Search results
Search for "leaving group" in Full Text gives 229 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Regioselective cobalt(II)-catalyzed [2 + 3] cycloaddition reaction of fluoroalkylated alkynes with 2-formylphenylboronic acids: easy access to 2-fluoroalkylated indenols
- Tatsuya Kumon,
- Miroku Shimada,
- Jianyan Wu,
- Shigeyuki Yamada and
- Tsutomu Konno
Beilstein J. Org. Chem. 2020, 16, 2193–2200, doi:10.3762/bjoc.16.184
- best of our knowledge, there are no reports on the practical synthesis of disubstituted 2-fluoroalkylated indenols so far. Transition-metal-catalyzed carbocyclization reactions of alkynes with benzene derivatives having a leaving group X (X = Br, I, OTs, B(OH)2) have been widely considered as one of

Graphical Abstract

Figure 1: Indenol skeleton.

Scheme 1: Synthesis of 2,3-disubstituted indene derivatives.

Scheme 2: Cobalt-catalyzed [2 + 3] cycloaddition reaction of the fluorinated alkynes 1 with various 2-formylp...

Scheme 3: Synthesis of the fluoroalkylated indenone 6 and the indanone 7 from the indenol 3aA. The yields wer...

Scheme 4: Stereochemical assignment of 5aA and 7 based on NMR techniques. The cross-peaks were observed throu...

Scheme 5: Proposed reaction mechanism.
Facile synthesis of 7-alkyl-1,2,3,4-tetrahydro-1,8-naphthyridines as arginine mimetics using a Horner–Wadsworth–Emmons-based approach
- Rhys A. Lippa,
- John A. Murphy and
- Tim N. Barrett
Beilstein J. Org. Chem. 2020, 16, 1617–1626, doi:10.3762/bjoc.16.134
- , affording phosphoramidate 9 in low yield, indicating good leaving group ability of the stabilised tetrahydronaphthyridine anion. No formation of phosphonate 10 was detected (Scheme 3). It was proposed that a deprotonation of 7-methyl-1,2,3,4-tetrahydro-1,8-naphthyridine (11) with two equivalents of sec-BuLi

Graphical Abstract

Figure 1: The Arg–Gly–Asp tripeptide sequence and examples of tetrahydro-1,8-naphthyridine-containing integri...

Scheme 1: Commonly used synthetic routes to tetrahydro-1,8-naphthyridine moieties by hydrogenation of saturat...

Scheme 2: Previous synthetic route to fluoropyrrolidine 6 utilising a Wittig reaction and the novel, higher y...

Scheme 3: Synthesis of phosphoramidate 9 from tetrahydro-1,8-naphthyridine 8. Conditions: s-BuLi (3 equiv), d...

Scheme 4: Mono- and diphosphorylation of tetrahydro-1,8-naphthyridine 11. Conditions: (i) s-BuLi (2 equiv), d...

Scheme 5: Synthesis of amine 6 from phosphonate 7 and aldehyde 5. Conditions: (i) T3P® (50% w/w in DCM, 3 equ...

Scheme 6: Monodeuteration of 13 as observed by 1H and 13C NMR. Conditions: s-BuLi (3 equiv), THF, −42 °C, 20 ...

Scheme 7: Sequential diphosphorylation of tetrahydronaphthyridine 11. Conditions: (i) iPrMgCl (1.5 equiv), TH...

Scheme 8: Possible mechanistic pathways for the formation of dimer 28. Conditions: KOt-Bu, THF, 1 h, 68% yiel...

Scheme 9: Alkylation of phosphoramidate 13 by iodide 29 to afford compound 30 and byproducts alcohol 31 and d...
Heterogeneous photocatalysis in flow chemical reactors
- Christopher G. Thomson,
- Ai-Lan Lee and
- Filipe Vilela
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125


Graphical Abstract

Figure 1: A) Bar chart of the publications per year for the topics “Photocatalysis” (49,662 instances) and “P...

Figure 2: A) Professor Giacomo Ciamician and Dr. Paolo Silber on their roof laboratory at the University of B...

Scheme 1: PRC trifluoromethylation of N-methylpyrrole (1) using hazardous gaseous CF3I safely in a flow react...

Figure 3: A) Unit cells of the three most common crystal structures of TiO2: rutile, brookite, and anatase. R...

Figure 4: Illustration of the key semiconductor photocatalysis events: 1) A photon with a frequency exceeding...

Figure 5: Photocatalytic splitting of water by oxygen vacancies on a TiO2(110) surface. Reprinted with permis...

Figure 6: Proposed adsorption modes of A) benzene, B) chlorobenzene, C) toluene, D) phenol, E) anisole, and F...

Figure 7: Structures of the sulfonate-containing organic dyes RB5 (3) and MX-5B (4) and the adsorption isothe...

Figure 8: Idealised triclinic unit cell of a g-C3N4 type polymer, displaying possible hopping transport scena...

Figure 9: Idealised structure of a perfect g-C3N4 sheet. The central unit highlighted in red represents one t...

Figure 10: Timeline of the key processes of charge transport following the photoexcitation of g-C3N4, leading ...

Scheme 2: Photocatalytic bifunctionalisation of heteroarenes using mpg-C3N4, with the selected examples 5 and ...

Figure 11: A) Structure of four linear conjugated polymer photocatalysts for hydrogen evolution, displaying th...

Figure 12: Graphical representation of the common methods used to immobilise molecular photocatalysts (PC) ont...

Figure 13: Wireless light emitter-supported TiO2 (TiO2@WLE) HPCat spheres powered by resonant inductive coupli...

Figure 14: Graphical representation of zinc–perylene diimide (Zn-PDI) supramolecular assembly photocatalysis v...

Scheme 3: Upconversion of NIR photons to the UV frequency by NaYF4:Yb,Tm nanocrystals sequentially coated wit...

Figure 15: Types of reactors employed in heterogeneous photocatalysis in flow. A) Fixed bed reactors and the s...

Figure 16: Electrochemical potential of common semiconductor, transition metal, and organic dye-based photocat...

Scheme 4: Possible mechanisms of an immobilised molecular photoredox catalyst by oxidative or reductive quenc...

Scheme 5: Scheme of the CMB-C3N4 photocatalytic decarboxylative fluorination of aryloxyacetic acids, with the...

Scheme 6: Scheme of the g-C3N4 photocatalytic desilylative coupling reaction in flow and proposed mechanism [208].

Scheme 7: Proposed mechanism of the radical cyclisation of unsaturated alkyl 2-bromo-1,3-dicarbonyl compounds...

Scheme 8: N-alkylation of benzylamine and schematic of the TiO2-coated microfluidic device [213].

Scheme 9: Proposed mechanism of the Pt@TiO2 photocatalytic deaminitive cyclisation of ʟ-lysine (23) to ʟ-pipe...

Scheme 10: A) Proposed mechanism for the photocatalytic oxidation of phenylboronic acid (24). B) Photos and SE...

Scheme 11: Proposed mechanism for the DA-CMP3 photocatalytic aza-Henry reaction performed in a continuous flow...

Scheme 12: Proposed mechanism for the formation of the cyclic product 32 by TiO2-NC HPCats in a slurry flow re...

Scheme 13: Reaction scheme for the photocatalytic synthesis of homo and hetero disulfides in flow and scope of...

Scheme 14: Reaction scheme for the MoOx/TiO2 HPCat oxidation of cyclohexane (34) to benzene. The graph shows t...

Scheme 15: Proposed mechanism of the TiO2 HPC heteroarene C–H functionalisation via aryl radicals generated fr...

