Search results
Search for "elimination" in Full Text gives 778 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Palladium nanoparticles supported on chitin-based nanomaterials as heterogeneous catalysts for the Heck coupling reaction
- Tony Jin,
- Malickah Hicks,
- Davis Kurdyla,
- Sabahudin Hrapovic,
- Edmond Lam and
- Audrey Moores
Beilstein J. Org. Chem. 2020, 16, 2477–2483, doi:10.3762/bjoc.16.201

- ChsNC in the +2 oxidation state (Figure 3). These results align with our previous work where ChsNCs tend to stabilize Au in the +1 oxidation state as opposed to metallic Au. Since the Heck coupling primarily follows a classic oxidative addition/reductive elimination pathway with Pd(0) being the active

Graphical Abstract

Scheme 1: Pathway for the formation of ChNC and subsequently ChsNCs from bulk chitin.

Figure 1: TEM micrographs of (a) ChNCs and (b) ChsNCs. Both samples were stained and prepared on glow-dischar...

Scheme 2: Catalyst fabrication method for the deposition of Pd NPs onto chitin (PdNP@ChNC) and chitosan (PdNP...

Figure 2: TEM micrographs of (a) PdNP@ChNCs and (b) PdNP@ChsNCs. The samples were placed on glow discharged T...

Figure 3: High-resolution X-ray photoelectron spectroscopy of the Pd 3d region of (a) PdNP@ChNC and (b) PdNP@...
Catalytic trifluoromethylation of iodoarenes by use of 2-trifluoromethylated benzimidazoline as trifluoromethylating reagent
- Tatsuhiro Uchikura,
- Nanami Kamiyama,
- Taisuke Ishikawa and
- Takahiko Akiyama
Beilstein J. Org. Chem. 2020, 16, 2442–2447, doi:10.3762/bjoc.16.198
- (I)–CF3 species, generated through the reaction of benzimidazoline 2 with CuI under basic conditions, underwent an oxidative addition reaction with the aryl iodide to generate a Cu(III) complex. A subsequent reductive elimination furnished the trifluoromethylarene and Cu(I). Because an electron

Graphical Abstract

Figure 1: Trifluoromethylation of aryl halides.

Figure 2: Scope of aryl iodides. Yields determined by 19F NMR spectroscopy and used ligand is given in parent...

Figure 3: Scope of heteroaryl iodides. Yields determined by 19F NMR spectroscopy and used ligand is given in ...

Figure 4: Time course of the trifluoromethylation reaction.

Figure 5: Proposed mechanism of the catalytic cycle.
Recent developments in enantioselective photocatalysis
- Callum Prentice,
- James Morrisson,
- Andrew D. Smith and
- Eli Zysman-Colman
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197


Graphical Abstract

Scheme 1: Amine/photoredox-catalysed α-alkylation of aldehydes with alkyl bromides bearing electron-withdrawi...

Scheme 2: Amine/HAT/photoredox-catalysed α-functionalisation of aldehydes using alkenes.

Scheme 3: Amine/cobalt/photoredox-catalysed α-functionalisation of ketones and THIQs.

Scheme 4: Amine/photoredox-catalysed α-functionalisation of aldehydes or ketones with imines. (a) Using keton...

Scheme 5: Bifunctional amine/photoredox-catalysed enantioselective α-functionalisation of aldehydes.

Scheme 6: Bifunctional amine/photoredox-catalysed α-functionalisation of aldehydes using amine catalysts via ...

Scheme 7: Amine/photoredox-catalysed RCA of iminium ion intermediates. (a) Synthesis of quaternary stereocent...

Scheme 8: Bifunctional amine/photoredox-catalysed RCA of enones in a radical chain reaction initiated by an i...

Scheme 9: Bifunctional amine/photoredox-catalysed RCA reactions of iminium ions with different radical precur...

Scheme 10: Bifunctional amine/photoredox-catalysed radical cascade reactions between enones and alkenes with a...

Scheme 11: Amine/photocatalysed photocycloadditions of iminium ion intermediates. (a) External photocatalyst u...

Scheme 12: Amine/photoredox-catalysed addition of acrolein (94) to iminium ions.

Scheme 13: Dual NHC/photoredox-catalysed acylation of THIQs.

Scheme 14: NHC/photocatalysed spirocyclisation via photoisomerisation of an extended Breslow intermediate.

Scheme 15: CPA/photoredox-catalysed aza-pinacol cyclisation.

Scheme 16: CPA/photoredox-catalysed Minisci-type reaction between azaarenes and α-amino radicals.

Scheme 17: CPA/photoredox-catalysed radical additions to azaarenes. (a) α-Amino radical or ketyl radical addit...

Scheme 18: CPA/photoredox-catalysed reduction of azaarene-derived substrates. (a) Reduction of ketones. (b) Ex...

Scheme 19: CPA/photoredox-catalysed radical coupling reactions of α-amino radicals with α-carbonyl radicals. (...

Scheme 20: CPA/photoredox-catalysed Povarov reaction.

Scheme 21: CPA/photoredox-catalysed reactions with imines. (a) Decarboxylative imine generation followed by Po...

Scheme 22: Bifunctional CPA/photocatalysed [2 + 2] photocycloadditions.

Scheme 23: PTC/photocatalysed oxygenation of 1-indanone-derived β-keto esters.

Scheme 24: PTC/photoredox-catalysed perfluoroalkylation of 1-indanone-derived β-keto esters via a radical chai...

Scheme 25: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 26: Bifunctional hydrogen bonding/photocatalysed intramolecular RCA cyclisation of a quinolone.

Scheme 27: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 28: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloaddition reactions. (a) First use of...

Scheme 29: Bifunctional hydrogen bonding/photocatalysed deracemisation of allenes.

Scheme 30: Bifunctional hydrogen bonding/photocatalysed deracemisation reactions. (a) Deracemisation of sulfox...

Scheme 31: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloaddition of coumarins....

Scheme 32: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloadditions of quinolones. (a) Intramo...

Scheme 33: Hydrogen bonding/photocatalysed formal arylation of benzofuranones.

Scheme 34: Hydrogen bonding/photoredox-catalysed dehalogenative protonation of α,α-chlorofluoro ketones.

Scheme 35: Hydrogen bonding/photoredox-catalysed reductions. (a) Reduction of 1,2-diketones. (b) Reduction of ...

Scheme 36: Hydrogen bonding/HAT/photocatalysed deracemisation of cyclic ureas.

Scheme 37: Hydrogen bonding/HAT/photoredox-catalysed synthesis of cyclic sulfonamides.

Scheme 38: Hydrogen bonding/photoredox-catalysed reaction between imines and indoles.

Scheme 39: Chiral cation/photoredox-catalysed radical coupling of two α-amino radicals.

Scheme 40: Chiral phosphate/photoredox-catalysed hydroetherfication of alkenols.

Scheme 41: Chiral phosphate/photoredox-catalysed synthesis of pyrroloindolines.

Scheme 42: Chiral anion/photoredox-catalysed radical cation Diels–Alder reaction.

Scheme 43: Lewis acid/photoredox-catalysed cycloadditions of carbonyls. (a) Formal [2 + 2] cycloaddition of en...

Scheme 44: Lewis acid/photoredox-catalysed RCA reaction using a scandium Lewis acid between α-amino radicals a...

Scheme 45: Lewis acid/photoredox-catalysed RCA reaction using a copper Lewis acid between α-amino radicals and...

Scheme 46: Lewis acid/photoredox-catalysed synthesis of 1,2-amino alcohols from aldehydes and nitrones using a...

Scheme 47: Lewis acid/photocatalysed [2 + 2] photocycloadditions of enones and alkenes.

Scheme 48: Meggers’s chiral-at-metal catalysts.

Scheme 49: Lewis acid/photoredox-catalysed α-functionalisation of ketones with alkyl bromides bearing electron...

