Search results
Search for "dimer" in Full Text gives 374 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Synthetic approaches to bowl-shaped π-conjugated sumanene and its congeners
Beilstein J. Org. Chem. 2020, 16, 2212–2259, doi:10.3762/bjoc.16.186

- have attempted various combinations of Ti(IV) and reducing agents but Cp2TiCl2 with Zn powder provided the better results compared to the other combinations [14][40]. Additionally, they prepared benzyl–benzyl coupled sumanene dimer 48 by means of oxidative dimerization via sumanenyl monoanion
- (DFT) calculations. As we are aware that if the carbene formed is a singlet then a C–H bond inserted product is predominating whereas if the dimer is the major product along with the minor C–H bond inserted product then the triplet carbene is generated. During their study, they obtained the C–H
- inserted product 61 in 65% along with the dimer 62 in 18% yield with cyclohexane, confirming that the reaction precedes via the singlet state, albeit it was a triplet species in the ground-state. They further reported the applicability of the carbene precursor 59 by reacting it with different thiocarbonyl
Isolation and structure determination of a tetrameric sulfonyl dilithio methandiide in solution based on crystal structure analysis and 6Li/13C NMR spectroscopic data
Beilstein J. Org. Chem. 2020, 16, 2057–2063, doi:10.3762/bjoc.16.172

- –dimer equilibrium. Bonding of the dianionic C atoms 6Li NMR spectroscopy of tetramer 2a-I had revealed a dianionic carbon atom, C1B, carrying two different Li atoms, a bonding situation which is reflected by the two different 6Li,13C coupling constants. It was thus of particular interest to see whether
The biomimetic synthesis of balsaminone A and ellagic acid via oxidative dimerization
Beilstein J. Org. Chem. 2020, 16, 2026–2031, doi:10.3762/bjoc.16.169
- aqueous conditions to prevent complexation of the reagent and the starting material. Of the Lewis acids used, stannic chloride proved to be the most effective oxidant for dimerization (Table 1). However, the hypervalent iodine reagents PIFA and PIDA gave better results overall, affording dimer 18 in 63
Pauson–Khand reaction of fluorinated compounds
Beilstein J. Org. Chem. 2020, 16, 1662–1682, doi:10.3762/bjoc.16.138

- could not be detected in the crude reaction mixtures. Instead, the major isolated species was dimer 45. However, the fact that 45 bears the cyclopentenone core suggests that the desired PKR does indeed take place, albeit as an intermediate before a secondary transformation to form the final dimer
Rearrangement of o-(pivaloylaminomethyl)benzaldehydes: an experimental and computational study
Beilstein J. Org. Chem. 2020, 16, 1636–1648, doi:10.3762/bjoc.16.136
- )benzaldehydes under acidic conditions resulted in the formation of the regioisomeric aldehydes and/or dimer-like products. Detailed NMR studies and single-crystal X-ray measurements supported the structure elucidation of the compounds. DFT calculations were also carried out to clarify the reaction mechanism
- , and to explain the observed product distributions and structural variances in the dimer-like products. Studies on the transformation of unsubstituted o-(pivaloylaminomethyl)benzaldehyde under similar conditions were presented as well. Keywords: DFT calculations; NMR; reaction mechanism; rearrangement
- , gave isomeric aldehyde 2a (48%) and the dimer-like racemic product 3a (11%). Both transformations were rationalized by the intermediacy of the isoindole 4a (Scheme 1). The formation of aldehyde 2a can be explained by a protonation of the ring tautomer 1a, followed by an acid-catalyzed water elimination
Facile synthesis of 7-alkyl-1,2,3,4-tetrahydro-1,8-naphthyridines as arginine mimetics using a Horner–Wadsworth–Emmons-based approach
Beilstein J. Org. Chem. 2020, 16, 1617–1626, doi:10.3762/bjoc.16.134
- available iodide 29. The formation of compound 30 proceeded in 21% yield, with alcohol 31 and dimer 32 also formed in 20% and 5% yield, respectively (Scheme 9). Indeed, when iodide 29 was replaced with bromide 33 and tosylate 34 no formation of compound 30 was observed, with alcohol 31 and dimer 32
