Search results
Search for "diols" in Full Text gives 176 result(s) in Beilstein Journal of Organic Chemistry.
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
- Minzan Zuo,
- Krishnasamy Velmurugan,
- Kaiya Wang,
- Xueqi Tian and
- Xiao-Yu Hu
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- between thiol-functionalized β-CD and oleic acid-protected CdS nanocrystals [29]. These spherical CdS–CD nanoparticles could be employed as a photocatalyst for the dehydrogenation of alcohols to aldehydes (at a low concentration of the reactant of 1 mM, ≥92% selectivity) or diols (at a high concentration

Graphical Abstract

Figure 1: Chemical structures of representative macrocycles.

Figure 2: Ba2+-induced intermolecular [2 + 2]-photocycloaddition of crown ether-functionalized substrates 1 a...

Figure 3: Energy transfer system constructed of a BODIPY–zinc porphyrin–crown ether triad assembly bound to a...

Figure 4: The sensitizer 5 was prepared by a flavin–zinc(II)–cyclen complex for the photooxidation of benzyl ...

Figure 5: Enantiodifferentiating Z–E photoisomerization of cyclooctene sensitized by a chiral sensitizer as t...

Figure 6: Structures of the modified CDs as chiral sensitizing hosts. Adapted with permission from [24], Copyrigh...

Figure 7: Supramolecular 1:1 and 2:2 complexations of AC with the cationic β-CD derivatives 16–21 and subsequ...

Figure 8: Construction of the TiO2–AuNCs@β-CD photocatalyst. Republished with permission of The Royal Society...

Figure 9: Visible-light-driven conversion of benzyl alcohol to H2 and a vicinal diol or to H2 and benzaldehyd...

Figure 10: (a) Structures of CDs, (b) CoPyS, and (c) EY. Republished with permission of The Royal Society of C...

Figure 11: Conversion of CO2 to CO by ReP/HO-TPA–TiO2. Republished with permission of The Royal Society of Che...

Figure 12: Thiacalix[4]arene-protected TiO2 clusters for H2 evolution. Reprinted with permission from [37], Copyri...

Figure 13: 4-Methoxycalix[7]arene film-based TiO2 photocatalytic system. Reprinted from [38], Materials Today Chem...

Figure 14: (a) Photodimerization of 6-methylcoumarin (22). (b) Catalytic cycle for the photodimerization of 22...

Figure 15: Formation of a supramolecular PDI–CB[7] complex and structures of monomers and the chain transfer a...

Figure 16: Ternary self-assembled system for photocatalytic H2 evolution (a) and structure of 27 (b). Figure 16 reprodu...

Figure 17: Structures of COP-1, CMP-1, and their substrate S-1 and S-2.

Figure 18: Supramolecular self-assembly of the light-harvesting system formed by WP5, β-CAR, and Chl-b. Reprod...

Figure 19: Photocyclodimerization of AC based on WP5 and WP6.
Recent progress in the synthesis of homotropane alkaloids adaline, euphococcinine and N-methyleuphococcinine
- Dimas J. P. Lima,
- Antonio E. G. Santana,
- Michael A. Birkett and
- Ricardo S. Porto
Beilstein J. Org. Chem. 2021, 17, 28–41, doi:10.3762/bjoc.17.4
- providing a mixture of monoprotected diols (−)-53 and 54 in a 10:1 ratio for the least sterically hindered alcohol in 98% yield. After chromatographic separation, (−)-53 underwent radical-induced Barton–McCombie deoxygenation (AIBN, Bu3SnH) to form (−)-55 in 82% yield. The double deprotection of (−)-55

Graphical Abstract

Figure 1: Homotropane (azabicyclononane) systems.

Figure 2: Alkaloids (−)-adaline (1), (+)-euphococcinine (2) and (+)-N-methyleuphococcinine (3).

Scheme 1: Synthetic strategies before 1995.

Scheme 2: Synthesis (±)-adaline (1) and (±)-euphococcinine (2). Reagents and conditions: i) 1. dihydropyran, ...

Scheme 3: Synthesis (+)-euphococcinine (2). Reagents and conditions: i) H2O2, SeO2 (cat), acetone, rt, 88%; i...

Scheme 4: Synthesis (+)-euphococcinine (2). Reagents and conditions: i) 2,4-bis(4-phenoxyphenyl)-1,3-dithia-2...

Scheme 5: Synthesis of (±)-euphococcinine precursor (±)-42. Reagents and conditions: i) Bu3SnH, AIBN, toluene...

Scheme 6: Synthesis of (−)-adaline (1). Reagents and conditions: i) LiH2NBH3, THF, 40 °C, 88%; ii) TPAP, NMO,...

Scheme 7: Synthesis of (−)-adaline (1) and (−)-euphococcinine (2). Reagents and conditions: i) 1. BuLi, t-BuO...

Scheme 8: Synthesis of (−)-adaline (1). Reagents and conditions: i) Ref. [52]; ii) Et3N, TBDMSOTf, CH2Cl2, 0 °C t...

Scheme 9: Synthesis of (+)-euphococcinine (2). Reagents and conditions: i) 1. Cp2ZrCl2,AlMe3, CH2Cl2; 2. p-me...

Scheme 10: Synthesis of (−)-adaline 1. Reagents and conditions: i) 1. CuBr.DMS, Et2O/DMS, -42 ºC; 2. 1-heptyne...

Scheme 11: Synthesis of (−)-euphococcinine (2) and (−)-adaline (1). Reagents and conditions: i) 102, KHMDS, Et2...

Scheme 12: Synthesis of N-methyleuphococcinine 3. Reagents and conditions: i) 108 (1.5 equiv), 3,5-di-F-C6H3B(...
The B & B approach: Ball-milling conjugation of dextran with phenylboronic acid (PBA)-functionalized BODIPY
- Patrizia Andreozzi,
- Lorenza Tamberi,
- Elisamaria Tasca,
- Gina Elena Giacomazzo,
- Marta Martinez,
- Mirko Severi,
- Marco Marradi,
- Stefano Cicchi,
- Sergio Moya,
- Giacomo Biagiotti and
- Barbara Richichi
Beilstein J. Org. Chem. 2020, 16, 2272–2281, doi:10.3762/bjoc.16.188

- related esters are relevant synthetic building blocks widely employed as cross-coupling reagents [14] as well as protecting groups for polyols and diamines [15][16]. Moreover, the reversible covalent interaction of boronic acids with specifically oriented cis-1,2 and 1,3-diols has been successfully
- nanoparticles, depending on the degree of substitution. For example, reaction of vicinal diols of dextran with hydrophobic PBA generates boronate esters, which form nanoparticles in water and are capable to host the anticancer drug doxorubicin [27]. In this report, the advantages of the milling process, such as
- ), by following a straightforward synthetic strategy recently reported by some of us [26]. Then, in order to assess the efficacy of the solid-state milling approach in the formation of boronate esters between the PBA moiety of 1 and the vicinal diols of dextran (Dex), both mechanochemical and

Graphical Abstract

Figure 1: Structure of PBA-BODIPY (1) and schematic representation of dextran (Dex) and PBA-BODIPY conjugated...

Scheme 1: Schematic representation of dextran/PBA-BODIPY bioconjugations in: A. conventional solution-based c...

Figure 2: A) Amount of recovered PBA-BODIPY (1, i.e., nonreacted 1) in the mixtures DMSO/EtOH and in the seri...

Figure 3: A) UV–vis absorption and B) fluorescence emission spectra (λexc = 380 nm) of the BODIPY-dextran con...

Figure 4: A) Hydrodynamic diameter of (nm) conjugate Dex-1b (at 1 mg/mL in H2O, black curve) and PBS (red cur...