Scheme 50: Bifunctional Lewis acid/photoredox-catalysed radical coupling reaction using α-chloroketones and α-...

Scheme 51: Lewis acid/photocatalysed RCA of enones. (a) Using aldehydes as acyl radical precursors. (b) Other ...

Scheme 52: Bifunctional Lewis acid/photocatalysis for a photocycloaddition of enones.

Scheme 53: Lewis acid/photoredox-catalysed RCA reactions of enones using DHPs as radical precursors.

Scheme 54: Lewis acid/photoredox-catalysed functionalisation of β-ketoesters. (a) Hydroxylation reaction catal...

Scheme 55: Bifunctional copper-photocatalysed alkylation of imines.

Scheme 56: Copper/photocatalysed alkylation of imines. (a) Bifunctional copper catalysis using α-silyl amines....

Scheme 57: Bifunctional Lewis acid/photocatalysed intramolecular [2 + 2] photocycloaddition.

Scheme 58: Bifunctional Lewis acid/photocatalysed [2 + 2] photocycloadditions (a) Intramolecular cycloaddition...

Scheme 59: Bifunctional Lewis acid/photocatalysed rearrangement of 2,4-dieneones.

Scheme 60: Lewis acid/photocatalysed [2 + 2] cycloadditions of cinnamate esters and styrenes.

Scheme 61: Nickel/photoredox-catalysed arylation of α-amino acids using aryl bromides.

Scheme 62: Nickel/photoredox catalysis. (a) Desymmetrisation of cyclic meso-anhydrides using benzyl trifluorob...

Scheme 63: Nickel/photoredox catalysis for the acyl-carbamoylation of alkenes with aldehydes using TBADT as a ...

Scheme 64: Bifunctional copper/photoredox-catalysed C–N coupling between α-chloro amides and carbazoles or ind...

Scheme 65: Bifunctional copper/photoredox-catalysed difunctionalisation of alkenes with alkynes and alkyl or a...

Scheme 66: Copper/photoredox-catalysed decarboxylative cyanation of benzyl phthalimide esters.

Scheme 67: Copper/photoredox-catalysed cyanation reactions using TMSCN. (a) Propargylic cyanation (b) Ring ope...

Scheme 68: Palladium/photoredox-catalysed allylic alkylation reactions. (a) Using alkyl DHPs as radical precur...

Scheme 69: Manganese/photoredox-catalysed epoxidation of terminal alkenes.

Scheme 70: Chromium/photoredox-catalysed allylation of aldehydes.

Scheme 71: Enzyme/photoredox-catalysed dehalogenation of halolactones.

Scheme 72: Enzyme/photoredox-catalysed dehalogenative cyclisation.

Scheme 73: Enzyme/photoredox-catalysed reduction of cyclic imines.

Scheme 74: Enzyme/photocatalysed enantioselective reduction of electron-deficient alkenes as mixtures of (E)/(Z...

Scheme 75: Enzyme/photoredox catalysis. (a) Deacetoxylation of cyclic ketones. (b) Reduction of heteroaromatic...

Scheme 76: Enzyme/photoredox-catalysed synthesis of indole-3-ones from 2-arylindoles.

Scheme 77: Enzyme/HAT/photoredox catalysis for the DKR of primary amines.

Scheme 78: Bifunctional enzyme/photoredox-catalysed benzylic C–H hydroxylation of trifluoromethylated arenes.
Synthesis of 1,4-benzothiazinones from acylpyruvic acids or furan-2,3-diones and o-aminothiophenol
- Ekaterina E. Stepanova,
- Maksim V. Dmitriev and
- Andrey N. Maslivets
Beilstein J. Org. Chem. 2020, 16, 2322–2331, doi:10.3762/bjoc.16.193
- the suspension of acid 2a with DCC, formation of the white precipitate of dicyclohexylurea was observed, which gave the evidence of intramolecular cyclization to furandione 5a with dicyclohexylurea elimination occurring. The formation of furandione 5a explained well our observations when DMAP was used

Graphical Abstract

Figure 1: Enaminones fused to heterocyclic moieties.

Scheme 1: Reported structure A of assayed compound [9] and its correct structure B.

Scheme 2: Known synthetic approaches to BTAs III.

Scheme 3: General synthetic approaches to enaminones I and II.

Scheme 4: Reported reactions of acylpyruvic acids or their esters IV with o-aminothiophenol (1a) [27,28].

Scheme 5: Plausible mechanism of the reaction of acylpyruvic acid 2a with o-aminothiophenol (1a) in the prese...

Scheme 6: The substrate scope of the optimized approach to BTAs 3a–n. Procedure: to a cooled to 0–5 °C stirri...

Scheme 7: The substrate scope of the optimized approach to compounds 4a–n. Procedure: to a stirring solution ...

Scheme 8: Plausible scheme of the formation of diketone 6.
Synthesis of 6,13-difluoropentacene
- Matthias W. Tripp and
- Ulrich Koert
Beilstein J. Org. Chem. 2020, 16, 2136–2140, doi:10.3762/bjoc.16.181
- 14 then rapidly decomposes to 6,13-pentacenequinone (15) after elimination of HF. The final aromatization of diol 13 to the target molecule 5 proceeded smoothly in 74% yield using SnCl2 in 1,4-dioxane and aqueous HCl [11][12]. F2PEN 5 can be stored under inertgas atmosphere at −20 °C for a month

Graphical Abstract

Figure 1: Structures of pentacene and fluorinated pentacenes.

Scheme 1: Retrosynthetic analysis of F2PEN 5.

Scheme 2: Synthesis of F2PEN 5.

Scheme 3: Decomposition of diol 13 in solution.

Figure 2: UV–vis spectrum of F2PEN 5 in CH2Cl2.
Access to highly substituted oxazoles by the reaction of α-azidochalcone with potassium thiocyanate
- Mysore Bhyrappa Harisha,
- Pandi Dhanalakshmi,
- Rajendran Suresh,
- Raju Ranjith Kumar and
- Shanmugam Muthusubramanian
Beilstein J. Org. Chem. 2020, 16, 2108–2118, doi:10.3762/bjoc.16.178
- substituted oxazoles and 2-aminothiazoles from α-azidochalcones and potassium thiocyanate employing potassium persulfate and ferric nitrate, respectively. This new route gains a streamlined workup and the elimination of air-sensitive techniques to afford the product in good yield in a greener medium over a

Graphical Abstract

Figure 1: Examples of biologically active oxazole and aminothiazole scaffolds.

Scheme 1: Strategies for the synthesis of 2,4,5-trisubstituted oxazole from azirine. a) I2, PPh3; b) NaH, 1H-...

Scheme 2: Scope of the α-azidochalcones. The reactions were carried out at reflux temperature, using 1 (1 mmo...

Scheme 3: Large-scale synthesis of 3i.

Figure 2: Large-scale synthesis of 3i. a) At the start of the reaction, b) after the reaction.

Scheme 4: Acetyl derivative of 3d.

Figure 3: ORTEP diagram of compound 5.

Scheme 5: Synthesis of S-methyl/benzylated products 6 and 7.

Scheme 6: Control experiments.

Scheme 7: Plausible mechanism proposed for the formation of 2,4,5-trisubstituted oxazoles 3.

Scheme 8: Reaction of vinyl azide 1 and 3 with ferric nitrate. Reactions were carried out at reflux temperatu...

Figure 4: X-ray crystal structure of 4h.
The biomimetic synthesis of balsaminone A and ellagic acid via oxidative dimerization
- Sharna-kay Daley and
- Nadale Downer-Riley
Beilstein J. Org. Chem. 2020, 16, 2026–2031, doi:10.3762/bjoc.16.169
- 16 in 29, 34, and 19% yields, respectively (Table 1). As anticipated, the reaction of naphthalene 17 with CAN under aqueous conditions resulted in preferential oxidative demethylation to give quinone 7, as opposed to oxidative dimerization. The elimination of water from the reaction posed a few

Graphical Abstract

Figure 1: Selected natural products synthesized via oxidative dimerization.

