Search results
Search for "macrocyclic" in Full Text gives 246 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Synthetic strategies of phosphonodepsipeptides
Beilstein J. Org. Chem. 2021, 17, 461–484, doi:10.3762/bjoc.17.41
- nucleophilic esterification of potassium 1-(N-benzyloxycarbonylamino)alkylphosphonates 172 with alkyl halides in the presence of 18-crown-6. They also prepared a phosphonodepsidipeptide 174 with ethyl chloroacetate (173) as an electrophile (Scheme 33) [53]. The macrocyclic peptidyl phosphonodepsipeptide 180
- was designed on the basis of the acyclic conformational analog bound to the aspartic protease penicillopepsin. Dimethyl N-Cbz-1-amino-2-(naphthalen-2-yl)ethylphosphonate 176 was first prepared from 7-bromo-3,4-dihydronaphthalen-1(2H)-one (175) and further transformed to the macrocyclic peptidyl
- phosphonic monomethyl ester 177. The latter compound was alkylated with methyl 3-phenyl-2-trifluoromethanesulfonyloxypropanoate (178) to produce the macrocyclic peptidyl phosphonodepsipeptide 179, which was selectively hydrolyzed with TMSBr and treated with Dowex-Na+ to afford the macrocyclic peptidyl
Au(III) complexes with tetradentate-cyclam-based ligands
Beilstein J. Org. Chem. 2021, 17, 186–192, doi:10.3762/bjoc.17.18
- binding properties [25] and reactions with bovine serum albumin [27]. Cyclam is known as a tetraamino-macrocyclic ligand, which binds strongly to give complexes with many transition metal cations. While catalytic applications of square planar cyclam complexes are reported for metals, such as Ni [30][31
- macrocyclic byproduct (14%) was formed by condensation of three units of diamine A and malonyl dichloride. To inhibit the formation of the trimer, we decided to prepare the cyclams in an indirect way. In fact, increased yields of cyclam derivative 2a (68% yield over three steps) were obtained by malonyl
Insight into functionalized-macrocycles-guided supramolecular photocatalysis
Beilstein J. Org. Chem. 2021, 17, 139–155, doi:10.3762/bjoc.17.15

- with various macrocyclic hosts as well as on the role of macrocyclic-hosts-assisted hybrid materials in energy transfer. To keep the clarity of this review, the macrocycles are categorized into the most commonly used supramolecular hosts, including crown ethers, cyclodextrins, cucurbiturils
- advantages of the macrocycles in photocatalysis including: i) substrate selectivity, ii) controllability of the rate of photochemical reactions, iii) the close proximity to substrates in a confined space, iv) the generation of a stabilized high-energy transition state, and v) macrocyclic-cavity-size
- via photophysical analyses is relatively important. Hence, this review aims to summarize the photochemical transformation and catalytic reactivity of different guests within the various macrocyclic hosts of various sizes and shapes based on molecular recognition as well as the role of macrocyclic-host
The fluorescence of a mercury probe based on osthol
Beilstein J. Org. Chem. 2021, 17, 22–27, doi:10.3762/bjoc.17.3
- Guangyan Luo Zhishu Zeng Lin Zhang Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.17.3 Abstract The ability of osthol (OST) to recognize mercury ions in aqueous solution was studied using
Selected peptide-based fluorescent probes for biological applications
Beilstein J. Org. Chem. 2020, 16, 2971–2982, doi:10.3762/bjoc.16.247

- insulin Schmuck and co-workers reported a supramolecular ensemble in combination of a pyrene-tagged amphiphilic peptide beacon (6) and a macrocyclic host (cucurbit[8]uril, CB[8]) for ratiometric fluorescent detection of amino acid derivatives, specific peptides, and proteins in aqueous media (Figure 6
Construction of pillar[4]arene[1]quinone–1,10-dibromodecane pseudorotaxanes in solution and in the solid state
Beilstein J. Org. Chem. 2020, 16, 2954–2959, doi:10.3762/bjoc.16.245

- architectures and chemical topology [12][13][14][15][16][17][18][19][20][21][22][23]. Seeking new systems to produce pseudorotaxanes is currently considered a “hot topic” in supramolecular chemistry. As a new class of supramolecular macrocyclic hosts, pillararenes have received extensive attention in recent
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- made towards switchable macrocyclic receptors, in which crown ethers are functionalized with a stimuli-responsive unit [14][15]. These studies were mainly motivated by a biomimetic approach and included examples such as crown ethers incorporating photo-responsive azobenzene [15][16] or redox-active
Host–guest interaction of cucurbit[8]uril with oroxin A and its effect on the properties of oroxin A
Beilstein J. Org. Chem. 2020, 16, 2332–2337, doi:10.3762/bjoc.16.194

