]. Moreover, Q[n]s can enhance the physical stability [17][18] and increase the solubility [19][20] of drug molecules. After Q[n]s forms a host–guest complex with drug molecules, it can also improve the bioavailability and delivery capacity
Design and synthesis of a bis-macrocyclic host and guests as building blocks for small molecular knots
Beilstein J. Org. Chem. 2020, 16, 2314–2321, doi:10.3762/bjoc.16.192
- . Keywords: alkynes; azides; host–guest; macrocycles; molecular knots; Introduction Macrocycles have played a central role in the development of molecular recognition, self-assembled molecular devices, and molecular topology [1][2][3][4][5][6]. For example, early work by Pedersen on crown ethers [1], Lehn
- 0.02 to 0.09 ppm for some aromatic resonances on the host. These shifts suggest at least some host–guest complex was formed, even in this competitive polar solvent mixture. The full TLC sequence was tested by reacting 1 and 2 (1:1 ratio) in a solvent mixture of acetonitrile/dichloromethane/methanol (1
Clickable azide-functionalized bromoarylaldehydes – synthesis and photophysical characterization
Beilstein J. Org. Chem. 2020, 16, 1683–1692, doi:10.3762/bjoc.16.139
- non-radiative transitions can be achieved by the host–guest method [50][51][52] or by crystallization [53][54][55][56][57]. In difference to the liquid phase, the highly ordered packing and the restriction of molecular motions in the crystalline state favor a persistent luminescence. The promotion of
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- stereoselective approaches of substrates and reagents or catalysts in a variety of processes. Examples include the substrate–enzyme recognition (the host–guest interaction) responsible for inducing pharmacological activity, the DNA-intercalation capable of generating biomolecular activity, molecular dynamics [63
Aryl-substituted acridanes as hosts for TADF-based OLEDs
Beilstein J. Org. Chem. 2020, 16, 989–1000, doi:10.3762/bjoc.16.88

- , high singlet and triplet energies (higher than those of the guest) are required for host compounds for qualifying host–guest energy transfer, thus implying restrict of the emissive excitons on the TADF emitters [11]. High glass-transition temperatures are also required for increasing the morphological
The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties
Beilstein J. Org. Chem. 2020, 16, 71–77, doi:10.3762/bjoc.16.9
- Xiaodong Zhang Jun Xie Zhiling Xu Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.16.9 Abstract The host–guest interactions between baicalein (BALE) and cucurbit[8]uril (Q[8]) and the
- corresponding properties of the inclusion complex were studied using 1H NMR, IR and UV–vis spectroscopy and DTA. The results showed that BALE forms an inclusion compound (1:1) with Q[8], and the properties of baicalein are changed by cucurbit[8]uril. Keywords: baicalein; cucurbit[8]uril; host–guest interaction
- compounds such as calixarenes, crown ethers and cyclodextrins, which can be used to form a stable inclusion complex with the drug, and improving the bioavailability of the drug [34][35]. Herein, we describe the results of the investigations of host–guest interactions between BALE and Q[8] in an aqueous
Photoreversible stretching of a BAPTA chelator marshalling Ca2+-binding in aqueous media
Beilstein J. Org. Chem. 2019, 15, 2801–2811, doi:10.3762/bjoc.15.273

- , as elucidated by the crystallographic structure of a 1:1 host–guest BAPTA system [2]. This moiety has been exploited in the development of various fluorescent supramolecular chemosensor systems and even molecular logic systems [3][4][5][6][7][8][9][10][11]. Equally, the incorporation of
A chiral self-sorting photoresponsive coordination cage based on overcrowded alkenes
Beilstein J. Org. Chem. 2019, 15, 2767–2773, doi:10.3762/bjoc.15.268

- between the three isomers. Two of the isomers were able to form host–guest complexes opening up new prospects toward stimuli-controlled substrate binding and release. Keywords: coordination cages; molecular motors; molecular switches; overcrowded alkene; palladium; Introduction Supramolecular
- metal centers has been used to control host–guest interactions [25][26][27][28], structural composition [29], and sol–gel transition [30]. However, dithienylethene undergoes a limited structural change upon photoisomerization and, up to now, photoswitchable ligands based on other types of photochromic
- formed revealing that a chiral self-sorting process takes place. In addition, two of the cage isomers can bind a tosylate anion in solution by formation of a host–guest complex. Results and Discussion Ligands Z-1 and E-1 (Scheme 1) were synthesized by a Suzuki cross-coupling reaction of 3
Anion-driven encapsulation of cationic guests inside pyridine[4]arene dimers
Beilstein J. Org. Chem. 2019, 15, 2486–2492, doi:10.3762/bjoc.15.241