Scheme 1: Proposed biosynthesis of balsaminone A (4) [19].

Scheme 2: Proposed biosynthesis of ellagic acid (5) [20].

Scheme 3: Previous syntheses of balsaminone A (4) [22] and ellagic acid (5) [23].

Scheme 4: Attempted synthesis of the biomimetic precursor 9. [O]: Act-C, K3[Fe(CN)6], or p-benzoquinone.

Scheme 5: Biomimetic synthesis of balsaminone A (4).

Scheme 6: Concise and efficient biomimetic synthesis of ellagic acid (5).
Syntheses of spliceostatins and thailanstatins: a review
- William A. Donaldson
Beilstein J. Org. Chem. 2020, 16, 1991–2006, doi:10.3762/bjoc.16.166
- -methyl-3-buten-1-yl tosylate in the presence of Grubbs’ 2nd generation catalyst yielded 59, which, upon elimination with potassium tert-butoxide led to the diene 50. The reductive amination of 50 afforded an inseparable mixture of the C-14 amines (6:1 ratio). However, the amidation of this mixture with
- and the amidation with 12c afforded 129. The synthesis was completed by the elimination of the 1° iodide, silyl ether cleavage, and hydration of the exocyclic enol ether. Of all the syntheses of FR901464 (1), the coupling of 91 with 48 is the most efficient sequence (6 steps, 45% yield). Nicolaou

Graphical Abstract

Figure 1: Structures of spliceostatins/thailanstatins.

Scheme 1: Synthetic routes to protected (2Z,4S)-4-hydroxy-2-butenoic acid fragments.

Scheme 2: Kitahara synthesis of the (all-cis)-2,3,5,6-tetrasubstituted tetrahydropyran.

Scheme 3: Koide synthesis of (all-cis)-2,3,5,6-tetrasubstituted tetrahydropyran.

Scheme 4: Nicolaou synthesis of the (all-cis)-2,3,5,6-tetrasubstituted tetrahydropyran.

Scheme 5: Jacobsen synthesis of the (all-cis)-2,3,5,6-tetrasubstituted tetrahydropyran.

Scheme 6: Unproductive attempt to generate the (all-cis)-tetrahydropyranone 50.

Scheme 7: Ghosh synthesis of the C-7–C-14 (all-cis)-tetrahydropyran segment.

Scheme 8: Ghosh’s alternative route to the (all-cis)-tetrahydropyranone 50.

Scheme 9: Alternative synthesis of the dihydro-3-pyrone 58.

Scheme 10: Kitahara’s 1st-generation synthesis of the C-1–C-6 fragment of FR901464 (1).

Scheme 11: Kitahara 1st-generation synthesis of the C-1–C-6 fragment of FR901464 (1).

Scheme 12: Nimura/Arisawa synthesis of the C-1-phenyl segment.

Scheme 13: Ghosh synthesis of the C-1–C-6 fragment of FR901464 (1) from (R)-glyceraldehyde acetonide.

Scheme 14: Jacobsen synthesis of the C-1–C-7 segment of FR901464 (1).

Scheme 15: Koide synthesis of the C-1–C-7 segment of FR901464 (1).

Scheme 16: Ghosh synthesis of the C-1–C-5 segment 102 of thailanstatin A (7).

Scheme 17: Nicolaou synthesis of the C-1–C-9 segments of spliceostatin D (9) and thailanstatins A (7) and B (5...

Scheme 18: Ghosh synthesis of the C-1–C-6 segment 115 of spliceostatin E (10).

Scheme 19: Fragment coupling via Wittig and modified Julia olefinations by Kitahara.

Scheme 20: Fragment coupling via cross-metathesis by Koide.

Scheme 21: The Ghosh synthesis of spliceostatin A (4), FR901464 (1), spliceostatin E (10), and thailanstatin m...

Scheme 22: Arisawa synthesis of a C-1-phenyl analog of FR901464 (1).

Scheme 23: Jacobsen fragment coupling by a Pd-catalyzed Negishi coupling.

Scheme 24: Nicolaou syntheses of thailanstatin A and B (7 and 5) and spliceostatin D (9) via a Pd-catalyzed Su...

Scheme 25: The Ghosh synthesis of spliceostatin G (11) via Suzuki–Miyaura coupling.
Synthesis of 3(2)-phosphonylated thiazolo[3,2-a]oxopyrimidines
- Ksenia I. Kaskevich,
- Anastasia A. Babushkina,
- Vladislav V. Gurzhiy,
- Dmitrij M. Egorov,
- Nataly I. Svintsitskaya and
- Albina V. Dogadina
Beilstein J. Org. Chem. 2020, 16, 1947–1954, doi:10.3762/bjoc.16.161
- -trifluoromethyl-2-thiouracil (1e) enhances the acidity of the N3H hydrogen by direct conjugation to the carbonyl moiety. As a result, 2-thiouracil 1e acts as an ambident nucleophile. Thus, the attack of the carbon atom attached to the chlorine by the N3 nitrogen atom is accompanied by the elimination of hydrogen

Graphical Abstract

Figure 1: Structure of ritanserin and setoperone drugs.

Scheme 1: One-pot synthesis of 5(7)-oxothiazolopyrimidine-6-carbonitriles.

Scheme 2: Synthesis of thiazolopyrimidine-5-ones through the reaction of 2-aminothiazoles with ethyl acetoace...

Scheme 3: Synthesis of 2-(benzo[d]thiazol-2-yl)-2-(7-R-5-oxo-5H-thiazolo[3,2-a]pyrimidin-3-yl)acetonitriles.

Scheme 4: Synthesis of 3-acyl-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-ones.

Scheme 5: Sonogashira coupling reaction of 6-amino-2-thiouracil with propargyl bromide.

Scheme 6: Reactions of 6-substituted 2-thiouracils 1a,b with chloroethynylphosphonates 2a–c.

Scheme 7: Reaction of 5-methyl-2-thiouracil (1c) with chloroethynylphosphonates 2a–c.

Scheme 8: Reaction of 2-thiouracil (1d) with chloroethynylphosphonates 2a–c.

Scheme 9: Reaction of 6-trifluoromethyl-2-thiouracil (1e) with chloroethynylphosphonates 2a–c.

Scheme 10: A plausible mechanism of the reaction between 6-trifluoromethyl-2-thiouracil (1e) and chloroethynyl...
Synergy between supported ionic liquid-like phases and immobilized palladium N-heterocyclic carbene–phosphine complexes for the Negishi reaction under flow conditions
- Edgar Peris,
- Raúl Porcar,
- María Macia,
- Jesús Alcázar,
- Eduardo García-Verdugo and
- Santiago V. Luis
Beilstein J. Org. Chem. 2020, 16, 1924–1935, doi:10.3762/bjoc.16.159
- authors, Pd–NHC complexes can evolve through two different pathways towards the formation of a catalytically active cocktail of Pd species. In the first one, a reductive elimination takes place from the Pd(II) intermediate with the concomitant release of NHC-containing byproducts. In the second pathway

Graphical Abstract

Scheme 1: Synthesis of NHC-supported catalysts.

Scheme 2: Negishi benchmark reaction.

Figure 1: Negishi reaction catalyzed by immobilized NHC–Pd complexes. Conditions: methyl 4-bromobenzoate (0.2...

Scheme 3: Synthesis of immobilized NHC–Pd–RuPhos.

Figure 2: Negishi model reaction between 5 and 6 under flow conditions catalyzed by 4b. V = 0.535 mL, 363 mg ...

Figure 3: Negishi model reaction under flow conditions catalyzed by 8a. V = 2.9 mL, 1.25 g of catalyst, resid...