Figure 5: Fluorescence emission spectra of pyrene (4.4 × 10−8 M) in water and in a water solution in the pres...
Selective preparation of tetrasubstituted fluoroalkenes by fluorine-directed oxetane ring-opening reactions
- Clément Q. Fontenelle,
- Thibault Thierry,
- Romain Laporte,
- Emmanuel Pfund and
- Thierry Lequeux
Beilstein J. Org. Chem. 2020, 16, 1936–1946, doi:10.3762/bjoc.16.160
- VIII was observed due to the difficulty of phosphorylation of the substrate by kinases [16]. The first kinase phosphorylation step is generally rate limiting, and the prior introduction of a phosphate or phosphonate function can circumvent this problem. The preparation of diols VIII was realized by
- olefination of a protected 1,3-dihydroxypropanone (Figure 3). However, the selective introduction of functional groups is not possible in these diols as the two hydroxy groups present similar chemical reactivity. Other approaches are available for a selective preparation of monofluoroalkenes including

Graphical Abstract

Figure 1: Representative fluorinated nucleos(t)ides and acyclonucleotides.

Figure 2: Acyclonucleotides as nucleotide surrogates.

Figure 3: Olefination approaches and ring-opening of oxetane derivatives.

Scheme 1: Preparation of fluoroakylidene-oxetanes and their ring-opening reactions.

Scheme 2: Synthesis of benzyloxy-substituted fluoroethylidene-oxetane derivative 8.

Scheme 3: Effect of the medium on the selective formation of derivative 10.

Scheme 4: Mechanism for the formation of dihydrofuran 10.

Scheme 5: Mechanism for the formation of unsaturated lactones 14 and 15.

Scheme 6: Opening reaction of ethyl 2-(oxetanyl-3-idene)acetate (16).

Scheme 7: Functionalization of bromomethyllactone 15 and its analogues.

Scheme 8: Functionalization by substitution reaction of the bromide E-1d vs ring-opening reaction of the oxet...

Scheme 9: Preparation of tetrasubstituted fluoroalkenes.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
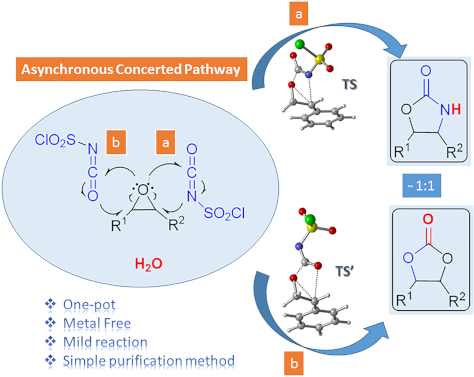
- shorter reaction times under mild conditions using a simple purification method. Apparently, our protocol describes a reasonable methodology for the conversion of epoxides to protected 1,2-diols and 2-amino alcohols. Attention is drawn on these 1,2-oxygen and/or nitrogen units since they are present in
- using a safe, inexpensive, metal-free reagent, a simple purification method and shorter reaction times via a one-pot reaction. The study presents a useful method for one-pot conversion of epoxides to protected 1,2-diols and 2-amino alcohols in one reaction. In the computational part of the study, the

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- , or disulfonates of alkane-1,3-diols with sodium sulfide. The intramolecular substitution of 3-mercaptoalkyl halides or sulfonates is a similar strategy for the preparation of thietanes [12][13][14]. Alternatively, inter- and intramolecular photochemical [2 + 2] cycloadditions (thia-Paternò–Büchi
- )-1-tosylazetidine (22) with sodium sulfide followed by the detosylation with Mg in MeOH afforded 1,6-thiazaspiro[3.3]heptane (24) [36] (Scheme 4). 2.1.2 Synthesis via double nucleophilic displacements of disulfonates of alkane-1,3-diols: Considering that 6-amino-3-azaspiro[3.3]heptane was evaluated
- thietanes. Indeed, the direct cyclization of the 3-mercaptopropan-1-ol unit in 60 with Ph3P(OEt)2 as a reagent was realized in the synthesis of the spirothietane derivative 61 [44] (Scheme 14). Also, 1,3-diols were considered as precursors of γ-mercaptoalkanols. A Japanese group developed a new method to

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Synthesis of 3-substituted isoxazolidin-4-ols using hydroboration–oxidation reactions of 4,5-unsubstituted 2,3-dihydroisoxazoles
- Lívia Dikošová,
- Júlia Laceková,
- Ondrej Záborský and
- Róbert Fischer
Beilstein J. Org. Chem. 2020, 16, 1313–1319, doi:10.3762/bjoc.16.112
- isoxazolidines by means of dihydroxylation [9][10] and epoxidation [11][12] reactions. Regarding the stereochemistry, almost all of the realized additions proceed with an excellent trans stereoselectivity relative to the substituent at C-3, giving isoxazolidine-4,5-diols and isoxazolidinyl epoxides with a C-3/4
- , respectively, according to our procedure [7][10]. The compounds 5a and 5b were first converted into the isoxazolidine-4,5-diols by the treatment with potassium osmate/N-methylmorpholine N-oxide (NMO). The dihydroxylation reactions proceeded with an excellent trans selectivity with respect to the substituent at
- the C-3 carbon atom. The obtained products were benzoylated with benzoyl chloride/pyridine in the presence of DMAP to give the fully benzoylated isoxazolidine-4,5-diols 6a and 6b, which were subsequently treated with Et3SiH (3 equiv) and TMSOTf (2 equiv) in anhydrous CH2Cl2 at room temperature for 2 h

Graphical Abstract

Figure 1: 3-Substituted isoxazolidin-4-ols resembling 3-hydroxypyrrolidines.

Scheme 1: Synthetic approach towards isoxazolidin-4-ols via the regioselective reductive cleavage of the C5–O...

Scheme 2: Hydroboration-oxidation of 4,5-unsubstituted 2,3-dihydroisoxazoles.

Figure 2: Selected NOE enhancements observed in the isoxazolidin-4-ol trans-8a. The arrows show the NOESY cor...

Scheme 3: Dess-Martin oxidation of isoxazolidin-4-ols to ketones.

Scheme 4: Inversion of the relative configuration of the isoxazolidine ring.

Figure 3: Selected NOE enhancements observed in the isoxazolidin-4-ol cis-10a. The arrows show the NOESY corr...

Scheme 5: N-debenzylation via N-Troc-protected isoxazolidines.
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- enantioselective and chemoselective oxidations of many organic compounds. Notably, the Gryko’s group recently described an enantio- and diastereoselective approach involving a porphyrin-based photooxygenation of aldehydes with sequential reduction to yield chiral diols in yields up to 91% and significant er (up to

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- energy [54]. The aromatic carbonyl compounds were dissolved in isopropanol and exposed to direct sunlight for 7–10 days to give the corresponding 1,2-diols 92 in high to moderate yield. The excitation of the carbonyl compound 87 was followed by hydrogen atom abstraction from the solvent 89, affording the

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
- Balaram S. Takale,
- Ruchita R. Thakore,
- Elham Etemadi-Davan and
- Bruce H. Lipshutz
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- silyl ethers 89–92 (Scheme 18), thus showcasing the synergistic relationship between Pd and Cu catalysis [43]. Driven by the success of earlier results, the authors utilized 78 for reductive couplings between ketones 93 and imines 97 as electrophiles to form unsymmetrical 1,2-diols 94–96 and 1,2-amino

Graphical Abstract

Scheme 1: Pharmaceuticals possessing a silicon or boron atom.