- and studied by multiple gas-phase techniques, ESI-QTOF-MS, IRMPD, and DT-IMMS experiments, as well as DFT calculations. The comparison of classical resorcinarenes with pyridinearenes by MS and NMR experiments reveals clear differences in their host–guest chemistry and implies that cation encapsulation
- synthesis of pyridine[4]arenes dates back to 2001 [4], their host–guest chemistry is still under-explored. Both macrocycles are concave and are known to form capsular assemblies via intermolecular hydrogen bonding [5][6]. Pyridine is significantly less electron-rich than benzene. Consequently, pyridinearene
- dimeric resorcin[4]arene and pyridine[4]arene capsules, we highlight here unique host–guest properties of pyridinearene capsules. In marked contrast to the corresponding resorcin[4]arene capsules, cation binding is clearly feasible, when anions bind in an exo-site and support cation encapsulation by
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- multiple host–guest correlations (see Figure S6 in Supporting Information File 1 for a full-range spectrum). For example, E-1’s protons Hd at 6.22 ppm correlate both with the resonances at 7.78 ppm and at 7.57 ppm, which all originate from 2’s protons (H2 + H3 and H5 + H7, respectively). Similarly, the Ha
- a premade complex of 2 with a tightly bound guest, tetra-o-fluoroazobenzene [49], no acceleration effect was observed. Conclusion In summary, we investigated the encapsulation and switching of a prototypical arylazopyrazole in the hydrophobic cavity of a water-soluble metal–organic cage. A host
- –guest inclusion complex incorporating two molecules of arylazopyrazole was obtained in a quantitative yield. The complex was characterized by a series of NMR techniques, which showed that the resonances of the guest protons were upfield-shifted by up to 2.2 ppm. An antiparallel configuration of the
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- -triazolium macrocycles; Review 1. Introduction Supramolecular chemistry – “The chemistry beyond the molecule” [1] – is an ever growing interdisciplinary area has emerged from the early host–guest chemistry to more elaborate bio-inspired supramolecular aggregates by exploiting various noncovalent
- interestingly, based on the results and control studies, Li’s group have proposed the ability of macrocycle 15 (triiodide form) to act as a nanoreactor by the photo-driven dimerization of tetracyano-para-quinodimethane (TCNQ). The proposed mechanism consists of 7 steps: the formation of a 1:1 host–guest [15
- interactions, compared to monovalent receptor–ligand interactions offer the advantage of multiple and sequential binding within the host–guest systems. These noncovalent interactions which can be controlled in a chemical or physical manner depend on the binding sites in the multivalent assembly [67
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- noncovalent interactions between anions and the tetrazine rings. Keywords: anion–π interactions; coronarenes; host–guest complexation; N-functionalized phthalimides; O6-corona[3]arene[3]tetrazines; Introduction Synthetic macrocycles [1][2] are always attractive and important because they are unique
- ability of 3a to bind various anion species in gas phase. To our delight, host molecule 3a co-crystalized with n-Bu4NX (X = Cl, Br) from diffusion of diethyl ether vapor into ethyl acetate solution at ambient temperature to give single crystals of the host–guest complexes (n-Bu4NX)3-3a (X = Cl, Br). X-ray
- crystallography then allowed us to understand the host–guest interactions at the molecular level. As illustrated in the molecular structures in Figure 4, above the centroid of each tetrazine ring there resided a chloride or a bromide. The distance of the anion to the centroid of tetrazine ranged from 3.060 Å to
Morphology-tunable and pH-responsive supramolecular self-assemblies based on AB2-type host–guest-conjugated amphiphilic molecules for controlled drug delivery
Beilstein J. Org. Chem. 2019, 15, 1925–1932, doi:10.3762/bjoc.15.188