Figure 4: Negishi reaction between 5 and 6 catalyzed by 8a in the presence of SILLPs. a) Yield (%) vs time fo...

Figure 5: TEM images of the polymers after the Negishi reaction between 5 and 6. a) 8a, bar scale 20 nm, PdNP...

Scheme 4: Pd species immobilized onto SILLPs. i) 1 g SILLP 10, 100 mg PdCl2 in milli-Q® water (100 mL 1% HCl,...

Figure 6: Negishi reaction between 5 and 6 catalyzed by 11. 1 equiv methyl 4-bromobenzoate (6, 0.25 mmol), 2 ...

Figure 7: Negishi reaction between 5 and 6 under flow conditions catalyzed by 8a in the presence of a scaveng...

Figure 8: Effect of the structure of the SILLP scavenger for the Negishi reaction between 5 and 6 under flow ...

Figure 9: TEM images of the polymer after the Negishi reaction between 5 and 6 under flow conditions. a) 8a + ...
Regiodivergent synthesis of functionalized pyrimidines and imidazoles through phenacyl azides in deep eutectic solvents
- Paola Vitale,
- Luciana Cicco,
- Ilaria Cellamare,
- Filippo M. Perna,
- Antonio Salomone and
- Vito Capriati
Beilstein J. Org. Chem. 2020, 16, 1915–1923, doi:10.3762/bjoc.16.158

- undergo a base-promoted loss of nitrogen to form α-imino ketones upon protonation [38]. A plausible mechanism for the formation of pyrimidine derivative 7a from 2a, in competition with imidazoles 3a/3a', is depicted in Scheme 5. The key intermediate 5a, formed by elimination of nitrogen from the enol
- intramolecular nucleophilic attack to the terminal imino group of 9a, provides cyclized adduct 10a, and finally pyrimidine derivative 7a by aromatization/elimination of NH3. To the best of our knowledge, this is the first one-pot synthesis of functionalized pyrimidines using phenacyl azides as the sole starting

Graphical Abstract

Scheme 1: One-pot synthesis of 2,5-diarylpyrazines (A) (path a) or 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles (B) ...

Scheme 2: Transformation of phenacyl bromide (1a) in ChCl/Gly into phenacyl azide (2a) and 2-benzoyl-(4 or 5)...

Scheme 3: Synthesis of 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles 3. Scope of the reaction. Typical conditions: 1 ...

Scheme 4: Proposed mechanism for the formation of 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles 3/3' from α-phenacyl ...

Scheme 5: Proposed mechanism for the formation of 2-benzoyl-(4 or 5)-phenyl-(1H)-imidazoles 3a/3a' and 2,4-di...

Scheme 6: Scope of the synthesis of 2,4-diaroyl-6-arylpyrimidines 7. Typical conditions: 2 (0.3 mmol), Et3N (...
One-pot and metal-free synthesis of 3-arylated-4-nitrophenols via polyfunctionalized cyclohexanones from β-nitrostyrenes
- Haruyasu Asahara,
- Minami Hiraishi and
- Nagatoshi Nishiwaki
Beilstein J. Org. Chem. 2020, 16, 1830–1836, doi:10.3762/bjoc.16.150
- products were obtained. The aromatization is considered to proceed as shown in Scheme 4. After the iodization at the 4-position, which leads to the formation of the intermediate 6, the aromatization is achieved by the successive elimination of hydrogen iodide and methanol, with concurrent tautomerism to

Graphical Abstract

Scheme 1: Synthetic scheme of the 3-arylated-4-nitrophenols 5.

Figure 1: X-ray crystallography of the major isomer of 4a. The thermal ellipsoids indicate 50% probability.

Scheme 2: Conversion from 3a to 4a and one-pot synthesis of 4a.

Scheme 3: Deuteration of cyclohexanone 4a.

Scheme 4: A plausible mechanism for the formation of 5a.

Figure 2: Resonance structure of nitroalkenes 1b and 1d.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
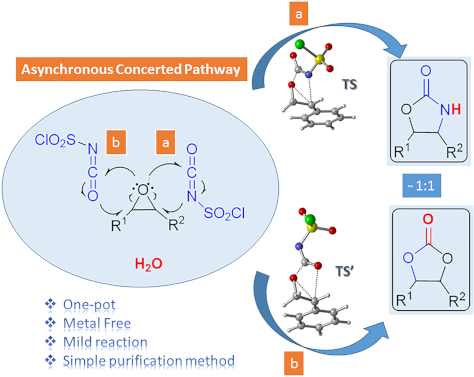
- elimination of hydrated HCl and formation of 13. The calculated free energy of activation was found to be 15.4 kcal/mol with respect to RC1 (7f+CSI) (Figure 5). The final step of path 2 takes place from RC5 (13+H2O) passing through TS5 and forming the target product 9f. This step requires an activation free
- corresponding barrier was calculated to be 13.0 kcal/mol relative to initial reactant complex RC6 (7f+CSI) (Figure 7). Elimination of 18, accompanied by C=O bond formation, constitutes the final step of the reaction observed. Optimized structures are given in Figure 10. The elimination reaction, via the

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
- Lucas Guillemard and
- Joanna Wencel-Delord
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- metal catalyst X2M into an aromatic C–H bond of a substrate (generally facilitated by the presence of a directing group (DG)), delivers a metal–aryl complex. Coordination and subsequent insertion of an alkene into the M–aryl bond then provides the desired coupling product after β-hydride elimination
- reaction media in order to transfer electrons from the low-valent metal complex formed in situ after reductive elimination of the product (Figure 4, right). In this way, the metalacyclic intermediate is reoxidized while the photosensitizer is reduced, thus completing the C–H activation catalytic cycle. By
- that both, the excited Ir photocatalyst and the superoxide anion generated during the transformation, were able to oxidize the low-valent Pd(0) species resulting from the reductive elimination (Figure 6). Under such dual catalysis protocol, various oxidant-sensitive functional groups were tolerated

Graphical Abstract

Figure 1: Concept of dual synergistic catalysis.

Figure 2: Classification of catalytic systems involving two catalysts.

Figure 3: General mechanism for the dual nickel/photoredox catalytic system.

Figure 4: General mechanisms for C–H activation catalysis involving different reoxidation strategies.

Figure 5: Indole synthesis via dual C–H activation/photoredox catalysis.

Figure 6: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 7: Oxidative Heck reaction on arenes via the dual catalysis.

Figure 8: Proposed mechanism for the Heck reaction on arenes via dual catalysis.

Figure 9: Oxidative Heck reaction on phenols via the dual catalysis.

Figure 10: Proposed mechanism for the Heck reaction on phenols via dual catalysis.

Figure 11: Carbazole synthesis via dual C–H activation/photoredox catalysis.

Figure 12: Proposed mechanism for the carbazole synthesis via dual catalysis.

Figure 13: Carbonylation of enamides via the dual C–H activation/photoredox catalysis.

Figure 14: Proposed mechanism for carbonylation of enamides via dual catalysis.

Figure 15: Annulation of benzamides via the dual C–H activation/photoredox catalysis.

Figure 16: Proposed mechanism for the annulation of benzamides via dual catalysis.

Figure 17: Synthesis of indoles via the dual C–H activation/photoredox catalysis.

Figure 18: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 19: General concept of dual catalysis merging C–H activation and photoredox catalysis.

Figure 20: The first example of dual catalysis merging C–H activation and photoredox catalysis.

Figure 21: Proposed mechanism for the C–H arylation with diazonium salts via dual catalysis.

Figure 22: Dual catalysis merging C–H activation/photoredox using diaryliodonium salts.

Figure 23: Direct arylation via the dual catalytic system reported by Xu.

Figure 24: Direct arylation via dual catalytic system reported by Balaraman.

Figure 25: Direct arylation via dual catalytic system reported by Guo.

Figure 26: C(sp3)–H bond arylation via the dual Pd/photoredox catalytic system.