- accounting for the major products. Acidic deprotection of phosphoramidate 30 afforded amine (R)-23 in 92% yield in >99% ee, offering an alternative route to the Horner–Wadsworth–Emmons-based approach. The mechanism of the formation of alcohol 31 and dimer 32 was not fully explored although it is likely to
- occur via a radical pathway. When iodide 29 was replaced by a superior oxidant in 1,2-dibromoethane, formation of dimer 32 increased (17% isolated yield). This supports the previously reported proposals that dimerisation occurs via single-electron oxidation of the 2-picolyl anion by the halide
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- the parallel dimer of CHC (p(CHC)2), which binds two molecules of the neurotransmitter N-acetylneuraminic acid (NANA) in a cooperative fashion (K1 = 143, K2 = 5.1 × 103 M−1). Recently we rationalized that since our peptide building blocks consist of the same binding motifs as binding proteins (amines
- combination of cyclic tripeptide dimer. Each peptide dimer has two peaks because of the two constitutional isomers. Note that intensities between different compounds are not directly comparable in mass spectrometry. Upon addition of certain templates, notable changes were found in the chromatograms of the CFC
- by co-elution with the FF template. This is a not an issue for the YY containing sample as the template peak maximum lays between the first and second tripeptide dimer peak. We therefore concluded that the binding affinities of the receptor template pairs should be FF > YY > AA. In order to
Oxime radicals: generation, properties and application in organic synthesis
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- radicals with the formation of a C–O bond (dimer 4b, oxidation of diisopropyl oxime 1b), an O–N bond (dimer 4c, oxidation of benzophenone oxime 1c), and an N–N bond (dimer 4d, oxidation of benzaldoxime 1d, see also [58]) was observed. As a rule, the initial dimers of iminoxyl radicals are unstable, which
- established that the studied iminoxyl radicals reversibly dimerized in the solution. For sterically unhindered dialkyliminoxyl radicals, the radical–dimer equilibrium was quickly reached, shifted toward the dimer, while a first-order decay kinetics of was observed for the iminoxyl radical. For sterically
- hindered tert-butylmethyliminoxyl and diisopropyliminoxyl radicals, as well as for diaryl and alkylaryliminoxyl radicals, the radical–dimer equilibrium was reached slowly, it was shifted toward the free radical, and a second-order decay kinetics was observed. The first synthesized long-lived iminoxyl
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- , this work reports the first example of a linearly connected porphyrin dimer, linked through the bridgehead carbons of triptycene. Symmetric and unsymmetric examples of these complexes are demonstrated and single crystal X-ray analysis of an unsymmetrically substituted porphyrin dimer highlights the
- material was purified via column chromatography using silica gel. The BODIPY dimer 7 was prepared using 6 [38] and was obtained as a fluorescent orange solution or as pink-orange crystals in a 30% yield. Similarly, the Pd-catalyzed Sonogashira cross-coupling reaction [39] was successful for the reaction
- . In an effort to synthesize an unsymmetrically substituted porphyrin dimer, triptycene 12 was utilized (Scheme 2). Firstly, the zinc porphyrin 13 [41] was coupled to triptycene 12 using Sonogashira methods that were previously successfully employed with compound 11. The desired compound 14 could be
Design and synthesis of diazine-based panobinostat analogues for HDAC8 inhibition
Beilstein J. Org. Chem. 2020, 16, 628–637, doi:10.3762/bjoc.16.59

- Biotek Synergy HTX multimodal plate reader after incubation for 30 minutes. Preparation of the HDAC8 receptor for docking The HDAC8 crystal structure (protein database pdb: 1W22) [32] was utilized as the docking receptor for all compounds. This receptor is crystalized as a dimer thus only the A chain was
Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions
Beilstein J. Org. Chem. 2020, 16, 305–316, doi:10.3762/bjoc.16.30