Scheme 2: The first Cu-catalyzed C(sp3)–Si bond formation.

Scheme 3: Conversion of benzylic phosphate 6 to the corresponding silane.

Scheme 4: Conversion of alkyl triflates to alkylsilanes.

Scheme 5: Conversion of secondary alkyl triflates to alkylsilanes.

Scheme 6: Conversion of alkyl iodides to alkylsilanes.

Scheme 7: Trapping of intermediate radical through cascade reaction.

Scheme 8: Radical pathway for conversion of alkyl iodides to alkylsilanes.

Scheme 9: Conversion of alkyl ester of N-hydroxyphthalimide to alkylsilanes.

Scheme 10: Conversion of gem-dibromides to bis-silylalkanes.

Scheme 11: Conversion of imines to α-silylated amines (A) and the reaction pathway (B).

Scheme 12: Conversion of N-tosylimines to α-silylated amines.

Scheme 13: Screening of diamine ligands.

Scheme 14: Conversion of N-tert-butylsulfonylimines to α-silylated amines.

Scheme 15: Conversion of aldimines to nonracemic α-silylated amines.

Scheme 16: Conversion of N-tosylimines to α-silylated amines.

Scheme 17: Reaction pathway [A] and conversion of aldehydes to α-silylated alcohols [B].

Scheme 18: Conversion of aldehydes to benzhydryl silyl ethers.

Scheme 19: Conversion of ketones to 1,2-diols (A) and conversion of imines to 1,2-amino alcohols (B).

Scheme 20: Ligand screening (A) and conversion of aldehydes to α-silylated alcohols (B).

Scheme 21: Conversion of aldehydes to α-silylated alcohols.

Scheme 22: 1,4-Additions to α,β-unsaturated ketones.

Scheme 23: 1,4-Additions to unsaturated ketones to give β-silylated derivatives.

Scheme 24: Additions onto α,β-unsaturated lactones to give β-silylated lactones.

Scheme 25: Conversion of α,β-unsaturated to β-silylated lactams.

Scheme 26: Conversion of N-arylacrylamides to silylated oxindoles.

Scheme 27: Conversion of α,β-unsaturated carbonyl compounds to silylated tert-butylperoxides.

Scheme 28: Catalytic cycle for Cu(I) catalyzed α,β-unsaturated compounds.

Scheme 29: Conversion of p-quinone methides to benzylic silanes.

Scheme 30: Conversion of α,β-unsaturated ketimines to regio- and stereocontrolled allylic silanes.

Scheme 31: Conversion of α,β-unsaturated ketimines to enantioenriched allylic silanes.

Scheme 32: Regioselective conversion of dienedioates to allylic silanes.

Scheme 33: Conversion of alkenyl-substituted azaarenes to β-silylated adducts.

Scheme 34: Conversion of conjugated benzoxazoles to enantioenriched β-silylated adducts.

Scheme 35: Conversion of α,β-unsaturated carbonyl indoles to α-silylated N-alkylated indoles.

Scheme 36: Conversion of β-amidoacrylates to α-aminosilanes.

Scheme 37: Conversion of α,β-unsaturated ketones to enantioenriched β-silylated ketones, nitriles, and nitro d...

Scheme 38: Regio-divergent silacarboxylation of allenes.

Scheme 39: Silylation of diazocarbonyl compounds, (A) asymmetric and (B) racemic.

Scheme 40: Enantioselective hydrosilylation of alkenes.

Scheme 41: Conversion of 3-acylindoles to indolino-silanes.

Scheme 42: Proposed mechanism for the silylation of 3-acylindoles.

Scheme 43: Silyation of N-chlorosulfonamides.

Scheme 44: Conversion of acyl silanes to α-silyl alcohols.

Scheme 45: Conversion of N-tosylaziridines to β-silylated N-tosylamines.

Scheme 46: Conversion of N-tosylaziridines to silylated N-tosylamines.

Scheme 47: Conversion of 3,3-disubstituted cyclopropenes to silylated cyclopropanes.

Scheme 48: Conversion of conjugated enynes to 1,3-bis(silyl)propenes.

Scheme 49: Proposed sequence for the Cu-catalyzed borylation of substituted alkenes.

Scheme 50: Cu-catalyzed synthesis of nonracemic allylic boronates.

Scheme 51: Cu–NHC catalyzed synthesis of α-substituted allylboronates.

Scheme 52: Synthesis of α-chiral (γ-alkoxyallyl)boronates.

Scheme 53: Cu-mediated formation of nonracemic cis- or trans- 2-substituted cyclopropylboronates.

Scheme 54: Cu-catalyzed synthesis of γ,γ-gem-difluoroallylboronates.

Scheme 55: Cu-catalyzed hydrofunctionalization of internal alkenes and vinylarenes.

Scheme 56: Cu-catalyzed Markovnikov and anti-Markovnikov borylation of alkenes.

Scheme 57: Cu-catalyzed borylation/ortho-cyanation/Cope rearrangement.

Scheme 58: Borylfluoromethylation of alkenes.

Scheme 59: Cu-catalyzed synthesis of tertiary nonracemic alcohols.

Scheme 60: Synthesis of densely functionalized and synthetically versatile 1,2- or 4,3-borocyanated 1,3-butadi...

Scheme 61: Cu-catalyzed trifunctionalization of allenes.

Scheme 62: Cu-catalyzed selective arylborylation of arenes.

Scheme 63: Asymmetric borylative coupling between styrenes and imines.

Scheme 64: Regio-divergent aminoboration of unactivated terminal alkenes.

Scheme 65: Cu-catalyzed 1,4-borylation of α,β-unsaturated ketones.

Scheme 66: Cu-catalyzed protodeboronation of α,β-unsaturated ketones.

Scheme 67: Cu-catalyzed β-borylation of α,β-unsaturated imines.

Scheme 68: Cu-catalyzed synthesis of β-trifluoroborato carbonyl compounds.

Scheme 69: Asymmetric 1,4-borylation of α,β-unsaturated carbonyl compounds.

Scheme 70: Cu-catalyzed ACB and ACA reactions of α,β-unsaturated 2-acyl-N-methylimidazoles.

Scheme 71: Cu-catalyzed diborylation of aldehydes.

Scheme 72: Umpolung pathway for chiral, nonracemic tertiary alcohol synthesis (top) and proposed mechanism for...

Scheme 73: Cu-catalyzed synthesis of α-hydroxyboronates.

Scheme 74: Cu-catalyzed borylation of ketones.

Scheme 75: Cu-catalyzed borylation of unactivated alkyl halides.

Scheme 76: Cu-catalyzed borylation of allylic difluorides.

Scheme 77: Cu-catalyzed borylation of cyclic and acyclic alkyl halides.

Scheme 78: Cu-catalyzed borylation of unactivated alkyl chlorides and bromides.

Scheme 79: Cu-catalyzed decarboxylative borylation of carboxylic acids.

Scheme 80: Cu-catalyzed borylation of benzylic, allylic, and propargylic alcohols.
Asymmetric synthesis of CF2-functionalized aziridines by combined strong Brønsted acid catalysis
- Xing-Fa Tan,
- Fa-Guang Zhang and
- Jun-An Ma
Beilstein J. Org. Chem. 2020, 16, 638–644, doi:10.3762/bjoc.16.60
- no enantioselectivity at all. As arylboronic acids have been harnessed to enhance the Brønsted acidity in asymmetric organocatalysis in combination with chiral diols or chiral aminoalcohols [40][41][42][43][44], we envisioned that the simultaneous use of arylboronic acids and chiral Brønsted acids

Graphical Abstract

Scheme 1: Preparation of chiral aziridines from fluorinated diazo reagents.