Figure 27: Acetanilide derivatives acylation via the dual C–H activation/photoredox catalysis.

Figure 28: Proposed mechanism for the C–H acylation with α-ketoacids via dual catalysis.

Figure 29: Acylation of azobenzenes via the dual catalysis C–H activation/photoredox.

Figure 30: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 31: Proposed mechanism for the C2-acylation of indoles with aldehydes via dual catalysis.

Figure 32: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 33: Perfluoroalkylation of arenes via the dual C–H activation/photoredox catalysis.

Figure 34: Proposed mechanism for perfluoroalkylation of arenes via dual catalysis.

Figure 35: Sulfonylation of 1-naphthylamides via the dual C–H activation/photoredox catalysis.

Figure 36: Proposed mechanism for sulfonylation of 1-naphthylamides via dual catalysis.

Figure 37: meta-C–H Alkylation of arenes via visible-light metallaphotocatalysis.

Figure 38: Alternative procedure for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 39: Proposed mechanism for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 40: C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 41: Proposed mechanism for C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 42: Undirected C–H aryl–aryl cross coupling via dual gold/photoredox catalysis.

Figure 43: Proposed mechanism for the undirected C–H aryl–aryl cross-coupling via dual catalysis.

Figure 44: Undirected C–H arylation of (hetero)arenes via dual manganese/photoredox catalysis.

Figure 45: Proposed mechanism for the undirected arylation of (hetero)arenes via dual catalysis.

Figure 46: Photoinduced C–H arylation of azoles via copper catalysis.

Figure 47: Photo-induced C–H chalcogenation of azoles via copper catalysis.

Figure 48: Decarboxylative C–H adamantylation of azoles via dual cobalt/photoredox catalysis.

Figure 49: Proposed mechanism for the C–H adamantylation of azoles via dual catalysis.

Figure 50: General mechanisms for the “classical” (left) and Cu-free variant (right) Sonogoshira reaction.

Figure 51: First example of a dual palladium/photoredox catalysis for Sonogashira-type couplings.

Figure 52: Arylation of terminal alkynes with diazonium salts via dual gold/photoredox catalysis.

Figure 53: Proposed mechanism for the arylation of terminal alkynes via dual catalysis.

Figure 54: C–H Alkylation of alcohols promoted by H-atom transfer (HAT).

Figure 55: Proposed mechanism for the C–H alkylation of alcohols promoted by HAT.

Figure 56: C(sp3)–H arylation of latent nucleophiles promoted by H-atom transfer.

Figure 57: Proposed mechanism for the C(sp3)–H arylation of latent nucleophiles promoted by HAT.

Figure 58: Direct α-arylation of alcohols promoted by H-atom transfer.

Figure 59: Proposed mechanism for the direct α-arylation of alcohols promoted by HAT.

Figure 60: C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 61: Proposed mechanism for the C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 62: C–H functionalization of nucleophiles via excited ketone/nickel dual catalysis.

Figure 63: Proposed mechanism for the C–H functionalization enabled by excited ketones.

Figure 64: Selective sp3–sp3 cross-coupling promoted by H-atom transfer.

Figure 65: Proposed mechanism for the selective sp3–sp3 cross-coupling promoted by HAT.

Figure 66: Direct C(sp3)–H acylation of amines via dual Ni/photoredox catalysis.

Figure 67: Proposed mechanism for the C–H acylation of amines via dual Ni/photoredox catalysis.

Figure 68: C–H hydroalkylation of internal alkynes via dual Ni/photoredox catalysis.

Figure 69: Proposed mechanism for the C–H hydroalkylation of internal alkynes.

Figure 70: Alternative procedure for the C–H hydroalkylation of ynones, ynoates, and ynamides.

Figure 71: Allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 72: Proposed mechanism for the allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 73: Asymmetric allylation of aldehydes via dual Cr/photoredox catalysis.

Figure 74: Proposed mechanism for the asymmetric allylation of aldehydes via dual catalysis.

Figure 75: Aldehyde C–H functionalization promoted by H-atom transfer.

Figure 76: Proposed mechanism for the C–H functionalization of aldehydes promoted by HAT.

Figure 77: Direct C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 78: Proposed mechanism for the C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 79: Direct C–H trifluoromethylation of strong aliphatic bonds promoted by HAT.

Figure 80: Proposed mechanism for the C–H trifluoromethylation of strong aliphatic bonds.
Pauson–Khand reaction of fluorinated compounds
- Jorge Escorihuela,
- Daniel M. Sedgwick,
- Alberto Llobat,
- Mercedes Medio-Simón,
- Pablo Barrio and
- Santos Fustero
Beilstein J. Org. Chem. 2020, 16, 1662–1682, doi:10.3762/bjoc.16.138

- stereochemical outcome of the overall process. A carbon monoxide ligand then undergoes migratory insertion into one of the Co–C bonds in cobaltacycle V, followed by reductive elimination to release the final product (Scheme 3). As mentioned above, the regiochemistry of this transformation is, in most cases
- corresponding mesylate 38b proved sufficient for the synthesis of N-tethered substrates 37 (Z = NTs), through simple nucleophilic substitution (via c). It is noteworthy that under standard PK reaction conditions, fluoroenyne 39 evolves to diene 40. The formation of compound 40 is possible by elimination of HF
- from the PK product 41, meaning that the fluoro-PKR does initially take place and that the desired product 41 could be isolated by avoiding the subsequent elimination reaction. This hypothesis was confirmed when the less basic DMSO was used as the promoter instead of NMO, allowing the successful

Graphical Abstract

Scheme 1: Schematic representation of the Pauson–Khand reaction.

Scheme 2: Substrates included in this review.

Scheme 3: Commonly accepted mechanism for the Pauson–Khand reaction.

Scheme 4: Regioselectivity of the PKR.

Scheme 5: Variability at the acetylenic and olefinic counterpart.

Scheme 6: Pauson–Khand reaction of fluoroolefinic enynes reported by the group of Ishizaki [46].

Scheme 7: PKR of enynes bearing fluorinated groups on the alkynyl moiety, reported by the group of Ishizaki [46]....

Scheme 8: Intramolecular PKR of 1,7-enynes reported by the group of Billard [47].

Scheme 9: Intramolecular PKR of 1,7-enynes reported by the group of Billard [48].

Scheme 10: Intramolecular PKR of 1,7-enynes by the group of Bonnet-Delpon [49]. Reaction conditions: i) Co(CO)8 (1...

Scheme 11: Intramolecular PKR of 1,6-enynes reported by the group of Ichikawa [50].

Scheme 12: Intramolecular Rh(I)-catalyzed PKR reported by the group of Hammond [52].

Scheme 13: Intramolecular PKR of allenynes reported by the group of Osipov [53].

Scheme 14: Intramolecular PKR of 1,7-enynes reported by the group of Osipov [53].

Scheme 15: Intramolecular PKR of fluorine-containing 1,6-enynes reported by the Konno group [54].

Scheme 16: Diastereoselective PKR with enantioenriched fluorinated enynes 34 [55].

Scheme 17: Intramolecular PKR reported by the group of Martinez-Solorio [56].

Scheme 18: Fluorine substitution at the olefinic counterpart.

Scheme 19: Synthesis of fluorinated enynes 37 [59].

Scheme 20: Fluorine-containing substrates in PKR [59].

Scheme 21: Pauson Khand reaction for fluorinated enynes by the Fustero group: scope and limitations [59].

Scheme 22: Synthesis of chloro and bromo analogues [59].

Scheme 23: Dimerization pathway [59].

Scheme 24: Synthesis of fluorine-containing N-tethered 1,7-enynes [61].

Scheme 25: Intramolecular PKR of chiral N-tethered fluorinated 1,7-enynes [61].

Scheme 26: Examples of further modifications to the Pauson−Khand adducts [61].

Scheme 27: Asymmetric synthesis the fluorinated enynes 53.