Scheme 16: Scheme of the oxidative coupling of benzylamines with the HOTT-HATN HPCat and selected examples of ...

Scheme 17: Photocatalysis oxidation of benzyl alcohol (40) to benzaldehyde (41) in a microflow reactor coated ...

Figure 17: Mechanisms of Dexter and Forster energy transfer.

Scheme 18: Continuous flow process for the isomerisation of alkenes with an ionic liquid-immobilised photocata...

Scheme 19: Singlet oxygen synthetic step in the total synthesis of canataxpropellane [265].

Scheme 20: Scheme and proposed mechanism of the singlet oxygen photosensitisation by CMP_X HPCats, with the st...

Scheme 21: Structures of CMP HPCat materials applied by Vilela and co-workers for the singlet oxygen photosens...

Scheme 22: Polyvinylchloride resin-supported TDCPP photosensitisers applied for singlet oxygen photosensitisat...

Scheme 23: Structure of the ionically immobilised TPP photosensitiser on amberlyst-15 ion exchange resins (TPP...

Scheme 24: Photosensitised singlet oxygen oxidation of citronellol (46) in scCO2, with automatic phase separat...

Scheme 25: Schematic of PS-Est-BDP-Cl2 being applied for singlet oxygen photosensitisation in flow. A) Pseudo-...

Scheme 26: Reaction scheme of the singlet oxygen oxidation of furoic acid (54) using a 3D-printed microfluidic...

Figure 18: A) Photocatalytic bactericidal mechanism by ROS oxidative cleavage of membrane lipids (R = H, amino...

Figure 19: A) Suggested mechanisms for the aqueous pollutant degradation by TiO2 in a slurry flow reactor [284-287]. B)...

Figure 20: Schematic of the flow system used for the degradation of aqueous oxytetracycline (56) solutions [215]. M...

Scheme 27: Degradation of a salicylic acid (57) solution by a coupled solar photoelectro-Fenton (SPEF) process...

Figure 21: A) Schematic flow diagram using the TiO2-coated NETmix microfluidic device for an efficient mass tr...
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- has been applied in the synthesis of thietanes as a nucleophile and generated phosphorothioate as a leaving group [24]. Some other cyclization methods have been reported in the synthesis of thietanes as well [25] (Figure 2). This review covers the methods outlined in Figure 2 and also some
- strongly favored over the ring closure between the thiolate and the 2-position, since the SN2 displacement of a mesylate leaving group adjacent to the anomeric center is known to be restricted [50] (Scheme 20). The same group synthesized a thietane-fused gulopyranoside starting from methyl 4,6-O
- . From a mechanistic point of view, 3-chloroallyl lithium (420) first coordinated with the thiiranes 419 followed by a nucleophilic ring opening and intramolecular substitution [115] (Scheme 92). One carbon-containing nucleophiles with a good leaving group should be another reagent for the nucleophilic

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
- Stephanie G. E. Amos,
- Marion Garreau,
- Luca Buzzetti and
- Jerome Waser
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- alkynes. They can also perform atom abstractions, in particular HATs and halogen abstractions [66]. The generation of such species generally occurs through the homolytic cleavage of a peripheral σ bond [67][68]. In the majority of the cases, a leaving group is reduced and fragments to the aryl radical

Graphical Abstract

Figure 1: Selected examples of organic dyes. Mes-Acr+: 9-mesityl-10-methylacridinium, DCA: 9,10-dicyanoanthra...

Scheme 1: Activation modes in photocatalysis.

Scheme 2: Main strategies for the formation of C(sp3) radicals used in organophotocatalysis.

Scheme 3: Illustrative example for the photocatalytic oxidative generation of radicals from carboxylic acids:...

Scheme 4: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from redoxactiv...

Figure 2: Common substrates for the photocatalytic oxidative generation of C(sp3) radicals.

Scheme 5: Illustrative example for the photocatalytic oxidative generation of radicals from dihydropyridines ...

Scheme 6: Illustrative example for the photocatalytic oxidative generation of C(sp3) radicals from trifluorob...

Scheme 7: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from benzylic h...

Scheme 8: Illustrative example for the photocatalytic generation of C(sp3) radicals via direct HAT: the cross...

Scheme 9: Illustrative example for the photocatalytic generation of C(sp3) radicals via indirect HAT: the deu...

Scheme 10: Selected precursors for the generation of aryl radicals using organophotocatalysis.

Scheme 11: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl diazoni...

Scheme 12: Illustrative examples for the photocatalytic reductive generation of aryl radicals from haloarenes:...

Scheme 13: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl halides...

Scheme 14: Illustrative example for the photocatalytic reductive generation of aryl radicals from arylsulfonyl...

Scheme 15: Illustrative example for the reductive photocatalytic generation of aryl radicals from triaryl sulf...

Scheme 16: Main strategies towards acyl radicals used in organophotocatalysis.

Scheme 17: Illustrative example for the decarboxylative photocatalytic generation of acyl radicals from α-keto...

Scheme 18: Illustrative example for the oxidative photocatalytic generation of acyl radicals from acyl silanes...

Scheme 19: Illustrative example for the oxidative photocatalytic generation of carbamoyl radicals from 4-carba...

Scheme 20: Illustrative example of the photocatalytic HAT approach for the generation of acyl radicals from al...

Scheme 21: General reactivity of a) radical cations; b) radical anions; c) the main strategies towards aryl an...

Scheme 22: Illustrative example for the oxidative photocatalytic generation of alkene radical cations from alk...

Scheme 23: Illustrative example for the reductive photocatalytic generation of an alkene radical anion from al...

Figure 3: Structure of C–X radical anions and their neutral derivatives.

Scheme 24: Illustrative example for the photocatalytic reduction of imines and the generation of an α-amino C(...

Scheme 25: Illustrative example for the oxidative photocatalytic generation of aryl radical cations from arene...

Scheme 26: NCR classifications and generation.

Scheme 27: Illustrative example for the photocatalytic reductive generation of iminyl radicals from O-aryl oxi...

Scheme 28: Illustrative example for the photocatalytic oxidative generation of iminyl radicals from α-N-oxy ac...

Scheme 29: Illustrative example for the photocatalytic oxidative generation of iminyl radicals via an N–H bond...

Scheme 30: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from Weinreb am...

Scheme 31: Illustrative example for the photocatalytic reductive generation of amidyl radicals from hydroxylam...

Scheme 32: Illustrative example for the photocatalytic reductive generation of amidyl radicals from N-aminopyr...

Scheme 33: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from α-amido-ox...

Scheme 34: Illustrative example for the photocatalytic oxidative generation of aminium radicals: the N-aryltet...

Scheme 35: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 36: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 37: Illustrative example for the photocatalytic oxidative generation of hydrazonyl radical from hydrazo...

Scheme 38: Generation of O-radicals.

Scheme 39: Illustrative examples for the photocatalytic generation of O-radicals from N-alkoxypyridinium salts...

Scheme 40: Illustrative examples for the photocatalytic generation of O-radicals from alkyl hydroperoxides: th...

Scheme 41: Illustrative example for the oxidative photocatalytic generation of thiyl radicals from thiols: the...

Scheme 42: Main strategies and reagents for the generation of sulfonyl radicals used in organophotocatalysis.

Scheme 43: Illustrative example for the reductive photocatalytic generation of sulfonyl radicals from arylsulf...

Scheme 44: Illustrative example of a Cl atom abstraction strategy for the photocatalytic generation of sulfamo...