Scheme 2: Substrate scope of chiral CF2-substituted aziridines from PhSO2CF2CHN2. General reaction conditions...

Scheme 3: Scale-up experiment to 4a and further synthetic transformations.
Synthesis of disparlure and monachalure enantiomers from 2,3-butanediacetals
- Adam Drop,
- Hubert Wojtasek and
- Bożena Frąckowiak-Wojtasek
Beilstein J. Org. Chem. 2020, 16, 616–620, doi:10.3762/bjoc.16.57
- the final product with ees not exceeding 95% [29], which is insufficient to be used as attractant for males of the Gypsy moth. In one of these methods the diols 5 and 7 were used as precursors in the synthesis of the cis-epoxides 1 and 3 (Scheme 1). A few methods for the synthesis of diols 5 and 7
- chiral centers for the synthesis of both enantiomers of disparlure 1 and 3 and monachalure 2 and 4. Compound 14 also offers the possibility of selective modification of one of the substituents as well as sequential introduction of aliphatic chains. After deprotection of the butanediacetal group, diols 5
- , giving compounds 22 and 23. The butanediacetal groups were then removed with p-toluenesulfonic acid and diols 5 and 6 were obtained with 79% and 64% yield, respectively. They were then used in a well-established three-step, one-pot procedure for epoxide ring closure [26][30][40][41]. Pure (+)-disparlure

Graphical Abstract

Scheme 1: Retrosynthesis of (+)-disparlure (1), (−)-disparlure (3), (+)-monachalure (2), and (−)-monachalure (...

Scheme 2: Isomerization of trans-2,3-butanediacetals 9–11 to cis-2,3-butanediacetals 12–14.

Scheme 3: Synthesis of diol 17 and its subsequent modifications.

Scheme 4: Synthesis of (+)-disparlure (1) and (+)-monachalure (2).

Scheme 5: Synthesis of (−)-disparlure (3) and (−)-monachalure (4).
Recent advances in photocatalyzed reactions using well-defined copper(I) complexes
- Mingbing Zhong,
- Xavier Pannecoucke,
- Philippe Jubault and
- Thomas Poisson
Beilstein J. Org. Chem. 2020, 16, 451–481, doi:10.3762/bjoc.16.42
- reaction. In the presence of 2 mol % of the catalyst, the Hantzsch ester (HEH), as a hydrogen atom donor, under blue light irradiation, a large panel of ketones and aldehydes was readily converted into the corresponding 1,2-diols in moderate to excellent yields. The functional group tolerance of the

Graphical Abstract

Scheme 1: [Cu(I)(dap)2]Cl-catalyzed ATRA reaction under green light irradiation.

Scheme 2: Photocatalytic allylation of α-haloketones.

Scheme 3: [Cu(I)(dap)2]Cl-photocatalyzed chlorosulfonylation and chlorotrifluoromethylation of alkenes.

Scheme 4: Photocatalytic perfluoroalkylchlorination of electron-deficient alkenes using the Sauvage catalyst.

Scheme 5: Photocatalytic synthesis of fluorinated sultones.

Scheme 6: Photocatalyzed haloperfluoroalkylation of alkenes and alkynes.

Scheme 7: Chlorosulfonylation of alkenes catalyzed by [Cu(I)(dap)2]Cl. aNo Na2CO3 was added. b1 equiv of Na2CO...

Scheme 8: Copper-photocatalyzed reductive allylation of diaryliodonium salts.

Scheme 9: Copper-photocatalyzed azidomethoxylation of olefins.

Scheme 10: Benzylic azidation initiated by [Cu(I)(dap)2]Cl.

Scheme 11: Trifluoromethyl methoxylation of styryl derivatives using [Cu(I)(dap)2]PF6. All redox potentials ar...

Scheme 12: Trifluoromethylation of silyl enol ethers.

Scheme 13: Synthesis of annulated heterocycles upon oxidation with the Sauvage catalyst.

Scheme 14: Oxoazidation of styrene derivatives using [Cu(dap)2]Cl as a precatalyst.

Scheme 15: [Cu(I)(dpp)(binc)]PF6-catalyzed ATRA reaction.

Scheme 16: Allylation reaction of α-bromomalonate catalyzed by [Cu(I)(dpp)(binc)]PF6 following an ATRA mechani...

Scheme 17: Bromo/tribromomethylation reaction using [Cu(I)(dmp)(BINAP)]PF6.

Scheme 18: Chlorotrifluoromethylation of alkenes catalyzed by [Cu(I)(N^N)(xantphos)]PF6.

Scheme 19: Chlorosulfonylation of styrene and alkyne derivatives by ATRA reactions.

Scheme 20: Reduction of aryl and alkyl halides with the complex [Cu(I)(bcp)(DPEPhos)]PF6. aIrradiation was car...

Scheme 21: Meerwein arylation of electron-rich aromatic derivatives and 5-exo-trig cyclization catalyzed by th...

Scheme 22: [Cu(I)(bcp)(DPEPhos)]PF6-photocatalyzed synthesis of alkaloids. aYield over two steps (cyclization ...

Scheme 23: Copper-photocatalyzed decarboxylative amination of NHP esters.

Scheme 24: Photocatalytic decarboxylative alkynylation using [Cu(I)(dq)(binap)]BF4.

Scheme 25: Copper-photocatalyzed alkylation of glycine esters.

Scheme 26: Copper-photocatalyzed borylation of organic halides. aUnder continuous flow conditions.

Scheme 27: Copper-photocatalyzed α-functionalization of alcohols with glycine ester derivatives.

Scheme 28: δ-Functionalization of alcohols using [Cu(I)(dmp)(xantphos)]BF4.

Scheme 29: Photocatalytic synthesis of [5]helicene and phenanthrene.

Scheme 30: Oxidative carbazole synthesis using in situ-formed [Cu(I)(dmp)(xantphos)]BF4.

Scheme 31: Copper-photocatalyzed functionalization of N-aryl tetrahydroisoquinolines.

Scheme 32: Bicyclic lactone synthesis using a copper-photocatalyzed PCET reaction.

Scheme 33: Photocatalytic Pinacol coupling reaction catalyzed by [Cu(I)(pypzs)(BINAP)]BF4. The ligands of the ...

Scheme 34: Azide photosensitization using a Cu-based photocatalyst.
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates
- Narasimhamurthy Rajeev,
- Toreshettahally R. Swaroop,
- Ahmad I. Alrawashdeh,
- Shofiur Rahman,
- Abdullah Alodhayb,
- Seegehalli M. Anil,
- Kuppalli R. Kiran,
- Chandra,
- Paris E. Georghiou,
- Kanchugarakoppal S. Rangappa and
- Maralinganadoddi P. Sadashiva
Beilstein J. Org. Chem. 2020, 16, 159–167, doi:10.3762/bjoc.16.18
- of xanthate esters with amines [18]. Furthermore, many methods have also been reported for the synthesis of cyclic thiocarbamates, and these include reactions of isothiocyanates with aldehydes in the presence of organocatalysts [19][20], reactions of vicinal diols with potassium thiocyanate [21

Graphical Abstract

Scheme 1: Synthesis of carbamothioates from xanthate esters and benzyl isocyanides.

Figure 1: Substrate scope for the synthesis of carbamothioates. Reaction conditions for methods A and B: sodi...

Figure 2: ORTEP diagram of O-benzyl (4-fluorobenzyl)carbamothioate (4c).

Figure 3: Rotamers of thionocarbamates 4 (top) and computer-minimized structures of 4c (bottom).

Scheme 2: Proposed general reaction mechanism for the formation of carbamothioates (e.g., 4a) from xanthate e...