Scheme 28: Intramolecular PKR of chiral N-tethered 1,7-enynes 53 [64].

Scheme 29: Intramolecular PKR of chiral N-tethered 1,7-enyne bearing a vinyl fluoride [64].

Scheme 30: Catalytic intramolecular PKR of chiral N-tethered 1,7-enynes [64].

Scheme 31: Model fluorinated alkynes used by Riera and Fustero [70].

Scheme 32: PKR with norbornadiene and fluorinated alkynes 58 [71].

Scheme 33: Nucleophilic addition/detrifluoromethylation and retro Diels-Alder reactions [70].

Scheme 34: Tentative mechanism for the nucleophilic addition/retro-aldol reaction sequence.

Scheme 35: Catalytic PKR with norbornadiene [70].

Scheme 36: Scope of the PKR of trifluoromethylalkynes with norbornadiene [72].

Scheme 37: DBU-mediated detrifluoromethylation [72].

Scheme 38: A simple route to enone 67, a common intermediate in the total synthesis of α-cuparenone.

Scheme 39: Effect of the olefin partner in the regioselectivity of the PKR with trifluoromethyl alkynes [79].

Scheme 40: Intermolecular PKR of trifluoromethylalkynes with 2-norbornene reported by the group of Konno [54].

Scheme 41: Intermolecular PKR of diarylalkynes with 2-norbornene reported by the group of Helaja [80].

Scheme 42: Intermolecular PKR reported by León and Fernández [81].

Scheme 43: PKR reported with cyclopropene 73 [82].
Rearrangement of o-(pivaloylaminomethyl)benzaldehydes: an experimental and computational study
- Csilla Hargitai,
- Györgyi Koványi-Lax,
- Tamás Nagy,
- Péter Ábrányi-Balogh,
- András Dancsó,
- Gábor Tóth,
- Judit Halász,
- Angéla Pandur,
- Gyula Simig and
- Balázs Volk
Beilstein J. Org. Chem. 2020, 16, 1636–1648, doi:10.3762/bjoc.16.136
- , gave isomeric aldehyde 2a (48%) and the dimer-like racemic product 3a (11%). Both transformations were rationalized by the intermediacy of the isoindole 4a (Scheme 1). The formation of aldehyde 2a can be explained by a protonation of the ring tautomer 1a, followed by an acid-catalyzed water elimination
- explained by the protonation of ring tautomers 9 to 10 (Scheme 4), followed by a water elimination, and subsequent deprotonation of cations 11 to afford isoindoles 4 [3][4][5]. The protonation of the latter [6], followed by the attack of water at position 1 of cations 12, and subsequent deprotonation of

Graphical Abstract

Scheme 1: Rearrangement of methylenedioxy-substituted aminoaldehyde 1a to regioisomer 2a and formation of the...

Scheme 2: Synthesis of 1-arylisoindoles 6 and formation of dimers 5.

Scheme 3: Rearrangement of aminoaldehydes 1 to regioisomers 2 and formation of dimer-like products 3 and 8.

Figure 1: X-ray structures of compounds 3b (left) and 8b (right).

Scheme 4: Proposed mechanism of the isomerization of aldehydes 1 via isoindoles 4.

Scheme 5: Proposed mechanism of the formation of dimer-like products 3a,b.

Scheme 6: Proposed mechanism for the formation of dimer-like products 8a,b.

Scheme 7: Dimerization of indole under acidic conditions.

Figure 2: Gibbs free energy diagram for the 1→2 rearrangement.

Scheme 8: Reaction of o-(pivaloylaminomethyl)benzaldehyde (1e) to give dimer-like products 23a and 23b.

Figure 3: X-ray structures of compounds 23a (left) and 23b (right).

Figure 4: Structures of the minimal energy conformer of stereoisomer 23a and those of two minimal energy conf...
Facile synthesis of 7-alkyl-1,2,3,4-tetrahydro-1,8-naphthyridines as arginine mimetics using a Horner–Wadsworth–Emmons-based approach
- Rhys A. Lippa,
- John A. Murphy and
- Tim N. Barrett
Beilstein J. Org. Chem. 2020, 16, 1617–1626, doi:10.3762/bjoc.16.134
- defluorination, presumably via a Tsuji–Trost-like elimination of the allylic fluoride [18][19]. This sequence represents a marked improvement from the Wittig-including route, lowering the number of synthetic steps and increasing the overall yield [2]. Furthermore, no problematic byproducts are formed, and good
- the exact mechanism is not known, it may involve a reactive carbene intermediate formed by α-elimination of the phosphonate as described in previous reports [23]. Alternatively, a base-promoted autoxidation of phosphonate 7 in the presence of trace amounts of oxygen, followed by olefination may be

Graphical Abstract

Figure 1: The Arg–Gly–Asp tripeptide sequence and examples of tetrahydro-1,8-naphthyridine-containing integri...

Scheme 1: Commonly used synthetic routes to tetrahydro-1,8-naphthyridine moieties by hydrogenation of saturat...

Scheme 2: Previous synthetic route to fluoropyrrolidine 6 utilising a Wittig reaction and the novel, higher y...

Scheme 3: Synthesis of phosphoramidate 9 from tetrahydro-1,8-naphthyridine 8. Conditions: s-BuLi (3 equiv), d...

Scheme 4: Mono- and diphosphorylation of tetrahydro-1,8-naphthyridine 11. Conditions: (i) s-BuLi (2 equiv), d...

Scheme 5: Synthesis of amine 6 from phosphonate 7 and aldehyde 5. Conditions: (i) T3P® (50% w/w in DCM, 3 equ...

Scheme 6: Monodeuteration of 13 as observed by 1H and 13C NMR. Conditions: s-BuLi (3 equiv), THF, −42 °C, 20 ...

Scheme 7: Sequential diphosphorylation of tetrahydronaphthyridine 11. Conditions: (i) iPrMgCl (1.5 equiv), TH...

Scheme 8: Possible mechanistic pathways for the formation of dimer 28. Conditions: KOt-Bu, THF, 1 h, 68% yiel...

Scheme 9: Alkylation of phosphoramidate 13 by iodide 29 to afford compound 30 and byproducts alcohol 31 and d...
Azidophosphonium salt-directed chemoselective synthesis of (E)/(Z)-cinnamyl-1H-triazoles and regiospecific access to bromomethylcoumarins from Morita–Baylis–Hillman adducts
- Soundararajan Karthikeyan,
- Radha Krishnan Shobana,
- Kamarajapurathu Raju Subimol,
- J. Helen Ratna Monica and
- Ayyanoth Karthik Krishna Kumar
Beilstein J. Org. Chem. 2020, 16, 1579–1587, doi:10.3762/bjoc.16.130
- protection and elimination of the allylic hydroxy group. We believe that this crucial strategy could be primarily resolved by a quaternary phosphonium salt. After the initial screening of various quaternary phosphonium salts, the azidophosphonium salt [Ph3P+CBr3]N3−, reported by Blanco and co-workers, was

Graphical Abstract

Scheme 1: Literature-reported cycloaddition reactions of MBH acetates involving azides and alkynes [24-28].

Scheme 2: Synthetic methodologies for triazolations of MBH adducts. a) Literature-reported indirect triazolat...

Scheme 3: Scope of the one-pot cascade reaction of the unprotected Morita–Baylis–Hillman adducts 3a–q.

Figure 1: Proposed mechanism for the synthesis of 1,4-disubstituted triazoles.

Scheme 4: Comparative analysis of the sequential one-pot reaction.