Scheme 45: Illustrative example for the oxidative photocatalytic generation of sulfonyl radicals from sulfinic...

Scheme 46: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...

Scheme 47: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...
Copper-catalysed alkylation of heterocyclic acceptors with organometallic reagents
- Yafei Guo and
- Syuzanna R. Harutyunyan
Beilstein J. Org. Chem. 2020, 16, 1006–1021, doi:10.3762/bjoc.16.90
- could also activate the leaving group. After testing several kinds of phosphoramidite ligands with copper salts, the catalyst system L17/CuTc was selected for further studies. The solvent was found to play a crucial role in this reaction, with MTBE as the solvent of choice. Various organoaluminium

Graphical Abstract

Scheme 1: Copper-catalysed ACA of organometallics to piperidones. A) addition of organozinc reagents; B) addi...

Scheme 2: Copper-catalysed ACA of alkenylalanes to N-substituted-2,3-dehydro-4-piperidones.

Scheme 3: Copper-catalysed asymmetric addition of dialkylzinc reagents to N-acyl-4-methoxypyridinium salts fo...

Scheme 4: Copper-catalysed ACA of organozirconium reagents to N-substituted 2,3-dehydro-4-piperidones and lac...

Scheme 5: Copper-catalysed ACA of Grignard reagents to chromones and coumarins and further derivatisation of ...

Scheme 6: Copper-catalysed ACA of Grignard reagents to N-protected quinolones.

Scheme 7: Copper-catalysed ACAs of organometallics to conjugated unsaturated lactams.

Scheme 8: Copper-catalysed ACA of Et2Zn to 5,6-dihydro-2-pyranone.

Scheme 9: Copper-catalysed ACA of Grignard reagents to pyranone and 5,6-dihydro-2-pyranone.

Scheme 10: Copper-catalysed AAA of an organozirconium reagent to heterocyclic acceptors.

Scheme 11: Copper-catalysed ring opening of an oxygen-bridged substrate with trialkylaluminium reagents.

Scheme 12: Copper-catalysed ring opening of oxabicyclic substrates with organolithium reagents (selected examp...

Scheme 13: Copper-catalysed ring opening of polycyclic meso hydrazines.

Scheme 14: Copper-catalysed ACA of Grignard reagents to alkenyl-substituted aromatic N-heterocycles.

Scheme 15: Copper-catalysed ACA of Grignard reagents to β-substituted alkenylpyridines.

Scheme 16: Copper-catalysed ACA of organozinc reagents to alkylidene Meldrum’s acids.
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
- Balaram S. Takale,
- Ruchita R. Thakore,
- Elham Etemadi-Davan and
- Bruce H. Lipshutz
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- )- and (E)-alkenes 311 afforded the (E)-alkene 312 as the major product. The targeted γ-borylated compounds (relative to the leaving group) were formed, each with high enantioselectivity, which can be used for further stereoselective C–C and C–X (X = heteroatom) bond formation. Catalytic Cu(NHC)-mediated

Graphical Abstract

Scheme 1: Pharmaceuticals possessing a silicon or boron atom.

Scheme 2: The first Cu-catalyzed C(sp3)–Si bond formation.

Scheme 3: Conversion of benzylic phosphate 6 to the corresponding silane.

Scheme 4: Conversion of alkyl triflates to alkylsilanes.

Scheme 5: Conversion of secondary alkyl triflates to alkylsilanes.

Scheme 6: Conversion of alkyl iodides to alkylsilanes.

Scheme 7: Trapping of intermediate radical through cascade reaction.

Scheme 8: Radical pathway for conversion of alkyl iodides to alkylsilanes.

Scheme 9: Conversion of alkyl ester of N-hydroxyphthalimide to alkylsilanes.

Scheme 10: Conversion of gem-dibromides to bis-silylalkanes.

Scheme 11: Conversion of imines to α-silylated amines (A) and the reaction pathway (B).

Scheme 12: Conversion of N-tosylimines to α-silylated amines.

Scheme 13: Screening of diamine ligands.

Scheme 14: Conversion of N-tert-butylsulfonylimines to α-silylated amines.

Scheme 15: Conversion of aldimines to nonracemic α-silylated amines.

Scheme 16: Conversion of N-tosylimines to α-silylated amines.

Scheme 17: Reaction pathway [A] and conversion of aldehydes to α-silylated alcohols [B].

Scheme 18: Conversion of aldehydes to benzhydryl silyl ethers.

Scheme 19: Conversion of ketones to 1,2-diols (A) and conversion of imines to 1,2-amino alcohols (B).

Scheme 20: Ligand screening (A) and conversion of aldehydes to α-silylated alcohols (B).

Scheme 21: Conversion of aldehydes to α-silylated alcohols.

Scheme 22: 1,4-Additions to α,β-unsaturated ketones.

Scheme 23: 1,4-Additions to unsaturated ketones to give β-silylated derivatives.

Scheme 24: Additions onto α,β-unsaturated lactones to give β-silylated lactones.

Scheme 25: Conversion of α,β-unsaturated to β-silylated lactams.

Scheme 26: Conversion of N-arylacrylamides to silylated oxindoles.

Scheme 27: Conversion of α,β-unsaturated carbonyl compounds to silylated tert-butylperoxides.

Scheme 28: Catalytic cycle for Cu(I) catalyzed α,β-unsaturated compounds.

Scheme 29: Conversion of p-quinone methides to benzylic silanes.

Scheme 30: Conversion of α,β-unsaturated ketimines to regio- and stereocontrolled allylic silanes.

Scheme 31: Conversion of α,β-unsaturated ketimines to enantioenriched allylic silanes.

Scheme 32: Regioselective conversion of dienedioates to allylic silanes.

Scheme 33: Conversion of alkenyl-substituted azaarenes to β-silylated adducts.

Scheme 34: Conversion of conjugated benzoxazoles to enantioenriched β-silylated adducts.

Scheme 35: Conversion of α,β-unsaturated carbonyl indoles to α-silylated N-alkylated indoles.

Scheme 36: Conversion of β-amidoacrylates to α-aminosilanes.

Scheme 37: Conversion of α,β-unsaturated ketones to enantioenriched β-silylated ketones, nitriles, and nitro d...

Scheme 38: Regio-divergent silacarboxylation of allenes.

Scheme 39: Silylation of diazocarbonyl compounds, (A) asymmetric and (B) racemic.

Scheme 40: Enantioselective hydrosilylation of alkenes.

Scheme 41: Conversion of 3-acylindoles to indolino-silanes.

Scheme 42: Proposed mechanism for the silylation of 3-acylindoles.

Scheme 43: Silyation of N-chlorosulfonamides.

Scheme 44: Conversion of acyl silanes to α-silyl alcohols.

Scheme 45: Conversion of N-tosylaziridines to β-silylated N-tosylamines.

Scheme 46: Conversion of N-tosylaziridines to silylated N-tosylamines.

Scheme 47: Conversion of 3,3-disubstituted cyclopropenes to silylated cyclopropanes.

Scheme 48: Conversion of conjugated enynes to 1,3-bis(silyl)propenes.

Scheme 49: Proposed sequence for the Cu-catalyzed borylation of substituted alkenes.

Scheme 50: Cu-catalyzed synthesis of nonracemic allylic boronates.

Scheme 51: Cu–NHC catalyzed synthesis of α-substituted allylboronates.

Scheme 52: Synthesis of α-chiral (γ-alkoxyallyl)boronates.