- Zhishu Zeng Jun Xie Guangyan Luo Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, No. 2708, South Section of Huaxi Avenue, Huaxi, Guiyang 550025, China 10.3762/bjoc.16.194 Abstract In this study, we investigated the host
- the inclusion compound with Q[8]. Keywords: cucurbit[8]uril; host–guest interaction; inclusion complex; oroxin A; properties; Introduction Cucurbit[n]urils (Q[n]s) are a family of macrocyclic cage compounds synthesized by the condensation of glycoluril and formaldehyde in a strong acidic solution [1
Design and synthesis of a bis-macrocyclic host and guests as building blocks for small molecular knots
Beilstein J. Org. Chem. 2020, 16, 2314–2321, doi:10.3762/bjoc.16.192
- molecular knots. The modular second-generation approach to small trefoil knots described herein involves electrostatic interactions between an electron-rich bis-macrocyclic host compound and electron-deficient guests in the threading step. The bis-macrocyclic host was synthesized in eight steps and 6.6
- ]. Herein the synthesis of a unique bis-macrocyclic host 1 is described (Figure 1). Host 1 was designed to be a second-generation building block in the thread–link–cut (TLC) approach to molecular knots [13]. The two 25-atom macrocycles are electron-rich and complementary to the electron-deficient guests bis
- ), whereas host 1 and guest 3 would lead to a 75 backbone-atom trefoil (and unknotted macrocycle). Results and Discussion The synthesis of bis-macrocyclic host 1 began by breaking the symmetry of naphthalene-1,5-diol (4) by alkylation of one of the alcohols with 2-azidoethyl mesylate to yield azide 5 in 27
Design, synthesis and application of carbazole macrocycles in anion sensors
Beilstein J. Org. Chem. 2020, 16, 1901–1914, doi:10.3762/bjoc.16.157

- that would allow the analyte to become protonated. In order to incorporate both functional parts of a carboxylate, a macrocyclic receptor architecture is desirable. By using a cyclic structure, it is possible to accommodate a solvophobic environment alongside polar functional groups. The benefits of
- positions 8 of the carbazole, using, e.g., amide bonds. Anion receptors containing carbazole and amide functionalities were investigated in numerous works [6][7][8][9]. In some cases, these functionalities were incorporated into macrocyclic systems, thereby offering valuable insight for design criteria. For
- example, a carbazole-urea macrocycle was reported previously [10], however, the binding of anions occurred outside the receptor due to modest dimensions of the macrocyclic cavity. Using click-chemistry, a carbazole-triazole macrocycle, “tricarb”, was prepared that showed the ability to form non-covalent
Five-component, one-pot synthesis of an electroactive rotaxane comprising a bisferrocene macrocycle
Beilstein J. Org. Chem. 2020, 16, 1564–1571, doi:10.3762/bjoc.16.128
- synthesis of macrocycle 2 was carried out using N,N'-dihexyl-1,4-butanediamide as the template, as represented in Figure 2. While the formation of some macrocyclic product was identified by 1H NMR and MS, it could not be completely separated from impurities (<29% yield after chromatographic purification
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- presence of PhSSPh, the new isomer 73 could also be converted to the desired (E,E)-isomer 71. In the total synthesis of the macrocyclic antibiotic antitumor agent (+)-hitachimycin, Smith used a disulfide-catalyzed isomerization [32] to synthesize the intermediate 75 with an E-configuration, which was
[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
Beilstein J. Org. Chem. 2020, 16, 1296–1304, doi:10.3762/bjoc.16.110
- the macrocyclic host affected the stereochemistry of the reaction. Moreover, we studied photodecarboxylation reactions initiated by the phthalimide chromophore [17][18][19] and applied them in cyclizations with memory of chirality [20] and diastereoselective peptide cyclizations [21
Anthelmintic drug discovery: target identification, screening methods and the role of open science
Beilstein J. Org. Chem. 2020, 16, 1203–1224, doi:10.3762/bjoc.16.105
- cattle treated annually is hundreds of millions [1]. Treatment of horses, other equids, and companion animals is also a major use of anthelmintics. Anthelmintic drug discovery has been a continued emphasis in the animal health industry, driven by the spread of resistance to the macrocyclic lactones [2
- macrocyclic lactone is an effective microfilaricide but does not kill the adult nematodes. Ivermectin must therefore be administered annually or twice-annually for many years to eliminate the parasite in the population. Progress has been impressive: onchocerciasis has been largely controlled as a public
- action of anthelmintic compounds, since it has been recognised that we do not fully understand how many anthelmintics work – for example the concentrations of macrocyclic lactones that paralyse worms in vitro are much greater than the concentration achieved by effective doses in vivo [147]. Several
The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties
Beilstein J. Org. Chem. 2020, 16, 71–77, doi:10.3762/bjoc.16.9
- Xiaodong Zhang Jun Xie Zhiling Xu Zhu Tao Qianjun Zhang Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China 10.3762/bjoc.16.9 Abstract The host–guest interactions between baicalein (BALE) and cucurbit[8]uril (Q[8]) and the
- , baicalein contains three phenolic hydroxy groups, which are easily oxidized to the quinone derivative and appear green, therefore, it has limited use in pharmaceuticals on account of its poor aqueous solubility and stability [31]. The cucurbit[n]urils (Q[n]s n = 5–8, 10, …) are macrocyclic hosts with a
- hydrophobic rigid cavity [32] (Figure 1). cucurbit[n]urils have a unique combination of properties including rigid highly symmetric structures, relatively large hydrophobic cavities and high thermal and chemical stability [33][34]. Cucurbit[n]urils are a type of macrocyclic drug carrier similar to macrocyclic
Understanding the role of active site residues in CotB2 catalysis using a cluster model
Beilstein J. Org. Chem. 2020, 16, 50–59, doi:10.3762/bjoc.16.7