Figure 2: Proposed mechanism for the synthesis of 3-(bromomethyl)coumarins.
NHC-catalyzed enantioselective synthesis of β-trifluoromethyl-β-hydroxyamides
- Alyn T. Davies,
- Mark D. Greenhalgh,
- Alexandra M. Z. Slawin and
- Andrew D. Smith
Beilstein J. Org. Chem. 2020, 16, 1572–1578, doi:10.3762/bjoc.16.129
- [2 + 2] cycloaddition with the trifluoroacetophenone derivative to give VI [60]. Elimination of the NHC catalyst completes the catalytic cycle and provides the corresponding β-lactone product. Subsequent ring opening with a suitable nucleophilic amine leads to the isolable β-trifluoromethyl-β

Graphical Abstract

Figure 1: Organocatalytic enantioselective aldol approaches using trifluoroacetophenone derivatives.

Figure 2: NHC-catalyzed approaches to β-lactones using trifluoroacetophenone derivatives.

Scheme 1: Reaction scope with respect to the nucleophile. aIsolated yield of the product in >95:5 dr. bDeterm...

Scheme 2: Reaction scope with respect to the trifluoroacetophenone derivative and α-aroyloxyaldehyde. aIsolat...

Scheme 3: Proposed mechanism.
Photoredox-catalyzed silyldifluoromethylation of silyl enol ethers
- Vyacheslav I. Supranovich,
- Vitalij V. Levin and
- Alexander D. Dilman
Beilstein J. Org. Chem. 2020, 16, 1550–1553, doi:10.3762/bjoc.16.126
- flash column chromatography on silica gel, presumably, owing to facile β-elimination of hydrogen fluoride. To isolate a stable product, the reaction mixture was treated with sodium borohydride in ethanol, which effected the reduction of the keto group affording the corresponding alcohol 4a in 52% yield

Graphical Abstract

Scheme 1: Reactions of (bromodifluoromethyl)trimethylsilane (1).

Scheme 2: Optimization studies. Yield determined by 19F NMR spectroscopy using an internal standard.

Figure 1: Reaction of silyl enol ethers. Yields refer to isolated yields. aReaction time 24 h; b1.0 equiv of ...

Scheme 3: Proposed mechanism of the fluoroalkylation reaction.
One-pot synthesis of 1,3,5-triazine-2,4-dithione derivatives via three-component reactions
- Gui-Feng Kang and
- Gang Zhang
Beilstein J. Org. Chem. 2020, 16, 1447–1455, doi:10.3762/bjoc.16.120
- nucleophilic addition of another molecule 2 takes place to furnish the tricomponent adduct 4, which undergoes elimination of ammonia to afford the corresponding ring-closed intermediate 7 [37]. The latter undergoes a proton-transfer process to form intermediate 10 [38]. Thereafter, the subsequent step involves

Graphical Abstract

Figure 1: Selected examples of triazinethione-containing bioactive compounds.

Scheme 1: Strategies for the synthesis of triazinethiones.

Scheme 2: Aldehyde substrate scope of three-component reaction of aldehydes, thiourea and trimethyl orthoform...

Scheme 3: Orthoformate substrate scope of the three component reaction of benzaldehyde, thiourea, and orthofo...

Scheme 4: Gram-scale synthesis of 6aa.

Figure 2: X-ray structure of 6-(methylthio)-4-phenyl-3,4-dihydro-1,3,5-triazine-2(1H)-thione (6aa) with therm...

Scheme 5: Control experiments for investigation of the mechanism.

Scheme 6: Plausible mechanism.
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
- Yeersen Patehebieke
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- cyclopropylcarbinyl radical 5. Then, the radical 5 opens to form the homoallylic radical 6, followed by the addition of olefins to this radical form the 5-hexenyl radical 7. The ring closure of the radical 7 and the elimination of a thiyl radical furnishes the final product 9. When it came to annulations of complex
- cyclization, and an elimination give the desired product 27 (Scheme 7). The use of PhSSPh obviated the undesired reactions, which occurred when using Ph3SnH and Bu3SnH, respectively, as the catalyst for this reaction. Oxidation reactions Not only can disulfide catalysts induce cycloadditions of olefins but

Graphical Abstract

Scheme 1: [3 + 2] cyclization catalyzed by diaryl disulfide.

Scheme 2: [3 + 2] cycloaddition catalyzed by disulfide.

Scheme 3: Disulfide-bridged peptide-catalyzed enantioselective cycloaddition.

Scheme 4: Disulfide-catalyzed [3 + 2] methylenecyclopentane annulations.

Scheme 5: Disulfide as a HAT cocatalyst in the [4 + 2] cycloaddition reaction.

Scheme 6: Proposed mechanism of the [4 + 2] cycloaddition reaction using disulfide as a HAT cocatalyst.

Scheme 7: Disulfide-catalyzed ring expansion of vinyl spiro epoxides.

Scheme 8: Disulfide-catalyzed aerobic oxidation of diarylacetylene.

Scheme 9: Disulfide-catalyzed aerobic photooxidative cleavage of olefins.

Scheme 10: Disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 11: Proposed mechanism of the disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 12: Disulfide-catalyzed oxidation of allyl alcohols.

Scheme 13: Disulfide-catalyzed diboration of alkynes.

Scheme 14: Dehalogenative radical cyclization catalyzed by disulfide.

Scheme 15: Hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 16: Plausible mechanism of the hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 17: Disulfide-cocatalyzed anti-Markovnikov olefin hydration reactions.

Scheme 18: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 19: Proposed mechanism of the disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 20: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 21: Disulfide-catalyzed conversion of maleate esters to fumarates and 5H-furanones.

Scheme 22: Disulfide-catalyzed isomerization of difluorotriethylsilylethylene.

Scheme 23: Disulfide-catalyzed isomerization of allyl alcohols to carbonyl compounds.

Scheme 24: Proposed mechanism for the disulfide-catalyzed isomerization of allyl alcohols to carbonyl compound...

Scheme 25: Diphenyl disulfide-catalyzed enantioselective synthesis of ophirin B.

Scheme 26: Disulfide-catalyzed isomerization in the total synthesis of (+)-hitachimycin.

Scheme 27: Disulfide-catalyzed isomerization in the synthesis of (−)-gloeosporone.
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- accompanied by elimination reactions. 3. Synthesis via cycloadditions Cycloadditions, especially the photochemical [2 + 2] cycloaddition (thia-Paternò–Büchi reaction) of thiones and thioamides with olefins [15][16][17][18], and formal cycloadditions are alternative routes for the construction of thietane

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Oxime radicals: generation, properties and application in organic synthesis
- Igor B. Krylov,
- Stanislav A. Paveliev,
- Alexander S. Budnikov and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- the radical 65 gives a C-centered radical 66, which is captured by TEMPO to form intermediate 67. Elimination of TEMPOH leads to a β-unsaturated oxime 68, which could undergo cyclization by ionic or radical mechanisms to give isoxazoline 63 [114][115]. A similar cyclization with the formation of
- , respectively (Scheme 48) [137]. Presumably, the C-centered radical formed after 5-exo-trig cyclization of the oxime radical recombines with TEMPO. The resulting adduct undergoes β-elimination of TEMPOH with the formation of final unsaturated compounds. The intermediate coupling product of the C-centered

Graphical Abstract

Figure 1: Imine-N-oxyl radicals (IV) discussed in the present review and other classes of N-oxyl radicals (I–...

Figure 2: The products of decomposition of iminoxyl radicals generated from oximes by oxidation with Ag2O.

Scheme 1: Generation of oxime radicals and study of the kinetics of their decay by photolysis of the solution...

Scheme 2: Synthesis of di-tert-butyliminoxyl radical and its decomposition products.

Scheme 3: The proposed reaction pathway of the decomposition of di-tert-butyliminoxyl radical (experimentally...

Scheme 4: Monomolecular decomposition of the tert-butyl(triethylmethyl)oxime radical.

Scheme 5: The synthesis and stability of the most stable dialkyl oxime radicals – di-tert-butyliminoxyl and d...

Scheme 6: The formation of iminoxyl radicals from β-diketones under the action of NO2.

Scheme 7: Synthesis of the diacetyliminoxyl radical.