- ]. Noteworthy, depending on the nature of the bidentate ligand used, the corresponding copper complex could be isolated as monomer or dimer, and both were air-stable. Among the new complexes, the reactivity of [(bpy)CuSeCF3]2 in trifluoromethylselenolations was thoroughly investigated using a large panel of
Reversible photoswitching of the DNA-binding properties of styrylquinolizinium derivatives through photochromic [2 + 2] cycloaddition and cycloreversion
Beilstein J. Org. Chem. 2020, 16, 111–124, doi:10.3762/bjoc.16.13
- Information File 1). However, it was observed that further irradiation of the nitro-substituted derivative 3d furnished the dimer in acetonitrile, as shown by the development of the characteristic cyclobutane protons at 4.85–4.95 ppm. In contrast, the NMR-spectroscopic analysis in D2O showed that the
- . Supporting Information File 1) that may have been followed by a [2 + 2] photodimerization (Scheme 2). However, it is difficult to explain why the photocycloaddition of the Z-isomers Z-3b and Z-3c led exclusively to the dimer, because such a selectivity has not been reported so far for (Z)-stilbenes. On the
- cyclobutane 4b at 315 nm in H2O, the monomer 3b formed, as indicated by the development of its characteristic absorption band (Figure 7B). After 30 min, the reaction was almost complete, however, dimer 4b still remained in solution in the photostationary state. Interactions of the photodimer 4b with DNA The
Halogen-bonding-induced diverse aggregation of 4,5-diiodo-1,2,3-triazolium salts with different anions
Beilstein J. Org. Chem. 2020, 16, 78–87, doi:10.3762/bjoc.16.10
- triclinic space group . The single crystal of 2-OAc crystallizes in the monoclinic space group P21/c. The package diagram shows a dimer which is almost a rectangle (Figure 4). As shown in Table 1, The C–I···O distances are 2.547(2) Å and 2.582(2) Å. The C–I···O angles are 174.60(7)° and 170.38(7)°. The RXB
- -triazolium salts with different anions. When the anion is chloride, bromide or iodide, the crystal is a tetramer. Strong XB was observed in these forms. When the anion is changed to tetrafluoroborate, it takes Chinese lantern shape as a trimer. Triazolium trifluoroacetate and acetate exist as a dimer, while
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
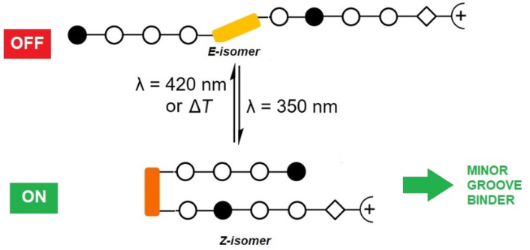
- -protected photoswitchable ω-amino acid 1 (Scheme 2A) was performed based on a procedure by Aemissegger et al. [35]. The ω-amino acid 1 was subsequently used in the solid-phase synthesis or for the synthesis of the dimer 7 in solution (Scheme 2B). The incorporation of β-alanine as a 'molecular spring' was
- –Im–OH dimer 5 was obtained [42][43]. Owing to the poor coupling results of Fmoc–Py–OH to the amino function of a terminal Im moiety, the Fmoc–Azo–Im–OH dimer 7 was synthesized in 73% yield by coupling to the amine 4, as shown in Scheme 2B. HBTU as a coupling reagent and DIPEA as a base were employed
- monomers 2–4, and Fmoc–Py–Im–OH 5. B) Synthesis of the dimer Fmoc–Azo–Im–OH 7: a) DCC, THF, rt, 5 min, then 4, rt, 15 h, 41%; b) BF3∙Et2O, dichloromethane, 0 °C→rt, 12 h, 73%. Binding constants and melting temperatures of the interaction of the polyamides with dsDNA obtained by circular dichroism
Pigmentosins from Gibellula sp. as antibiofilm agents and a new glycosylated asperfuran from Cordyceps javanica
Beilstein J. Org. Chem. 2019, 15, 2968–2981, doi:10.3762/bjoc.15.293

- with pigmentosin A, a 3,4-dihydro-α-naphthopyrone dimer with a 7,7′-dimethoxy pattern, by comparing its spectroscopic data with the published data for pigmentosin A [12]. Nevertheless, the chirality of the stereogenic centers C-3/C-3′ as well as the atropisomerism at the 6,6′ axis of pigmentosin A (1
- side of the dimer (Figure 3). Thus, compound 2 was determined to be a new asymmetrical dimer of 3,4-dihydro-α-naphthopyrone and 3-(propan-2-ol)-3,4-dihydro-α-naphthopyrone, a new member of the bis(naphtho-α-pyrone) group [12][13], for which we propose the trivial name pigmentosin B. To determine the
Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1H-pyridines by Michael addition–cyclocondensation sequences
Beilstein J. Org. Chem. 2019, 15, 2684–2703, doi:10.3762/bjoc.15.262