Scheme 53: Cu-mediated formation of nonracemic cis- or trans- 2-substituted cyclopropylboronates.

Scheme 54: Cu-catalyzed synthesis of γ,γ-gem-difluoroallylboronates.

Scheme 55: Cu-catalyzed hydrofunctionalization of internal alkenes and vinylarenes.

Scheme 56: Cu-catalyzed Markovnikov and anti-Markovnikov borylation of alkenes.

Scheme 57: Cu-catalyzed borylation/ortho-cyanation/Cope rearrangement.

Scheme 58: Borylfluoromethylation of alkenes.

Scheme 59: Cu-catalyzed synthesis of tertiary nonracemic alcohols.

Scheme 60: Synthesis of densely functionalized and synthetically versatile 1,2- or 4,3-borocyanated 1,3-butadi...

Scheme 61: Cu-catalyzed trifunctionalization of allenes.

Scheme 62: Cu-catalyzed selective arylborylation of arenes.

Scheme 63: Asymmetric borylative coupling between styrenes and imines.

Scheme 64: Regio-divergent aminoboration of unactivated terminal alkenes.

Scheme 65: Cu-catalyzed 1,4-borylation of α,β-unsaturated ketones.

Scheme 66: Cu-catalyzed protodeboronation of α,β-unsaturated ketones.

Scheme 67: Cu-catalyzed β-borylation of α,β-unsaturated imines.

Scheme 68: Cu-catalyzed synthesis of β-trifluoroborato carbonyl compounds.

Scheme 69: Asymmetric 1,4-borylation of α,β-unsaturated carbonyl compounds.

Scheme 70: Cu-catalyzed ACB and ACA reactions of α,β-unsaturated 2-acyl-N-methylimidazoles.

Scheme 71: Cu-catalyzed diborylation of aldehydes.

Scheme 72: Umpolung pathway for chiral, nonracemic tertiary alcohol synthesis (top) and proposed mechanism for...

Scheme 73: Cu-catalyzed synthesis of α-hydroxyboronates.

Scheme 74: Cu-catalyzed borylation of ketones.

Scheme 75: Cu-catalyzed borylation of unactivated alkyl halides.

Scheme 76: Cu-catalyzed borylation of allylic difluorides.

Scheme 77: Cu-catalyzed borylation of cyclic and acyclic alkyl halides.

Scheme 78: Cu-catalyzed borylation of unactivated alkyl chlorides and bromides.

Scheme 79: Cu-catalyzed decarboxylative borylation of carboxylic acids.

Scheme 80: Cu-catalyzed borylation of benzylic, allylic, and propargylic alcohols.
Microwave-assisted efficient and facile synthesis of tetramic acid derivatives via a one-pot post-Ugi cascade reaction
- Yong Li,
- Zheng Huang,
- Jia Xu,
- Yong Ding,
- Dian-Yong Tang,
- Jie Lei,
- Hong-yu Li,
- Zhong-Zhu Chen and
- Zhi-Gang Xu
Beilstein J. Org. Chem. 2020, 16, 663–669, doi:10.3762/bjoc.16.63
- , a convertible isonitrile promoted the cyclization, but restricted the scope of structural diversity. For this reason, it is desirable to replace the amide with a better leaving group, an ester group, which will undergo the Dieckmann reaction more effectively to form a five-membered ring. It is worth

Graphical Abstract

Figure 1: Structures of natural tetramic acid derivatives with more clinical relevance.

Scheme 1: Synthetic strategy of compound 7a.

Scheme 2: Scope of the Ugi/Dieckmann cyclization reaction route to lead to pyrrolopyridinones 7a–l. aYield of...

Scheme 3: Postulated reaction mechanism.
Rhodium-catalyzed reductive carbonylation of aryl iodides to arylaldehydes with syngas
- Zhenghui Liu,
- Peng Wang,
- Zhenzhong Yan,
- Suqing Chen,
- Dongkun Yu,
- Xinhui Zhao and
- Tiancheng Mu
Beilstein J. Org. Chem. 2020, 16, 645–656, doi:10.3762/bjoc.16.61
- most suitable Rh salt for the conversion. In addition, in spite of low yields, Rh species with valence states of 0, +1, +2 or +3 all promoted the reaction at least to some extent. It is worth noting that as a good leaving group, I− tends to leave in an alkaline environment, and dehalogenation and

Graphical Abstract

Figure 1: Rhodium-catalyzed reductive carbonylation of iodobenzene with CO and H2 to afford benzaldehyde. a) ...

Scheme 1: Scaled-up experiment of the reductive carbonylation of iodobenzene to benzaldehyde under the optimi...

Scheme 2: Catalytic species participating in the catalytic process.

Scheme 3: Substrate scope for the Rh-catalyzed reductive carbonylation of aryl iodides using CO and H2. React...

Scheme 4: Isotope-labeling experiments.

Scheme 5: Proposed reaction mechanism for the Rh-catalyzed reductive carbonylation of aryl iodides using CO a...
Synthesis of 4-amino-5-fluoropyrimidines and 5-amino-4-fluoropyrazoles from a β-fluoroenolate salt
- Tobias Lucas,
- Jule-Philipp Dietz and
- Till Opatz
Beilstein J. Org. Chem. 2020, 16, 445–450, doi:10.3762/bjoc.16.41
- a yield of 86%, the pyrazolo derivative 10p could only be obtained in a moderate yield of 38%. This might be explained by the tendency of the pyrazolo moiety to act as a leaving group and a following addition–elimination reaction with methanol can take place (the corresponding 2-methoxy-substituted

Graphical Abstract

Figure 1: The structures of 5-fluorouracil (1), 5-fluorocytosine (2), emtricitabine (3) and capecitabine (4).

Scheme 1: Synthesis of potassium (Z)-2-cyano-2-fluoroethenolate (8) by Dietz et al. [36].

Scheme 2: Scope of the cyclization reaction. All yields are those of the purified products. aNo further purif...

Scheme 3: Cyclization with phenylhydrazine (12a) to obtain the desired pyrazole 13a and the byproducts 13b an...
Copper-catalyzed enantioselective conjugate addition of organometallic reagents to challenging Michael acceptors
- Delphine Pichon,
- Jennifer Morvan,
- Christophe Crévisy and
- Marc Mauduit
Beilstein J. Org. Chem. 2020, 16, 212–232, doi:10.3762/bjoc.16.24
- –86%) and yield (44–63%) remained decent. The efficiency of TolBINAP (L3)/CuI was also demonstrated in the ECA of Grignard reagents to the 4-chloro-α,β-unsaturated thioester 22 [31]. Interestingly, the presence of the internal chloro leaving group allowed a powerful tandem conjugate addition–enolate

Graphical Abstract

Scheme 1: Competitive side reactions in the Cu ECA of organometallic reagents to α,β-unsaturated aldehydes.

Scheme 2: Cu-catalyzed ECA of α,β-unsaturated aldehydes with phosphoramidite- (a) and phosphine-based ligands...

Scheme 3: One-pot Cu-catalyzed ECA/organocatalyzed α-substitution of enals.

Scheme 4: Combination of copper and amino catalysis for enantioselective β-functionalizations of enals.

Scheme 5: Optimized conditions for the Cu ECAs of R2Zn, RMgBr, and AlMe3 with α,β-unsaturated aldehydes.

Scheme 6: CuECA of Grignard reagents to α,β-unsaturated thioesters and their application in the asymmetric to...

Scheme 7: Improved Cu ECA of Grignard reagents to α,β-unsaturated thioesters, and their application in the as...