Figure 4: Optimized geometries of the reactants, transition states, intermediates, and products of the propos...

Figure 5: Relative energies of the reactants, transition states (TS1–TS3), and intermediates (Int1–Int3) of t...
Why do thioureas and squaramides slow down the Ireland–Claisen rearrangement?
- Dominika Krištofíková,
- Juraj Filo,
- Mária Mečiarová and
- Radovan Šebesta
Beilstein J. Org. Chem. 2019, 15, 2948–2957, doi:10.3762/bjoc.15.290
- demanding ones, e.g., C6, C9 or C11. In addition, the acidity of the hydrogen-bond-donating moiety also ranges over a rather large area from pKa (H2O) 1 for phosphoric acid C10 to pKa (DMSO) 28 of diols C7 and C8. However, neither steric factors, nor the acidity of the H-bond-donor moiety seemed to play a

Graphical Abstract

Scheme 1: Ireland–Claisen rearrangement of allyl esters 1a–c.

Scheme 2: Ireland–Claisen rearrangement of 1c mediated by tertiary amines.

Figure 1: Organocatalysts used in this study. Conditions: typical procedure: 1. Et3N (4.9 equiv), DCM, −60 °C...

Scheme 3: Solvent-free Ireland–Claisen rearrangement of cinnamyl esters.

Figure 2: ωB97X-D/6-31G* calculated uncatalyzed Ireland–Claisen rearrangement of 1c. Charges on allylic oxyge...

Figure 3: ωB97X-D/6-31G* calculated Schreiner thiourea (12)-catalyzed Ireland–Claisen rearrangement of 1c. Ch...

Figure 4: ωB97X-D/6-31G* calculated Ph-thiourea (top) and squaramide-catalyzed (bottom) Ireland–Claisen rearr...

Figure 5: a) Rate of product formation; b) reaction profile without catalyst determined by 1H NMR.
A green, economical synthesis of β-ketonitriles and trifunctionalized building blocks from esters and lactones
- Daniel P. Pienaar,
- Kamogelo R. Butsi,
- Amanda L. Rousseau and
- Dean Brady
Beilstein J. Org. Chem. 2019, 15, 2930–2935, doi:10.3762/bjoc.15.287
- solvents was successfully exploited using inexpensive KOt-Bu to obtain a variety of β-ketonitriles and trifunctionalized building blocks, including useful O-unprotected diols. It was discovered that lactones react to produce the corresponding derivatized cyclic hemiketals. Furthermore, the addition of a
- diols 1 and 8, thereby indicating that the hemiketals are still fully reducible under standard ketone reduction conditions. Purification of 6 afforded only a 10% overall yield of the diol 8, but the direct conversion of crude intermediate product 6 resulted in a doubling of the overall yield of the diol
- preparation of trifunctionalized building blocks (hydroxylated β-ketonitriles) and valuable β-ketonitriles (including enolizable compounds) in modest to good yields from lactones and esters. We are currently investigating novel applications of diols 1 and 8, as well as the application of this methodology for

Graphical Abstract

Scheme 1: Proposed retrosynthesis of the free diol 1.

Scheme 2: Preparation of O-unprotected, trifunctionalized synthons from lactones.
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
- Munmun Ghosh,
- Valmik S. Shinde and
- Magnus Rueping
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- felt electrodes for successful application in asymmetric electroorganic transformations, Kashiwagi’s group came up with two distinct protocols for asymmetric electrochemical lactonization of differently substituted diols. In 1996, they disclosed an asymmetric electrocatalytic method for lactonization
- of methyl-substituted diols 51 on a TEMPO-modified graphite felt electrode in the presence of the chiral base (−)-sparteine (43) with excellent enantioselectivity (conditions A, Scheme 20) [51]. Later in 2003, they reported another protocol for a graphite felt electrode for asymmetric electrochemical
- lactonization of diols 51. However, in this method instead of a chiral base, they used 1-azaspiro[5.5]undecane N-oxyl 52 as a radical mediator for modifying the electrodes which resulted in optically active lactones 53 with enantiopurity of up to 99% (conditions B, Scheme 20) [52]. Chiral medium In this section

Graphical Abstract

Figure 1: General classification of asymmetric electroorganic reactions.

Scheme 1: Asymmetric reduction of 4-acetylpyridine using a modified graphite cathode.

Scheme 2: Asymmetric hydrogenation of ketones using Raney nickel powder electrodes modified with optically ac...

Scheme 3: Asymmetric reduction of prochiral activated olefins with a poly-ʟ-valine-coated graphite cathode.

Scheme 4: Asymmetric reduction of prochiral carbonyl compounds, oximes and gem-dibromides on a poly-ʟ-valine-...

Scheme 5: Asymmetric hydrogenation of prochiral ketones with poly[RuIII(L)2Cl2]+-modified carbon felt cathode...

Scheme 6: Asymmetric hydrogenation of α-keto esters using chiral polypyrrole film-coated cathode incorporated...

Scheme 7: Quinidine and cinchonidine alkaloid-induced asymmetric electroreduction of acetophenone.

Scheme 8: Asymmetric electroreduction of 4- and 2-acetylpyridines at a mercury cathode in the presence of a c...

Scheme 9: Enantioselective reduction of 4-methylcoumarin in the presence of catalytic yohimbine.

Scheme 10: Cinchonine-induced asymmetric electrocarboxylation of 4-methylpropiophenone.

Scheme 11: Enantioselective hydrogenation of methyl benzoylformate using an alkaloid entrapped silver cathode.

Scheme 12: Alkaloid-induced enantioselective hydrogenation using a Cu nanoparticle cathode.

Scheme 13: Alkaloid-induced enantioselective hydrogenation of aromatic ketones using a bimetallic Pt@Cu cathod...

Scheme 14: Enantioselective reduction of ketones at mercury cathode using N,N'-dimethylquininium tetrafluorobo...

Scheme 15: Asymmetric synthesis of an amino acid using an electrode modified with amino acid oxidase and elect...

Scheme 16: Asymmetric oxidation of p-tolyl methyl sulfide using chemically modified graphite anode.

Scheme 17: Asymmetric oxidation of unsymmetric sulfides using poly(amino acid)-coated electrodes.

Scheme 18: Enantioselective, electocatalytic oxidative coupling on TEMPO-modified graphite felt electrode in t...

Scheme 19: Asymmetric electrocatalytic oxidation of racemic alcohols on a TEMPO-modified graphite felt electro...

Scheme 20: Asymmetric electrocatalytic lactonization of diols on TEMPO-modified graphite felt electrodes.

Scheme 21: Asymmetric electrochemical pinacolization in a chiral solvent.

Scheme 22: Asymmetric electroreduction using a chiral supporting electrolyte.

Scheme 23: Asymmetric anodic oxidation of enol acetates using chiral supporting electrolytes.

Scheme 24: Kinetic resolution of primary amines using a chiral N-oxyl radical mediator.

Scheme 25: Chiral N-oxyl-radical-mediated kinetic resolution of secondary alcohols via electrochemical oxidati...

Scheme 26: Chiral iodoarene-mediated asymmetric electrochemical lactonization.

Scheme 27: Os-catalyzed electrochemical asymmetric dihydroxylation of olefins using the Sharpless ligand and i...

Scheme 28: Asymmetric electrochemical epoxidation of olefins catalyzed by a chiral Mn-salen complex.

Scheme 29: Asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.

Scheme 30: Mechanism of asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper cat...

Scheme 31: Enantioselective electrocarboxylation catalyzed by an electrogenerated chiral [CoI(salen)]− complex....