- substituent in the 3-position [29]. Most syntheses generating 1H-pyridines make use of ethyl cyanoactate as a starting material. It can react with itself and forms a dimer by selfcondensation, catalyzed by transition metals [30][31]. Intrigued by the unusual pseudo-four-component AMAC synthesis we
- conditions as for the 1H-pyridine from alkynone 3a was conducted, but only starting material could be isolated. Another option for the formation of the 1H-pyridine 5a was envisioned by an in situ generation of a dimer of ethyl cyanoacetate (4). The dimer 7 can be synthesized by iridium catalysis [30]. With
- dimer 7 in hand, we performed the reaction at 75 °C for 16 h, but we only could isolate 1H-pyridine 8a, which still contains an ester group (Scheme 8). Therefore, the in situ formation of the dimer starting from the alkynone 3a and ethyl cyanoacetate (4) was excluded for the formation of the 1H-pyridine
Formation of alkyne-bridged ferrocenophanes using ring-closing alkyne metathesis on 1,1’-diacetylenic ferrocenes
Beilstein J. Org. Chem. 2019, 15, 2534–2543, doi:10.3762/bjoc.15.246

- performed RCAM with a higher substrate concentration of 1a of 21 mM. When further increasing the concentration of 1a to 125 mM a product mixture of the monomeric ferrocenophane 2a, the fully ring-closed dimeric compound as well as the open dimer can be identified with the help of mass spectrometry (see also
- analogue of the formula [Fe{C5H4COO(CH2)3 C≡}2]2 in a ratio of approximately 4:1. Related NMR spectra can be found in Supporting Information File 1 (Figures S9 and S10). Separation of the monomer 2b could be achieved with column chromatography on silica gel with a yield of 53%. The ring-closed dimer
Anion-driven encapsulation of cationic guests inside pyridine[4]arene dimers
Beilstein J. Org. Chem. 2019, 15, 2486–2492, doi:10.3762/bjoc.15.241

- to the electron-poor nature of the pyridine ring. Herein, we demonstrate the encapsulation of Me4N+ cations inside a dimeric hydrogen-bonded pyridine[4]arene capsule, which contradicts with earlier assumptions. The complexation of a cationic guest inside the pyridine[4]arene dimer has been detected
- could not be verified and the anions were assumed to interact with the pyridinearene cavity. Inclusion complexes of anions within pyridinearene dimers were also theoretically studied by DFT calculations, but using a truncated pyridinearene dimer model [8]. However, previous studies have also shown that
- -rim alkyl chains [11]. A PF6− anion was bound to the lower rim of the pyridine[4]arene dimer via CH–anion and CH–F interactions, while the neutral guest was hosted inside the dimer. Calculated electrostatic potential (ESP) surfaces revealed that the cavity does not possess any significant partial
Combining the Ugi-azide multicomponent reaction and rhodium(III)-catalyzed annulation for the synthesis of tetrazole-isoquinolone/pyridone hybrids
Beilstein J. Org. Chem. 2019, 15, 2447–2457, doi:10.3762/bjoc.15.237
- the relatively cheap complex (p-cymene)ruthenium(II) chloride dimer, in the presence of copper(II) acetate as oxidant under conventional heating. Despite all effort put in this attempt, the isolated yields were in the range of 14–62%, with the highest yield achieved after 24 h of reaction using 10 mol
- use of pentamethylcyclopentadienylrhodium(III) chloride dimer, [Cp*RhCl2]2, allowed reducing both the loading to 5 mol % and the reaction time to 12 h (Table 1). The addition of cesium acetate appeared to be required, probably to convert the pre-catalyst into the active catalyst. After some additional
Photochromic diarylethene ligands featuring 2-(imidazol-2-yl)pyridine coordination site and their iron(II) complexes
Beilstein J. Org. Chem. 2019, 15, 2428–2437, doi:10.3762/bjoc.15.235
- that bond. Two iron(II) centers are 8.057 Å apart in the dimer. The ligand 6 appears in the open-ring form and the parallel conformation [8], with the α-methyl groups pointing in similar directions. While still appearing in a distorted half-chair conformation, the phenyl substituent of the
- 2.1190(19) Å. The pyridine and imidazole Fe–N bond distances are 2.2467(19) Å and 2.1793(18) Å, which are very similar to those found for the dimer 8. On the contrary, the Fe–O bond distances to the β-keto ester moiety differ much less with 2.0812(15) Å and 2.0098(15) Å. The iron–donor bond distances are