- -responsive supramolecular self-assemblies have been constructed, the controlled drug delivery induced by morphology transitions of these supramolecular self-assemblies on the basis of host–guest-conjugated monomers (HGCMs) are few reported. In this paper, the self-assembly behaviors of AB2-type HGCMs, e.g
- varied morphology could inhibit cancer cell proliferation, indicating their potential application in the field of drug delivery. Keywords: β-cyclodextrin; controlled drug delivery; host–guest interaction; stimuli-responsive; supramolecular self-assemblies; Introduction Supramolecular self-assemblies
- based on noncovalent interactions with dynamic nature and reversible property have attracted increasing attention in the fields of biomedicine [1][2][3][4][5][6][7], smart materials [8][9][10], etc. As one common noncovalent interaction [11], host–guest interaction has been used to effectively create
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- a hydrophobic effect, while the other one has a more directional character, or application of multidentate interaction sites [3]. Macrocyclic compounds with persistent hydrophobic cavities constitute a fundamental class of scaffolds for the construction of supramolecular host–guest complexes in
- manifested in chirality transfer. We will also present a crucial and non-intuitive solvent dependence. RSAs (e.g., 1) can be considered as analogues of calix[4]arene sulfonic acids (CSAs) – the class of macrocycles widely studied in the context of various host–guest interactions, especially with various
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- face with primary hydroxy groups and a wider secondary face with secondary hydroxy groups [6][7]. With the hydrophobic cavity and the hydrophilic hydroxy groups, CDs efficiently form host–guest complexes with different hydrophobic guests in aqueous medium [8][9]. Among the common CDs, γ-CD possesses a
- relatively large cavity size that could accommodate up to two aromatic guests to form 1:2 host–guest complexes, in contrast to the usual formation of 1:1 complex by α-CD and β-CD [10][11]. In addition to host–guest chemistry, the favourable binding of CDs to common organic scaffolds has also made CDs popular
- efficiently obtained by using both CB[6] and γ-CD which are interlocked onto the axle by orthogonal interactions. Of note, the γ-CD in all the rotaxanes are only singly threaded, suggesting that further guest molecules could bind to the γ-CD cavity to give interesting host–guest complexes with the [n
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- ; macrocycles; macrocyclic arene; Introduction Macrocyclic host molecules [1][2] play a significant role in host–guest chemistry. Compared with noncyclic molecules, the structures of macrocyclic hosts can greatly enhance the host–guest complexation ability through preorganization. Moreover, cyclic structures
- methenyl groups. They have been a kind of important macrocyclic host molecules during the last decades due to their unique structures and a wide range of applications in host–guest chemistry [13][14][15][16][17][18], self-assembly [19], biomedicine [20] and materials science [21][22]. The derivatives of
- macrocyclic arenes with diverse functional groups are also important for the development of various new host–guest supramolecular systems [23][24][25][26][27][28][29]. Helic[6]arenes [30], a new kind of macrocyclic arenes, are composed of 2,6-dihydroxy-substituted triptycene subunits bridged by methylene
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
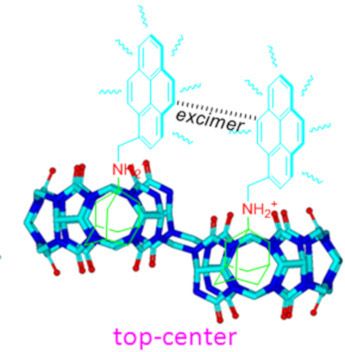
- ]. Keywords: fluorescent; host–guest interaction; macrocycles; molecular recognition; nor-seco-cucurbit[10]uril; pyrene; Introduction Host–guest interactions that trigger molecular recognition are a current topic of interest. For example, understanding the protein–ligand molecular recognition is of paramount
- importance in the study of enzymatic catalysis and allosteric regulation of cell signaling, as well as in the design of efficient drugs that utilize host–guest interactions [1]. Cucurbit[n]urils (Q[n]s or CB[n]s) [2][3] having been viewed to have high potential use in host–guest chemistry in aqueous solution
- ] was functionalized with imidazolidone (host-2, Scheme 1) [28]. Herein, we report the supramolecular host–guest interactions of the two cavities of NS-CB[10]-based host-1 and host-2 with two unsymmetrical ADA-based derivatives (G1 and G2, Scheme 1). As expected, hosts-1 and -2 are capable of
Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- guest receptors [4][5]. Planar-chiral macrocyclic molecules are particularly interesting in the context of the host–guest complexation properties [6][7][8]. Pillararenes are typical examples of this type of compounds and have attracted considerable attention due to their facile chemical synthesis and
- was 4.8 ns, which is very similar to DPA-6 (5.0 ns) and the non-substituted DPA (5.3 ns) [42]. The fact that grafting DPA units in one host did not influence the fluorescent quantum yield together with the appealing host–guest properties make P5A-DPA an ideal candidate for applications as acceptor for
- adding water into the THF solution. This work presented a new strategy for achieving versatile planar chiral hosts, and these hosts will have potential applications in various fields such as supramolecular sensing, host–guest recognition and triplet–triplet annihilation upconversion. Experimental General
Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding
Beilstein J. Org. Chem. 2019, 15, 1592–1600, doi:10.3762/bjoc.15.163

- supramolecular host–guest structures. The water content of CDs has been a subject of numerous investigations. The data (both experimental and theoretical) are scattered and, still, no consensus has been reached on the number and position of water molecules, and the energetics of the hydration/dehydration of
2,3-Dibutoxynaphthalene-based tetralactam macrocycles for recognizing precious metal chloride complexes
Beilstein J. Org. Chem. 2019, 15, 1460–1467, doi:10.3762/bjoc.15.146

- , hydrogen bonds are detected between the amide protons and H6 of 1 and the chloride ions of the guest (H···Cl distance: 2.48, 2.77 and 2.90 Å). Consequently, the electrostatic interaction and hydrogen bonds are the main driving forces for the host–guest complex. It is noted that the four amide protons are
- macrocycle, which make it conformationally adaptive to maximize the binding affinities. In addition, the macrocycle shows fluorescent quenching when adding the chloride metal complexes in its solution and may be used as a fluorescent sensor for the detection of these coordination complexes. Keywords: host
- –guest chemistry; macrocycles; molecular recognition; precious metal chloride complexes; Introduction Macrocyclic receptors are the major workhorses in supramolecular chemistry. Design and synthesis of new macrocyclic receptors with new properties is always attractive but is also challenging [1























































































































































































































