Scheme 8: Catalytic enantioselective synthesis of vicinal dialkyl arrays via Cu ECA of Grignard reagents to γ...

Scheme 9: 1,6-Cu ECA of MeMgBr to α,β,γ,δ-bisunsaturated thioesters: an iterative approach to deoxypropionate...

Scheme 10: Tandem Cu ECA/intramolecular enolate trapping involving 4-chloro-α,β-unsaturated thioester 22.

Scheme 11: Cu ECA of Grignard reagents to 3-boronyl α,β-unsaturated thioesters.

Scheme 12: Cu ECA of alkylzirconium reagents to α,β-unsaturated thioesters.

Scheme 13: Conversion of acylimidazoles into aldehydes, ketones, acids, esters, amides, and amines.

Scheme 14: Cu ECA of dimethyl malonate to α,β-unsaturated acylimidazole 31 with triazacyclophane-based ligand ...

Scheme 15: Cu/L13-catalyzed ECA of alkylboranes to α,β-unsaturated acylimidazoles.

Scheme 16: Cu/hydroxyalkyl-NHC-catalyzed ECA of dimethylzinc to α,β-unsaturated acylimidazoles.

Scheme 17: Stereocontrolled synthesis of 3,5,7-all-syn and anti,anti-stereotriads via iterative Cu ECAs.

Scheme 18: Stereocontrolled synthesis of anti,syn- and anti,anti-3,5,7-(Me,OR,Me) units via iterative Cu ECA/B...

Scheme 19: Cu-catalyzed ECA of dialkylzinc reagents to α,β-unsaturated N-acyloxazolidinones.

Scheme 20: Cu/phosphoramidite L16-catalyzed ECA of dialkylzincs to α,β-unsaturated N-acyl-2-pyrrolidinones.

Scheme 21: Cu/(R,S)-Josiphos (L9)-catalyzed ECA of Grignard reagents to α,β-unsaturated amides.

Scheme 22: Cu/Josiphos (L9)-catalyzed ECA of Grignard reagents to polyunsaturated amides.

Scheme 23: Cu-catalyzed ECA of trimethylaluminium to N-acylpyrrole derivatives.
Understanding the role of active site residues in CotB2 catalysis using a cluster model
- Keren Raz,
- Ronja Driller,
- Thomas Brück,
- Bernhard Loll and
- Dan T. Major
Beilstein J. Org. Chem. 2020, 16, 50–59, doi:10.3762/bjoc.16.7

- , Hong and Tantillo [38] and Sato and co-workers [39] investigated the CotB2 mechanism using QM tools. According to Meguro and co-workers [41], the cyclization process commences with the dissociation of the pyrophosphate leaving group of GGPP, forming an allylic carbocation, and two subsequent

Graphical Abstract

Scheme 1: Mechanism for formation of cyclooctat-9-en-7-ol, published similarly in [42].

Figure 1: Computed electronic energy profiles (kcal/mol) for the CotB2 cyclase mechanism. The calculations us...

Figure 2: Intermediates A–I in the active site model. Interactions are marked by dashed orange lines, the int...

Figure 3: TS structures TS_A_B–TS_G/H_I in the active site model. Interactions are marked by dashed orange li...

Figure 4: Comparison between gas phase and active site model conformations. A) Intermediate D. B) Intermediat...
Bacterial terpene biosynthesis: challenges and opportunities for pathway engineering
- Eric J. N. Helfrich,
- Geng-Min Lin,
- Christopher A. Voigt and
- Jon Clardy
Beilstein J. Org. Chem. 2019, 15, 2889–2906, doi:10.3762/bjoc.15.283

- ) Terpene biosynthesis. LG: leaving group, black ball: acyl carrier protein. Abundance and distribution of bacterial terpene biosynthetic gene clusters as determined by genome mining (black) compared to experimentally characterized bacterial terpene BGCs (red). Terpenoid biosynthesis. Terpenoid biosynthesis

Graphical Abstract

Figure 1: Examples of bioactive terpenoids.

Figure 2: Repetitive electrophilic and nucleophilic functionalities in terpene and type II PKS-derived polyke...

Figure 3: Abundance and distribution of bacterial terpene biosynthetic gene clusters as determined by genome ...

Figure 4: Terpenoid biosynthesis. Terpenoid biosynthesis is divided into two phases, 1) terpene scaffold gene...

Figure 5: Mechanisms for type I, type II, and type II/type I tandem terpene cyclases. a) Tail-to-head class I...

Figure 6: Functional TC characterization. a) Different terpenes were produced when hedycaryol (18) synthase a...

Figure 7: Selected examples of terpene modification by bacterial CYPs. a) Hydroxylation [89]. b) Carboxylation, h...

Figure 8: Off-target effects observed during heterologous expression of terpenoid BGCs. Unexpected oxidation ...

Figure 9: TC promiscuity and engineering. a) Spata-13,17-diene (39) synthase (SpS) can take C15 and C25 oligo...

Figure 10: Substrate promiscuity and engineering of CYPs. a) Selected examples from using a CYP library to oxi...

Figure 11: Engineering of terpenoid pathways. a) Metabolic network of terpenoid biosynthesis. Toxic intermedia...
Iodine-mediated hydration of alkynes on keto-functionalized scaffolds: mechanistic insight and the regiospecific hydration of internal alkynes
- Zachary Lee,
- Brandon R. Jones,
- Nyochembeng Nkengbeza,
- Michael Phillips,
- Kayla Valentine,
- Alexis Stewart,
- Brandon Sellers,
- Nicholas Shuber and
- Karelle S. Aiken
Beilstein J. Org. Chem. 2019, 15, 2747–2752, doi:10.3762/bjoc.15.265
- promise for further development of the reaction. The iodo group is an excellent leaving group and therefore, the generation of α-substituted hydration products through the addition of suitable nucleophiles to the reaction mixture is a strong possibility. Having proven the existence of the α-iodo

Graphical Abstract

Scheme 1: Proposed mechanism for the iodine-mediated hydration of terminal alkynes 1 [15].

Figure 1: 1H NMR investigations on the iodine-mediated hydration of 8 (the range of 1.75–5.25 ppm is displaye...

Figure 2: 1H—13C HSQC spectrum for α-iodo intermediate 9 in CD3CN in the range of 0.90–5.00 ppm (for 1H NMR s...

Scheme 2: Possible outcomes of the iodine-mediated hydration of asymmetric, internal alkynes with neighboring...

Scheme 3: Iodine-mediated hydration of asymmetric, internal alkynes 11a–e.
Synthesis of aryl-substituted thieno[3,2-b]thiophene derivatives and their use for N,S-heterotetracene construction
- Nadezhda S. Demina,
- Nikita A. Kazin,
- Nikolay A. Rasputin,
- Roman A. Irgashev and
- Gennady L. Rusinov
Beilstein J. Org. Chem. 2019, 15, 2678–2683, doi:10.3762/bjoc.15.261
- should be noted that the initial 3-aminoesters 1 are known compounds and can be obtained by the Fiesselmann thiophene synthesis from aryl-substituted acrylonitriles bearing a good leaving group at the C-3 position [22][23][24]. Methyl 3-chlorothiophene-2-carboxylates 2a–k were further involved in the

Graphical Abstract

Figure 1: An example of an earlier developed S,N-heterohexacene [13] and general structure of compounds synthesiz...

Scheme 1: Synthesis of aryl-substituted TT derivatives 3a–k, product scope, and yields.

Scheme 2: Synthesis of thieno[3,2-b]thiophen-3(2H)-one 4a–k, product scope, and yields.