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- release of "high-energy" water molecules from the cavity of 2 [50][51]. Determining the X-ray structure was challenging; the difficulty lies in the fact that both the cage and the encapsulated guest dimer exist in the form of two different conformations. Figure 3 shows (E-1)22 that features the main
- with any of the three methyl groups. Furthermore, we note that the resonance due to Hb – very broad in complex (E-1)22 as a consequence of dimer formation – becomes as sharp as those of the other protons after UV irradiation (compare spectra i and ii in Figure S23, Supporting Information File 1). Taken
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- ). CotB2 is arranged as a homo-dimer in crystallo [36][38] and it was demonstrated that CotB2 exists as a homo-dimer in solution as well [38]. The two active sites of CotB2 are arranged in an antiparallel fashion, resembling the arrangement initially observed for the monoterpene (+)-bornyl diphosphate
- synthase [47] and the sesquiterpene trichodiene synthase [48], but is in contrast to the parallel dimer as described for farnesyl diphosphate synthase [49]. Structurally, CotB2 is most closely related to the fungal monoterpene synthase aristolochene (PDB-ID 2OA6; [50]), and epi-isozizaene synthase (PDB-ID
Fluorescent phosphorus dendrimers excited by two photons: synthesis, two-photon absorption properties and biological uses
Beilstein J. Org. Chem. 2019, 15, 2287–2303, doi:10.3762/bjoc.15.221

- derivatives grafted on the surface of phosphorhydrazone dendrimers. In this case, a β-diketone functionalized by a phenol was first grafted on the surface of the dendrimer [47], then BF3 was added to obtain the dioxaborines (Scheme 3). Monomer, dimer, and all generations of the dendrimer from zero (6 terminal
- dimer and 33% for the G1 dendrimer, but a 15% decrease of the TPA response was measured for the G2 dendrimer [53]. In addition, in that case, also the fluorescence quantum yield was found to decrease dramatically with an increasing number of fluorophores. TPA fluorophore as core of dendrimers None of
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups
Beilstein J. Org. Chem. 2019, 15, 2213–2270, doi:10.3762/bjoc.15.218
- Scheme 45a. Cyclooctadiene iridium chloride dimer, [IrCl(COD)]2, was an effective catalyst to promote this fluorination with Et3N·3HF, forming allylic fluorides in moderate to good yields. This facile method shows a good regioselectivity to gain the branched isomer within 1 h. Later in 2017, they
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- [TCNQ]−· radical anion, dragging the second [TCNQ]− near to the cavity by the help of the electrostatic stabilization supplied by the 1,2,3-triazolium cations, coupling of the two [TCNQ]−· anion radicals in the cavity and rapid formation of the sigma-connected dimer, reduction of the photogenerated
- porphyrin cation by the iodide counterions of the triazolium macrocycle, either before or after the constraining of the second [TCNQ]−· anion radical, finally, the dimer is either ejected from the host or is displaced by the [TCNQ]−· anion radical. It was clearly shown that the macrocycle 15 acts as a
- compound 8. Chemical structures of compound 9. Chemical structures of compound 10, 11 and 12. Chemical structure of compound 13. Chemical structure of compound 15 including the sigma-connected TCNQ dimer. Chemical structure of compound 16 for the kinetic resolution of epoxides. Chemical structure of






















































































































































































































































































































































































































































































































































