Scheme 32: Asymmetric oxidative cross coupling of 2-acylimidazoles with silyl enol ethers.

Scheme 33: Ni-catalyzed asymmetric electroreductive cleavage of allylic β-keto ester 89.

Scheme 34: Asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.

Scheme 35: Mechanism of asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst....

Scheme 36: Asymmetric epoxidation by electrogenerated percarbonate and persulfate ions in the presence of chir...

Scheme 37: α-Oxyamination of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 38: The α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 39: Mechanism of α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 40: Electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.

Scheme 41: Mechanism of electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehyd...

Scheme 42: Asymmetric cross-dehydrogenative coupling of tertiary amines with simple ketones via an electrochem...

Scheme 43: Electroenzymatic asymmetric reduction using enoate reductase.

Scheme 44: Assymetric reduction using alcohol dehydrogenase as the electrocatalyst.

Scheme 45: Asymmetric electroreduction catalyzed by thermophilic NAD-dependent alcohol dehydrogenase.

Scheme 46: Asymmetric epoxidation of styrene by electrochemical regeneration of flavin-dependent monooxygenase....

Scheme 47: Asymmetric electroreduction using a chloroperoxidase catalyst.

Scheme 48: Asymmetric electrochemical transformation mediated by hydrophobic vitamin B12.

Scheme 49: Diastereoselective cathodic reduction of phenylglyoxalic acids substituted with amines as chiral au...

Scheme 50: Ni-catalyzed asymmetric electroreductive cross coupling of aryl halides with α-chloropropanoic acid...

Scheme 51: Electrochemical Mannich addition of silyloxyfuran to in situ-generated N-acyliminium ions.

Scheme 52: Stereoselective electroreductive homodimerization of cinnamates attached to a camphor-derived chira...

Scheme 53: Diastereoselective electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives.

Scheme 54: Electrocatalytic stereoselective conjugate addition of chiral β-dicarbonyl compounds to methyl viny...

Scheme 55: Stereoselective electrochemical carboxylation of chiral cinnamic acid derivatives under a CO2 atmos...

Scheme 56: Electrochemical diastereoselective α-alkylation of pyrrolidines attached with phosphorus-derived ch...

Scheme 57: Electrogenerated cyanomethyl anion-induced synthesis of chiral cis-β-lactams from amides bearing ch...

Scheme 58: Diastereoselective anodic oxidation followed by intramolecular cyclization of ω-hydroxyl amides bea...

Scheme 59: Electrochemical deprotonation of Ni(II) glycinate containing (S)-BPB as a chiral auxiliary: diaster...

Scheme 60: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compound...

Scheme 61: Asymmetric total synthesis of ropivacaine and its analogues using a electroorganic reaction as a ke...

Scheme 62: Asymmetric total synthesis of (−)-crispine A and its natural enantiomer via anodic cyanation of tet...

Scheme 63: Asymmetric oxidative electrodimerization of cinnamic acid derivatives as key step for the synthesis...
α-Photooxygenation of chiral aldehydes with singlet oxygen
- Dominika J. Walaszek,
- Magdalena Jawiczuk,
- Jakub Durka,
- Olga Drapała and
- Dorota Gryko
Beilstein J. Org. Chem. 2019, 15, 2076–2084, doi:10.3762/bjoc.15.205
- University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland 10.3762/bjoc.15.205 Abstract Organocatalytic α-oxygenation of chiral aldehydes with photochemically generated singlet oxygen allows synthesizing chiral 3-substituted 1,2-diols. Stereochemical results indicate that the reaction in the presence
- of diarylprolinol silyl ethers is highly diastereoselective and that the configuration of a newly created stereocenter at the α-position depends predominantly on the catalyst structure. The absolute configuration of chiral 1,2-diols has been unambiguously established based on electronic circular
- dichroism (ECD) and TD-DFT methods. Keywords: 1,2-diols; ECD; enamines; organocatalysis; porphyrins; silyl ethers of diarylprolinols; singlet oxygen; Introduction Carbonyl compounds are one of the most important building blocks in organic synthesis. As a consequence, there is a constant need for new

Graphical Abstract

Scheme 1: Asymmetric α-photooxygenation of chiral aldehydes.

Scheme 2: α-Photooxygenation of β-substituted aldehydes.

Scheme 3: Synthesis and α-photooxygenation of 3,4-diphenylbutanal (1).

Scheme 4: Stereoselective α-photooxygenation of 3,4-diphenylbutanal (1) with 1O2.

Scheme 5: Schematic representation of the in situ methodology and preferred conformation of diols with Mo2 co...

Figure 1: ECD spectra of diols syn-6 and anti’-6 recorded a) with 19 in DMSO and b) in acetonitrile compared ...

Scheme 6: Asymmetric synthesis of 3,4-diphenylbutane-1,2-diol.
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
- Annika Stein,
- Dave Compera,
- Bianka Karge,
- Mark Brönstrup and
- Jakob Franke
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- -oxidative Grob fragmentation could make use of a push–pull mechanism between C-17 and C-22, building on acid–base catalysis. Alternatively, an enzyme could cleave the C17–C20 diol oxidatively. Several P450 enzymes have been reported to be capable of cleaving diols, presumably via a ferric peroxo

Graphical Abstract

Figure 1: Withanolides from Physalis peruviana. A) Structures of the newly characterised truncated withanolid...

Figure 2: Key NMR correlations. (A) COSY and HMBC correlations for irinan A (2). (B) COSY and HMBC correlatio...

Figure 3: Structures and biosynthesis of androstanes. (A) Androstane backbone and androsterone (7) as a typic...

Figure 4: Intrinsic reactivity of 4ß-hydroxywithanolide E (1) under acidic/basic and oxidative conditions, re...
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
- Meng-Di Gu,
- Yao Lu and
- Mei-Xiang Wang
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- study 3,6-dihydroxyphthalimide derivatives as aromatic diols to construct functionalized O6-corona[3]arene[3]tetrazins. Being different from terephthalate in terms of substitution pattern, we envisioned that the phthalimide unit would flip freely owing to the less steric hindrance. In addition, N

Graphical Abstract

Scheme 1: Synthesis of phthalimide-containing O6-corona[3]arene[3]tetrazines.

Figure 1: X-ray molecular structure of 3a (CCDC 1913907) with side (left) and top (right) views. All solvent ...

Figure 2: 1H (top) and 13C (bottom) NMR spectra of 3a in acetone-d6 at 25 °C.

Figure 3: Normalized cyclic voltammograms (left) and differential pulse voltammograms (right) of 3a. CV and D...

Figure 4: X-ray molecular structures of complexes (n-Bu4NCl)3-3a (1913908) (top) and (n-Bu4NBr)3-3a (1913909)...
Application of chiral 2-isoxazoline for the synthesis of syn-1,3-diol analogs
- Juanjuan Feng,
- Tianyu Li,
- Jiaxin Zhang and
- Peng Jiao
Beilstein J. Org. Chem. 2019, 15, 1840–1847, doi:10.3762/bjoc.15.179
- methods using Claisen condensation. (b) Our new method using cycloaddition. Attempted oxidations of 4. Preparations of 16 and related syn-1,3-diol compounds. Attempted oxidations of 6'. Attempted selective protections of internal 1,3-hydroxy groups: (a) acetonizations of 1,3-diols; (b) removal of co

Graphical Abstract

Scheme 1: Accesses to tert-butyl 3,5-O-isopropylidene-3,5-dihydroxyhexanoates. (a) Previous methods using Cla...

Scheme 2: Attempted oxidations of 4.

Scheme 3: Preparations of 16 and related syn-1,3-diol compounds.

Scheme 4: Attempted oxidations of 6'.