Scheme 3: Synthesis of TTI derivatives 6a–o, substrate and product scopes, and yields.

Scheme 4: Alkylation of TTI 6d.

Figure 2: ORTEP diagram for the X-ray structure of compound 7d. Thermal ellipsoids of 50% probability are sho...
1,5-Phosphonium betaines from N-triflylpropiolamides, triphenylphosphane, and active methylene compounds
- Vito A. Fiore,
- Chiara Freisler and
- Gerhard Maas
Beilstein J. Org. Chem. 2019, 15, 2603–2611, doi:10.3762/bjoc.15.253
- broad O–H absorption bands in their IR spectra (≈3400–3500 cm−1) and the presence of hydrogen-bonded water molecules in the crystal structure of E-3e (vide infra) indicate. The formation of betaines 3 includes the elimination of the N-phenyltriflamide anion, which is known as an excellent leaving group
- phosphonium betaines, which can formally be named 1,5-phosphonium-carbabetaines, but because of the extended delocalization of the negative charge are better described as 1-phosphonium-5-oxabetaines. The excellent leaving group character of the N-phenyltriflylamide anion ([N(Ph)SO2CF3]−) is a key factor in

Graphical Abstract

Scheme 1: Stable betaines I–IV.

Scheme 2: Reactions of N-triflyl-propiolamides 1 with N- and P-nucleophiles. Tf = SO2CF3.

Scheme 3: Synthesis of betaines 3 by a three-component reaction. N-triflylpropiolamides 1: R = Ph (a), 4-Cl-C6...

Figure 1: Phosphonium betaines 3 prepared. An E:Z ratio of 100:0 means that only the E-isomer was observed in...

Scheme 4: Unexpected synthesis of E-3o.

Scheme 5: Betaines 3 from propiolic acid chlorides.

Scheme 6: Two mechanistic scenarios for the formation of betaines 3.

Figure 2: Solid-state structure of E-3a (ORTEP plot), two symmetry-independent molecules in the triclinic uni...

Figure 3: Solid-state structure of E-3b·CH2Cl2 (ORTEP plot); CH2Cl2 solvate molecule not shown.

Figure 4: Solid-state structure of E-3e·H2O·CH2Cl2 (ORTEP plot). The CH2Cl2 solvate molecule is disordered. H...

Figure 5: Solid-state structure of Z-3e (ORTEP plot).

Scheme 7: Resonance structures describing the bonding in 1,2-oxaphospholes/1,5-betaines 8.
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
- Silvie Rimpelová,
- Michal Jurášek,
- Lucie Peterková,
- Jiří Bejček,
- Vojtěch Spiwok,
- Miloš Majdl,
- Michal Jirásko,
- Miloš Buděšínský,
- Juraj Harmatha,
- Eva Kmoníčková,
- Pavel Drašar and
- Tomáš Ruml
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
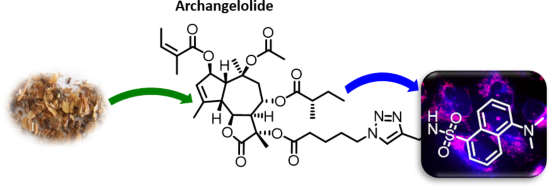
- hydroxy group more acidic and thus a better leaving group in the reaction. Next, the quaternary 11α-hydroxy group was acylated by freshly prepared 5-azidopentanoic anhydride in the presence of 4-DMAP at room temperature. Finally, the azidopentanoate 4 was successively introduced into a click reaction with

Graphical Abstract

Figure 1: The structure of the sesquiterpene lactones archangelolide (1) and trilobolide (2).

Scheme 1: Reagents and conditions: a) MeOH, TEA, 48 h, yield 32%; b) (i) 5-azidopentanoic acid, DCC, DCM, 90 ...

Figure 2: Intracellular localization of archangelolide-dansyl (5) in human cells from osteosarcoma (U-2 OS). ...

Figure 3: Co-localization of dansylarchangelolide 5 with a marker of endoplasmic reticulum (top row) and with...

Figure 4: Cartoon representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A, C) archang...

Figure 5: Molecular surface representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A) ...

Figure 6: Structural formulae of (i) thapsigargin, (ii) trilobolide (2), and (iii) archangelolide (1). Red pa...

Figure 7: Viability of rat peritoneal cells treated with archangelolide (1), dansylarchangelolide 5 and dansy...

Figure 8: NO production in primary rat macrophages. The cells were treated with archangelolide (1) and dansyl...

Figure 9: Evaluation of cytokine TNF-α secretion in rat peritoneal cells. Stimulation of primary cells was in...

Figure 10: Structure of laserolide.
The cyclopropylcarbinyl route to γ-silyl carbocations
- Xavier Creary
Beilstein J. Org. Chem. 2019, 15, 1769–1780, doi:10.3762/bjoc.15.170
- ). These cations 11 capture solvent molecules to give exclusively products 12 with net retention of configuration, a characteristic of carbocations that are stabilized by this type of rear lobe interaction. A series of cyclopropylcarbinyl substrates 13 and 14 (Figure 2), where X is a leaving group and R is

Graphical Abstract

Scheme 1: Solvolyses of cyclopropylcarbinyl and cyclobutyl substrates.

Scheme 2: The cyclopropylcarbinyl–cyclobutyl–homoallyl cation manifold.

Figure 1: Electron-deficient carbocations.

Scheme 3: Solvolyses of γ-trimethylsilylcyclobutyl substrates.

Figure 2: Substrates of interest.

Scheme 4: Synthesis of mesylates 19 and 20.

Scheme 5: Reaction of mesylate 19 in CD3CO2D.

Scheme 6: Reaction of mesylate 20 in CD3CO2D.

Figure 3: M062X/6-311+G** calculated structures and relative energies of cations 24, 27, and transition state ...

Scheme 7: Synthesis of mesylates 31 and 32.

Scheme 8: Reaction of mesylate 31 in CD3CO2D.

Scheme 9: Reaction of mesylate 32 in CD3CO2D.

Scheme 10: Reaction of trifluoroacetate 48 in CD3CO2D.

Scheme 11: Bicyclobutane formation from a γ-trimethylsilyl cation.

Scheme 12: Formation of triflates 60 and 61.

Scheme 13: Formation of triflates 67, 68, and 69.

Scheme 14: Reactions of substrates with electron-withdrawing groups in CD3CO2D.

Figure 4: γ-Trimethylsilyl cations.

Scheme 15: Bicyclobutane formation from mesylate 76 in CH3CO2H.

Scheme 16: Reactions of triflates 60 and 67 in CD3CO2D.
An improved synthesis of adefovir and related analogues
- David J. Jones,
- Eileen M. O’Leary and
- Timothy P. O’Sullivan
Beilstein J. Org. Chem. 2019, 15, 801–810, doi:10.3762/bjoc.15.77
- synthesis of adefovir dipivoxil (2) exploiting phosphonate 33. Impact of the leaving group on in situ conversion of 4 to 6. Impact of base, solvent and temperature on reaction performance and regioselectivity. Supporting Information Supporting Information File 506: Experimental part and NMR spectra.

Graphical Abstract

Figure 1: Adefovir (1) and its prodrug 2.

Scheme 1: Literature syntheses of 1.

Figure 2: Retrosynthetic analysis of 6 to synthons 9 and 10.

Scheme 2: Forward synthesis of 6 from 9 and 10.

Figure 3: Retrosynthesis of 6 to synthons 14 and 3.