Scheme 8: Examples of long-living oxime radicals with electron-withdrawing groups and the conditions for thei...

Figure 3: The electronic structure iminoxyl radicals and their geometry compared to the corresponding oximes.

Figure 4: Bond dissociation enthalpies (kcal/mol) of oximes and N,N-disubstituted hydroxylamines calculated o...

Scheme 9: Examples demonstrating the low reactivity of the di-tert-butyliminoxyl radical towards the substrat...

Scheme 10: The reactions of di-tert-butyliminoxyl radical with unsaturated hydrocarbons involving hydrogen ato...

Scheme 11: Possible mechanisms of reaction of di-tert-butyliminoxyl radical with alkenes.

Scheme 12: Products of the reaction between di-tert-butyliminoxyl radical and phenol derivatives.

Scheme 13: The reaction of di-tert-butyliminoxyl radical with amines.

Scheme 14: Reaction of di-tert-butyliminoxyl radicals with organolithium reagents.

Scheme 15: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of mang...

Scheme 16: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of Cu(BF...

Scheme 17: Oxidative C–O coupling of benzylmalononitrile (47) with 3-(hydroxyimino)pentane-2,4-dione (19).

Scheme 18: The proposed mechanism of the oxidative coupling of benzylmalononitrile (47) with diacetyl oxime (19...

Scheme 19: Oxidative C–O coupling of pyrazolones with oximes under the action of Fe(ClO4)3.

Scheme 20: The reaction of diacetyliminoxyl radical with pyrazolones.

Scheme 21: Oxidative C–O coupling of oximes with acetonitrile, ketones, and esters.

Scheme 22: Intramolecular cyclizations of oxime radicals to form substituted isoxazolines or cyclic nitrones.

Scheme 23: TEMPO-mediated oxidative cyclization of oximes with C–H bond cleavage.

Scheme 24: Proposed reaction mechanism of oxidative cyclization of oximes with C–H bond cleavage.

Scheme 25: Selectfluor/Bu4NI-mediated C–H oxidative cyclization of oximes.

Scheme 26: Oxidative cyclization of N-benzyl amidoximes to 1,2,4-oxadiazoles.

Scheme 27: The formation of quinazolinone 73a from 5-phenyl-4,5-dihydro-1,2,4-oxadiazole 74 under air.

Scheme 28: DDQ-mediated oxidative cyclization of thiohydroximic acids.

Scheme 29: Plausible mechanism of the oxidative cyclization of thiohydroximic acids.

Scheme 30: Silver-mediated oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl compounds.

Scheme 31: Possible pathway of one-pot oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl com...

Scheme 32: T(p-F)PPT-catalyzed oxidative cyclization of oximes with the formation of 1,2,4-oxadiazolines.

Scheme 33: Intramolecular cyclization of iminoxyl radicals involving multiple C=C and N=N bonds.

Scheme 34: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes employing the DEAD or TEMPO/DEAD system wi...

Scheme 35: Cobalt-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 36: Manganese-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 37: Visible light photocatalytic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 38: TBAI/TBHP-mediated radical cascade cyclization of the β,γ-unsaturated oximes.

Scheme 39: TBAI/TBHP-mediated radical cascade cyclization of vinyl isocyanides with β,γ-unsaturated oximes.

Scheme 40: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of an ...

Scheme 41: Transformation of unsaturated oxime to oxyiminomethylisoxazoline via the confirmed dimeric nitroso ...

Scheme 42: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of a n...

Scheme 43: Synthesis of cyano-substituted oxazolines from unsaturated oximes using the TBN/[RuCl2(p-cymene)]2 ...

Scheme 44: Synthesis of trifluoromethylthiolated isoxazolines from unsaturated oximes.

Scheme 45: Copper-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with the introduction of an azido ...

Scheme 46: TBHP-mediated oxidative cascade cyclization of β,γ-unsaturated oximes and unsaturated N-arylamides.

Scheme 47: Copper-сatalyzed oxidative cyclization of unsaturated oximes with the introduction of an amino grou...

Scheme 48: TEMPO-mediated oxidative cyclization of unsaturated oximes followed by elimination.

Scheme 49: Oxidative cyclization of β,γ-unsaturated oximes with the introduction of a trifluoromethyl group.

Scheme 50: Oxidative cyclization of unsaturated oximes with the introduction of a nitrile group.

Scheme 51: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a nitrile ...

Scheme 52: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a sulfonyl...

Scheme 53: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes to isoxazolines with the introduction of a...

Scheme 54: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a thiocyan...

Scheme 55: PhI(OAc)2-mediated oxidative cyclization of oximes with C–S and C–Se bond formation.

Scheme 56: PhI(OAc)2-mediated oxidative cyclization of unsaturated oximes accompanied by alkoxylation.

Scheme 57: PhI(OAc)2-mediated cyclization of unsaturated oximes to methylisoxazolines.

Scheme 58: Oxidative cyclization-alkynylation of unsaturated oximes.

Scheme 59: TEMPO-mediated oxidative cyclization of C-glycoside ketoximes to C-glycosylmethylisoxazoles.

Scheme 60: Silver-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with formation of fluoroalkyl isox...

Scheme 61: Oxidative cyclization of β,γ-unsaturated oximes with the formation of haloalkyl isoxazolines.

Scheme 62: Cyclization of β,γ-unsaturated oximes into haloalkyl isoxazolines under the action of the halogenat...

Scheme 63: Synthesis of haloalkyl isoxazoles and cyclic nitrones via oxidative cyclization and 1,2-halogen shi...

Scheme 64: Electrochemical oxidative cyclization of diaryl oximes.

Scheme 65: Copper-сatalyzed cyclization and dioxygenation oximes containing a triple C≡C bond.

Scheme 66: Photoredox-catalyzed sulfonylation of β,γ-unsaturated oximes by sulfonyl hydrazides.

Scheme 67: Oxidative cyclization of β,γ-unsaturated oximes with introduction of sulfonate group.

Scheme 68: Ultrasound-promoted oxidative cyclization of β,γ-unsaturated oximes.
Synthesis of pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinolines via three-component [3 + 2] cycloaddition followed by one-pot N-allylation and intramolecular Heck reactions
- Xiaoming Ma,
- Suzhi Meng,
- Xiaofeng Zhang,
- Qiang Zhang,
- Shenghu Yan,
- Yue Zhang and
- Wei Zhang
Beilstein J. Org. Chem. 2020, 16, 1225–1233, doi:10.3762/bjoc.16.106
- oxidative addition of the Pd(0) species to alkene intermediate 8a leads to Pd-complex I. Intramolecular coordination of Pd-complex I with the C–C double bond forms complex II which is followed by the syn insertion of alkene to give complex III [50][51]. Subsequent β-hydride elimination of III gives complex

Graphical Abstract

Figure 1: Bioactive pyrrolo[2,1-a]isoquinolines and hexahydropyrrolo[2,1-a]isoquinolines.

Scheme 1: [3 + 2] Cycloaddition with amino esters or amino acids.

Scheme 2: Scaffolds derived from the initial [3 + 2] adducts.

Scheme 3: [3 + 2] Cycloaddition with amino esters or amino acids. Conditions: 1:3:4 (1.2:1:1.1), Et3N (1.5 eq...

Scheme 4: Synthesis of pyrrolo[2,1-a]isoquinolines 9. Reaction conditions: 5 (0.5 mmol, 1 equiv), 7 (3 equiv)...

Scheme 5: Synthesis of pyrrolo[2,1-a]isoquinolines 11. Reaction conditions: 6 (0.5 mmol, 1 equiv), 7 (3 equiv...

Scheme 6: Synthesis of pyrrolo[2,1-a]isoquinolines 12. Reaction conditions: 5 or 6 (0.5 mmol, 1 equiv), cinna...

Scheme 7: Plausible mechanism for the synthesis of 9a.