Scheme 5: Attempted selective protections of internal 1,3-hydroxy groups: (a) acetonizations of 1,3-diols; (b...
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
- Iwona E. Głowacka,
- Aleksandra Trocha,
- Andrzej E. Wróblewski and
- Dorota G. Piotrowska
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- the same potency as N,N-dimethylsphingosine (DMS) being completely inactive toward hSphK1. 2-Amino-1,3-diols A 2-amino-1,3-dihydroxypropyl fragment 12 of sphingosine and ceramides of the required 2S,3R configuration can also originate from the aziridine alcohol, e.g., (2S,1'R,1''R)-87 prepared from
- synthesized from aziridine-2-methanols either by functionalization at C3 (Scheme 12 and Scheme 13) or by opening of the aziridine ring to form 2-amino-1,3-diols 12 (Schemes 22–24) combined with the removal of the secondary hydroxy group when simple amino acids (R = alkyl, aryl) are to be prepared. For the
- diols with 160 preponderating (Scheme 41) [34]. The regioselective aziridine ring opening with methanol and catalytic hydrogenation in the presence of formalin gave the final product (2S,3S,4S)-159. The innovative application of the aldehyde (2S,1'R)-6 in syntheses of nonproteinogenic γ-amino hydroxy

Graphical Abstract

Figure 1: Examples of three-carbon chirons.

Figure 2: Structures of derivatives of N-(1-phenylethyl)aziridine-2-carboxylic acid 5–8.

Figure 3: Synthetic equivalency of aziridine aldehydes 6.

Scheme 1: Synthesis of N-(1-phenylethyl)aziridine-2-carboxylates 5. Reagents and conditions: a) TEA, toluene,...

Scheme 2: Absolute configuration at C2 in (2S,1'S)-5a. Reagents and conditions: a) 20% HClO4, 80 °C, 30 h the...

Scheme 3: Major synthetic strategies for a 2-ketoaziridine scaffold [R* = (R)- or (S)-1-phenylethyl; R′ = Alk...

Scheme 4: Synthesis of cyanide (2S,1'S)-13. Reagents and conditions: a) NH3, EtOH/H2O, rt, 72 h; b) Ph3P, CCl4...

Scheme 5: Synthesis of key intermediates (R)-16 and (R)-17 for (R,R)-formoterol (14) and (R)-tamsulosin (15)....

Scheme 6: Synthesis of mitotic kinesin inhibitors (2R/S,1'R)-23. Reagents and conditions: a) H2, Pd(OH)2, EtO...

Scheme 7: Synthesis of (R)-mexiletine ((R)-24). Reagents and conditions: a) TsCl, TEA, DMAP, CH2Cl2, rt, 1 h;...

Scheme 8: Synthesis of (−)-cathinone ((S)-27). Reagents and conditions: a) PhMgBr, ether, 0 °C; b) H2, 10% Pd...

Scheme 9: Synthesis of N-Boc-norpseudoephedrine ((1S,2S)-(+)-29) and N-Boc-norephedrine ((1R,2S)-29). Reagent...

Scheme 10: Synthesis of (−)-ephedrine ((1R,2S)-31). Reagents and conditions: a) TfOMe, MeCN then NaBH3CN, rt; ...

Scheme 11: Synthesis of xestoaminol C ((2S,3R)-35), 3-epi-xestoaminol C ((2S,3S)-35) and N-Boc-spisulosine ((2S...

Scheme 12: Synthesis of ʟ-tryptophanol ((S)-41). Reagents and conditions: a) CDI, MeCN, rt, 1 h then TMSI, MeC...

Scheme 13: Synthesis of ʟ-homophenylalaninol ((S)-42). Reagents and conditions: a) NaH, THF, 0 °C to −78 °C, 1...

Scheme 14: Synthesis of ᴅ-homo(4-octylphenyl)alaninol ((R)-47) and a sphingolipid analogue (R)-48. Reagents an...

Scheme 15: Synthesis of florfenicol ((1R,2S)-49). Reagents and conditions: a) (S)-1-phenylethylamine, TEA, MeO...

Scheme 16: Synthesis of natural tyroscherin ((2S,3R,6E,8R,10R)-55). Reagents and conditions: a) I(CH2)3OTIPS, t...

Scheme 17: Syntheses of (−)-hygrine (S)-61, (−)-hygroline (2S,2'S)-62 and (−)-pseudohygroline (2S,2'R)-62. Rea...

Scheme 18: Synthesis of pyrrolidine (3S,3'R)-68, a fragment of the fluoroquinolone antibiotic PF-00951966. Rea...

Scheme 19: Synthesis of sphingolipid analogues (R)-76. Reagents and conditions: a) BnBr, Mg, THF, reflux, 6 h;...

Scheme 20: Synthesis of ᴅ-threo-PDMP (1R,2R)-81. Reagents and conditions: a) TMSCl, NaI, MeCN, rt, 1 h 50 min,...

Scheme 21: Synthesis of the sphingolipid analogue SG-14 (2S,3S)-84. Reagents and conditions: a) LiAlH4, THF, 0...

Scheme 22: Synthesis of the sphingolipid analogue SG-12 (2S,3R)-88. Reagents and conditions: a) 1-(bromomethyl...

Scheme 23: Synthesis of sphingosine-1-phosphate analogues DS-SG-44 and DS-SG-45 (2S,3R)-89a and (2S,3R)-89a. R...

Scheme 24: Synthesis of N-Boc-safingol ((2S,3S)-95) and N-Boc-ᴅ-erythro-sphinganine ((2S,3R)-95). Reagents and...

Scheme 25: Synthesis of ceramide analogues (2S,3R)-96. Reagents and conditions: a) NaBH4, ZnCl2, MeOH, −78 °C,...

Scheme 26: Synthesis of orthogonally protected serinols, (S)-101 and (R)-102. Reagents and conditions: a) BnBr...

Scheme 27: Synthesis of N-acetyl-3-phenylserinol ((1R,2R)-105). Reagents and conditions: a) AcOH, CH2Cl2, refl...

Scheme 28: Synthesis of (S)-linezolid (S)-107. Reagents and conditions: a) LiAlH4, THF, 0 °C to reflux; b) Boc2...

Scheme 29: Synthesis of (2S,3S,4R)-2-aminooctadecane-1,3,4-triol (ᴅ-ribo-phytosphingosine) (2S,3S,4R)-110. Rea...

Scheme 30: Syntheses of ᴅ-phenylalanine (R)-116. Reagents and conditions: a) AcOH, CH2Cl2, reflux, 4 h; b) MsC...

Scheme 31: Synthesis of N-Boc-ᴅ-3,3-diphenylalanine ((R)-122). Reagents and conditions: a) PhMgBr, THF, −78 °C...

Scheme 32: Synthesis of ethyl N,N’-di-Boc-ʟ-2,3-diaminopropanoate ((S)-125). Reagents and conditions: a) NaN3,...

Scheme 33: Synthesis of the bicyclic amino acid (S)-(+)-127. Reagents and conditions: a) BF3·OEt2, THF, 60 °C,...

Scheme 34: Synthesis of lacosamide, (R)-2-acetamido-N-benzyl-3-methoxypropanamide (R)-130. Reagents and condit...

Scheme 35: Synthesis of N-Boc-norfuranomycin ((2S,2'R)-133). Reagents and conditions: a) H2C=CHCH2I, NaH, THF,...

Scheme 36: Synthesis of MeBmt (2S,3R,4R,6E)-139. Reagents and conditions: a) diisopropyl (S,S)-tartrate (E)-cr...