Scheme 3: Application of related alkyl iodide 15 [52].

Scheme 4: Synthesis of 6 and 20 via iodide 14.

Scheme 5: Synthesis of phosphonate 6 using novel salt 21.

Scheme 6: Application of iodide 14 in the synthesis of adefovir analogues.

Figure 4: HMBC spectrum confirms N7-selectivity for the major product 29.

Scheme 7: Attempted synthesis of adefovir dipivoxil (2) exploiting phosphonate 33.
Diastereo- and enantioselective preparation of cyclopropanol derivatives
- Marwan Simaan and
- Ilan Marek
Beilstein J. Org. Chem. 2019, 15, 752–760, doi:10.3762/bjoc.15.71
- general structure M–O–LG, with a metal and a leaving group connected to an oxygen atom, have been shown to be an excellent electrophilic oxygen source for nucleophilic organometallic species [72]. Since the original discovery of Müller and Töpel of lithiated peroxides [73], several studies have been

Graphical Abstract

Scheme 1: Various strategies leading to the formation of cyclopropanols.

Scheme 2: General approach to the preparation of cyclopropanol and cyclopropylamine derivatives.

Figure 1: Prerequisite for a regio- and diastereoselective carbometalation.

Scheme 3: Preparation of cyclopropenyl methyl ethers 3a–d.

Scheme 4: Regio- and diastereoselective carbocupration of cyclopropenyl methyl ethers 3a,c.

Scheme 5: Diastereoselective formation of cyclopropanols.

Scheme 6: Diastereoselective carbometalation/oxidation of nonfunctionalized cyclopropenes 6.

Scheme 7: Preparation of diastereoisomerically pure and enantioenriched cyclopropanols and cyclopropylamines.
Tuning the stability of alkoxyisopropyl protection groups
- Zehong Liang,
- Henna Koivikko,
- Mikko Oivanen and
- Petri Heinonen
Beilstein J. Org. Chem. 2019, 15, 746–751, doi:10.3762/bjoc.15.70
- directly reflected by the observed hydrolysis rate, whereas the changes in polarity of the leaving alcohol do not give marked changes on the rate. The latter is due to the fact that the kinetic effects on the protonation step and the leaving group ability largely cancel each other. In our study, the

Graphical Abstract

Figure 1: Reagents for acetal protections.

Scheme 1: Synthesis of 2-alkoxyprop-2-yl-protected thymidines. Reagents and conditions (i) 7 equiv 1a–e, 0.5 ...

Figure 2: Enol ether 8a: R1 = TBS and 8b: R1 = H.

Scheme 2: Proposed acetal hydrolysis pathways.
An efficient and concise access to 2-amino-4H-benzothiopyran-4-one derivatives
- Peng Li,
- Yongqi Wu,
- Tingting Zhang,
- Chen Ma,
- Ziyun Lin,
- Gang Li and
- Haihong Huang
Beilstein J. Org. Chem. 2019, 15, 703–709, doi:10.3762/bjoc.15.65
- be the optimum leaving group by thorough investigations on the elimination of sulfide, sulfinyl, and sulfonyl groups at the 2-position of benzothiopyranone. Most 2-aminobenzothiopyranones were obtained in good to excellent yields under refluxing in isopropanol within 36 h. This method is base-free
- -imidazolylbenzothiopyranones [10]. It is well known that the sulfide, sulfinyl and sulfonyl groups are generally used as the leaving group for the synthesis of 2-substituted 4H-chromen-4-ones [11][12][13][14][15][16][17][18][19][20][21][22]. Due to the higher electronegativity of the oxygen atom compared to the sulfur atom
- provided a much higher product yield (Table 2, entries 1, 3 and 5 vs entries 2, 4 and 6, respectively) and sometimes required a shorter reaction time (Table 2, entry 3 vs entry 4) than the corresponding substrate 3a. To further verify the advantage of the sulfinyl group as the optimal leaving group, the

Graphical Abstract

Scheme 1: Representative strategies for the synthesis of N-substituted 2-aminobenzothiopyranones.

Scheme 2: The synthesis of sulfide 1, sulfoxide 2, and sulfone 3.

Scheme 3: Scope of the synthesis of versatile 2-aminobenzothiopyranones. All reactions were performed with 1....

Scheme 4: The gram-scale synthesis of 2-aminobenzothiopyranones 4a and 4d.
Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives
- Mikhail V. Makarov and
- Marie E. Migaud
Beilstein J. Org. Chem. 2019, 15, 401–430, doi:10.3762/bjoc.15.36

- riboside. The stereochemical outcomes of the synthesis (anomeric ratio) depends on the nature and the stereochemistry of the leaving group X in sugar B (α- or β-anomer), on the nature of the substituents at the amide nitrogen atom in Nam, and on the conditions of glycosylation, such as solvent and

Graphical Abstract

Figure 1: Structural formulas of Nam, NA, NR+, NMN, and NAD+.

Figure 2: Main synthetic routes to nicotinamide riboside (NR+X−).

Scheme 1: Synthesis of NR+Cl− based on the reaction of peracylated chlorosugars with Nam.

Figure 3: Predominant formation of β-anomer over α-anomer of NR+X−.

Scheme 2: Synthesis of NR+Cl− by reacting 3,5-di-O-benzoyl-D-ribofuranosyl chloride (5) with Nam (1a).

Figure 4: Mechanism of the formation of the β-anomer of the glycosylated product in the case of the reaction ...

Scheme 3: Synthesis of NR+Br− by reacting bromosugars with Nam (1a).

Scheme 4: Synthesis of NR+OTf− based on the glycosylation of Nam (1a) with tetra-O-acetyl-β-D-ribofuranose (2a...

Scheme 5: Improved synthesis of NR+OTfˉ and NAR+OTfˉ based on the glycosylation of pre-silylated Nam or NA wi...

Scheme 6: Synthesis of triacetylated NAR+OTf− by glycosylation of nicotinic acid trimethylsilyl ester with te...

Scheme 7: Synthesis of NR+Cl− from NR+OTf− by means of ion exchange with sodium chloride solution.

Scheme 8: Synthesis of acylated NR+OTf− by means of ion exchange with sodium chloride.

Scheme 9: Synthesis of triacetylated derivatives of NAR+ by glycosylation of nicotinic acid esters with ribos...

Scheme 10: Synthesis of NR+OTf− from the triflate salt of ethyl nicotinate-2,3,5-triacetyl-β-D-riboside in met...

Scheme 11: Reaction of 2,3,5-tri-O-acetyl-β-phenyl nicotinate riboside triflate salt with secondary and tertia...

Scheme 12: Synthesis of NMN based on the Zincke reaction of N-(2,4-dinitrophenyl)-3-carbamoylpyridinium chlori...

Scheme 13: Synthesis of NMN based on the Zincke reaction of N-(2,4-dinitrophenyl)-3-carbamoylpyridinium chlori...

Scheme 14: Efficacious protection of 2′,3′-hydroxy groups of NR+X−.

Scheme 15: Protection of the 2′,3′-hydroxy groups of NR+Cl– with a mesitylmethylene acetal group.

Figure 5: Reduction of derivatives of NR+Xˉ into corresponding 1,2-; 1,4-; 1,6-NRH derivatives.

Figure 6: Mechanism of the reduction of the pyridinium core with dithionite as adapted from [67].

Scheme 16: Reduction of triacylated NR+OTf– derivatives by sodium dithionite followed by complete removal of a...

Figure 7: Structural formulas of iridium and rhodium catalysts (a)–(d) for regeneration of NAD(P)H from NAD(P)...

Figure 8: Two approaches to synthesis of 5′-derivatives of NR+.

Scheme 17: Synthesis of NMN starting from NR+ salt.

Scheme 18: Efficient synthesis of NMN by phosphorylation of 2′,3′-O-isopropylidene-NR+ triflate followed by re...

Scheme 19: Synthesis of a bisphosphonate analogue of β-NAD+ based on DCC-induced conjugation of 2′,3′-O-isopro...

Scheme 20: Synthesis of 5′-acyl and 2′,3′,5′-triacyl derivatives of NR+.

Figure 9: Structural formulas of NMN analogues 39–41.

Scheme 21: Synthesis of 5′-phosphorylated derivatives of NR+ using a “reduction–modification–oxidation” approa...

Scheme 22: Synthesis of 5′-phosphorylated derivatives of NR+ using a “reduction–modification–reoxidation” appr...

Figure 10: Structural formulas of 5′-phosphorylated derivatives of NR+.

Scheme 23: Synthesis of 5′-phosphorylated derivatives of NR+ using a direct NR+ phosphorylation approach.

Figure 11: Structural formulas of amino acid NR+ conjugates.

Scheme 24: Synthesis of amino acid NR+ conjugates using NRH and protected amino acid under CDI-coupling condit...

Figure 12: Chemical structures of known isotopically labelled NR+ analogues and derivatives.

Scheme 25: Synthesis of [2′-3H]-NR+ and [2′-3H]-NMN.

Scheme 26: Synthesis of α- and β-anomers of [1′-2H]-NMN.
Silanediol versus chlorosilanol: hydrolyses and hydrogen-bonding catalyses with fenchole-based silanes
- Falco Fox,
- Jörg M. Neudörfl and
- Bernd Goldfuss
Beilstein J. Org. Chem. 2019, 15, 167–186, doi:10.3762/bjoc.15.17

- Nucleophilic substitution at silicon is already discussed with SN2 mechanism, following a backside attack opposite of the leaving group, as well as a front side attack near the leaving group [62][63][64][65][66][67][68]. A backside attack at the silicon in dichlorosilane 7 and monochlorosilanol 8 is blocked by

Graphical Abstract

Figure 1: Hydrogen-bonding silanediols, i.e., di(1-naphthyl)silanediol (1) [39], silanediols 2 [41-43], binaphthylsilane...

Scheme 1: Hydrogen-bond-catalyzed N-acyl Mannich reaction of in situ-generated isoquinolin derivative 10 with...

Scheme 2: Synthesis of BIFOXSiCl2, starting with BIFOL (5) [52,54] yielding dichlorosilane 7.

Scheme 3: Hydrolysis of BIFOXSiCl2 (7) yielding the corresponding silanediol 9 and controlled hydrolysis of B...

Scheme 4: Hydrolysis of dichlorosilanes 13 and 14 to their corresponding silanediols 1 and 15 [51,60].

Figure 2: Hydrolyses of dichlorosilane 7 and 14 to BIFOXSi(OH)2 (9, green circle) and bis(2,4,6-tri-tert-buty...

Figure 3: Hydrolyses of BIFOXSiCl2 (7) to BIFOXSi(OH)2 (9, green circle), bis(2,4,6-tri-tert-butylphenoxy)dic...

Scheme 5: Two investigated pathways for the hydrolysis of the dichlorosilanes. Front attack mechanism (front)...

Figure 4: Three transition structures each, for the hydrolysis of BIFOXSiCl2 (7) and BIFOXSiCl(OH) (8) consid...

Figure 5: Computed hydrolyses of BIFOXSiCl2 (7) to BIFOXSiCl(OH) 8ax and BIFOXSiCl(OH) 8eq and subsequent com...

Figure 6: Transition state leading to 8eq following front1 attack (Ea = 32.6 kcal mol−1, Figure 5, Table 3, entry 1). Breaki...

Figure 7: Transition state leading to 8ax following front2 attack (Ea = 33.2 kcal mol−1, Figure 5, Table 3, entry 2). Breaki...

Figure 8: Transition state leading to 8eq following side attack (Ea = 37.4 kcal mol−1, Figure 5, Table 3, entry 3). Breaking...

Figure 9: Transition state leading to 9 following side attack (Ea = 31.4 kcal mol−1, Figure 5, Table 3, entry 6). Breaking a...

Figure 10: Transition state leading to 9 following front1 attack (Ea = 33.4 kcal mol−1, Figure 5, Table 3, entry 4). Breaking...

Figure 11: Transition state leading to 9 following front2 attack (Ea = 40.2 kcal mol−1, Figure 5, Table 3, entry 5). Breaking...

Figure 12: X-ray crystal structure of BIFOXSiCl2 (7). H atoms on the chiral backbone are omitted for clarity i...

Figure 13: X-ray crystal structure of BIFOXSiCl(OH) (8). H atoms on the chiral backbone are omitted for clarit...

Figure 14: X-ray crystal structure ofrac-BIFOXSi(OH)2 (9) forming dimers. H atoms on the chiral backbone are o...

Figure 15: X-ray crystal structure of BIFOXSi(OH)2 (9) forming a tetramer. H atoms on the chiral backbone are ...

Figure 16: X-ray crystal structure of BIFOXSi(OH)2 (9) forming a dimeric structure with two bridged acetone mo...

Figure 17: X-ray crystal structure of BIFOXSiCl(OH) (8), binding an acetone molecule. H atoms on the chiral ba...

Scheme 6: Hydrogen-bond-catalyzed N-acyl Mannich reaction of in situ-generated 10 with different silyl ketene...

Scheme 7: Hydrogen-bond-catalyzed nucleophilic substitution of 18 with BIFOXSi(OH)2 (9) and nucleophile silyl...

Scheme 8: Nucleophilic substitution of 20 with BIFOXSi(OH)2 (9) and nucleophile silyl ketene acetals 11, 20 a...
Synthesis of a tubugi-1-toxin conjugate by a modulizable disulfide linker system with a neuropeptide Y analogue showing selectivity for hY1R-overexpressing tumor cells
- Rainer Kufka,
- Robert Rennert,
- Goran N. Kaluđerović,
- Lutz Weber,
- Wolfgang Richter and
- Ludger A. Wessjohann
Beilstein J. Org. Chem. 2019, 15, 96–105, doi:10.3762/bjoc.15.11

- (Scheme 1) shows that, in addition to tubugi-1 itself, only the readily accessible building block 4 is required to construct the activated compound tubugi-1-SSPy (3) as a universal precursor for peptide–toxin conjugate syntheses [57]. The pyridyl disulfide is a leaving group which can be substituted by

Graphical Abstract

Figure 1: Tubulysin A (1) and tubugi-1 (2).

Scheme 1: Retrosynthetic analysis of the modular attachment linker tubugi-1-SSPy (3).

Scheme 2: Synthesis of tubugi-1-SSPy (3): a) LiOH·H2O, THF/H2O, 0 °C → rt; b) Ac2O, py; c) 4, HBTU, DMF, DIPE...

Scheme 3: Synthesis of the tubugi-1–NPY conjugate [K4(C(tubugi-1)-βA-),F7,L17,P34]-hNPY (8).

Scheme 4: Toxin liberation by disulfide linker cleavage from the activated toxin conjugate under reductive co...

Figure 2: Reduction of viability and proliferation of SK-N-MC, MDA-MB-468, MDA-MB-231 cancer cell lines, and ...