Scheme 37: Synthesis of (+)-polyoxamic acid (2S,3S,4S)-144. Reagents and conditions: a) AD-mix-α, MeSO2NH2, t-...

Scheme 38: Synthesis of the protected 3-hydroxy-ʟ-glutamic acid (2S,3R)-148. Reagents and conditions: a) LiHMD...

Scheme 39: Synthesis of (+)-isoserine (R)-152. Reagents and conditions: a) AcCl, MeCN, rt, 0.5 h then Na2CO3, ...

Scheme 40: Synthesis of (3R,4S)-N3-Boc-3,4-diaminopentanoic acid (3R,4S)-155. Reagents and conditions: a) Ph3P...

Scheme 41: Synthesis of methyl (2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoate (2S,3S,4S)-159. ...

Scheme 42: Syntheses of methyl (3S,4S) 4,5-di-N-Boc-amino-3-hydroxypentanoate ((3S,4S)-164), methyl (3S,4S)-4-N...

Scheme 43: Syntheses of (3R,5S)-5-(aminomethyl)-3-(4-methoxyphenyl)dihydrofuran-2(3H)-one ((3R,5S)-168). Reage...

Scheme 44: Syntheses of a series of imidazolin-2-one dipeptides 175–177 (for R' and R'' see text). Reagents an...

Scheme 45: Syntheses of (2S,3S)-N-Boc-3-hydroxy-2-hydroxymethylpyrrolidine ((2S,3S)-179). Reagents and conditi...

Scheme 46: Syntheses of enantiomers of 1,4-dideoxy-1,4-imino-ʟ- and -ᴅ-lyxitols (2S,3R,4S)-182 and (2R,3S,4R)-...

Scheme 47: Synthesis of 1,4-dideoxy-1,4-imino-ʟ-ribitol (2S,3S,4R)-182. Reagents and conditions: a) AcOH, CH2Cl...

Scheme 48: Syntheses of 1,4-dideoxy-1,4-imino-ᴅ-arabinitol (2R,3R,4R)-182 and 1,4-dideoxy-1,4-imino-ᴅ-xylitol ...

Scheme 49: Syntheses of natural 2,5-imino-2,5,6-trideoxy-ʟ-gulo-heptitol ((2S,3R,4R,5R)-184) and its C4 epimer...

Scheme 50: Syntheses of (−)-dihydropinidine ((2S,6R)-187a) (R = C3H7) and (2S,6R)-isosolenopsins (2S,6R)-187b ...

Scheme 51: Syntheses of (+)-deoxocassine ((2S,3S,6R)-190a, R = C12H25) and (+)-spectaline ((2S,3S,6R)-190b, R ...

Scheme 52: Synthesis of (−)-microgrewiapine A ((2S,3R,6S)-194a) and (+)-microcosamine A ((2S,3R,6S)-194b). Rea...

Scheme 53: Syntheses of ʟ-1-deoxynojirimycin ((2S,3S,4S,5R)-200), ʟ-1-deoxymannojirimycin ((2S,3S,4S,5S)-200) ...

Scheme 54: Syntheses of 1-deoxy-ᴅ-galacto-homonojirimycin (2R,3S,4R,5S)-211. Reagents and conditions: a) MeONH...

Scheme 55: Syntheses of 7a-epi-hyacinthacine A1 (1S,2R,3R,7aS)-220. Reagents and conditions: a) TfOTBDMS, 2,6-...

Scheme 56: Syntheses of 8-deoxyhyacinthacine A1 ((1S,2R,3R,7aR)-221). Reagents and conditions: a) H2, Pd/C, PT...

Scheme 57: Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, L...

Scheme 58: Syntheses of 8-epi-swainsonine (1S,2R,8S,8aR)-231. Reagents and conditions: a) Ph3P=CHCOOMe, MeOH, ...

Scheme 59: Synthesis of a protected vinylpiperidine (2S,3R)-237, a key intermediate in the synthesis of (−)-sw...

Scheme 60: Synthesis of a modified carbapenem 245. Reagents and conditions: a) AcOEt, LiHMDS, THF, −78 °C, 1.5...
Borylation and rearrangement of alkynyloxiranes: a stereospecific route to substituted α-enynes
- Ruben Pomar Fuentespina,
- José Angel Garcia de la Cruz,
- Gabriel Durin,
- Victor Mamane,
- Jean-Marc Weibel and
- Patrick Pale
Beilstein J. Org. Chem. 2019, 15, 1416–1424, doi:10.3762/bjoc.15.141
- tetrasubstituted alkenes [6][7]. Capitalizing on these precedents, Aggarwal et al. showed that oxiranes could be homologated to diols through lithiation, borylation, rearrangement and oxidation of the so-formed β-hydroxyboranes [8]. More recently, Blakemore et al. applied this sequence to sulfinyloxiranes [9

Graphical Abstract

Scheme 1: Stereospecific formation of α-enynes from alkynyloxiranes.

Scheme 2: Trapping experiments of the oxiranyllithium derived from cis or trans-alkynyloxiranes 1b, and their...

Scheme 3: Proposed mechanism for the rearrangement of alkynyloxiranes to α-enynes through metalation and bory...
Catalytic asymmetric oxo-Diels–Alder reactions with chiral atropisomeric biphenyl diols
- Chi-Tung Yeung,
- Wesley Ting Kwok Chan,
- Wai-Sum Lo,
- Ga-Lai Law and
- Wing-Tak Wong
Beilstein J. Org. Chem. 2019, 15, 955–962, doi:10.3762/bjoc.15.92
- Polytechnic University, Hung Hom, Kowloon, Hong Kong 10.3762/bjoc.15.92 Abstract New chiral atropisomeric biphenyl diols 3, 4 and 6 containing additional peripheral chiral centers with different steric bulkiness and/or electronic properties were synthesized. The X-ray crystal structure of 3 shows the
- formation of a supramolecular structure whereas that of 6, containing additional CF3 substituents, shows the formation of a monomeric structure. Diols 1–6 were found to be active organocatalysts in oxo-Diels–Alder reactions in which 2 recorded a 72% ee with trimethylacetaldehyde as a substrate. Keywords
- supramolecular helices or dimers through intermolecular hydrogen bonding of two axially chiral biphenyl hybrid diols (1 and 2 in Scheme 1) which contain point chirality at the side arms and axial chirality at the biphenyl backbone [37]. We envisage the structural similarity and the ability of our scaffold to

Graphical Abstract

Scheme 1: Chiral biphenyl diol organocatalysts 1–6.

Scheme 2: Synthesis of 3.

Figure 1: (a) Single crystal X-ray structure of 3: showing intra- and intermolecular hydrogen bonds (green da...

Scheme 3: Synthesis of 4.

Scheme 4: Synthesis of 6.

Figure 2: X-ray crystal structure of (P)-(S,S)-6 at two different orientations to show (a) P atropselectiviti...
Easy, efficient and versatile one-pot synthesis of Janus-type-substituted fullerenols
- Marius Kunkel and
- Sebastian Polarz
Beilstein J. Org. Chem. 2019, 15, 901–905, doi:10.3762/bjoc.15.87

- the characterization of Janus-type fullerenol amphiphiles more than one method is needed to perfectly identify the compounds. Polyhydroxylation reaction of the fullerene can lead to a mixture of several oxygen moieties like hydroxy groups, diols, ketones, hemiketals, epoxides and ethers. The general

Graphical Abstract

Scheme 1: Reaction scheme for the one-pot reaction of C60Cl6 to produce Janus-type fullerenols (OH)19+/−3C60(...

Figure 1: Characterization of fullerenol amphiphile with substituent 1. a) ESIMS in positive mode, molecular ...