- different cellular compartments [1][2]. More specifically, the enigmatic class of terpene cyclases is responsible for converting linear aliphatic oligoprenyl diphosphates into various chemically complex macrocyclic products. The resulting terpene scaffolds and their functionalized terpenoid analogues
Starazo triple switches – synthesis of unsymmetrical 1,3,5-tris(arylazo)benzenes
Beilstein J. Org. Chem. 2020, 16, 22–31, doi:10.3762/bjoc.16.4

- their isomerization properties [3][4]. Also, the incorporation of AB units into cyclic [5] or macrocyclic structures can control the switching, depending, i.a., on symmetry and ring strain [6][7][8][9]. By combining these approaches, half-lives can be tuned from milliseconds to years. The incorporation
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- was based on the conservation of the 16-membered macrocyclic scaffold and the apolar tripeptidyl moiety found in the solonamides. Both features are important to guarantee the interference with S. aureus QS [12][13][14][15]. The ester linkage of the lactone core was substituted by the sulfide group
- peptides [40]. Noteworthy, the detected ions A–C always showed the –SCH2CH=NH moiety, confirming the formation of the sulfide group (Schemes S4–S11, Supporting Information File 1). The NMR experiments also confirmed the macrocyclic structure (Supporting Information File 1). The 1H NMR spectra of compounds
Anion-driven encapsulation of cationic guests inside pyridine[4]arene dimers
Beilstein J. Org. Chem. 2019, 15, 2486–2492, doi:10.3762/bjoc.15.241

- , cation binding to pyridinearene is clearly not as strong as with resorcin[4]arene, which is known for its excellent cation receptor properties. A comparison of these two macrocyclic hosts reveals significant differences in their binding properties. Pyridine[4]arene appears to have a better affinity
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
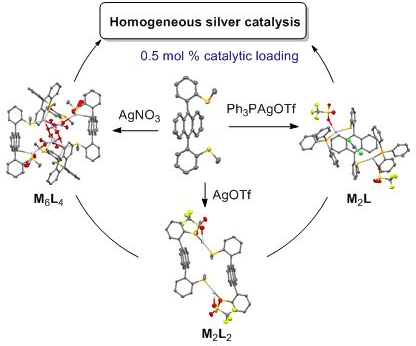
- -atropisomer, as revealed by X-ray diffraction. This alkylaryl thioether ligand (L) formed different macrocyclic complexes by coordination with silver(I) salts depending on the nature of the anion: M2L2 for AgOTf and AgOTFA, M6L4 for AgNO3. A discrete M2L complex was obtained in the presence of bulky PPh3AgOTf
- silver(I) catalysts based on sulfur ligands were reported so far, although alkyl thioethers are soft σ-donor ligands such as crown thioethers that were largely developed as macrocyclic ligands for silver(I) [35][36][37][38][39][40][41][42][43]. Interestingly, depending on their design, these known silver
- /cycloisomerization was previously described in high yields (>95%) using 5 mol % catalyst loadings starting from 2-(alkynyl)quinoline-3-carbaldehyde [60][61] with AgOTf catalyst and starting from 2-alkynylbenzaldehyde derivatives [62] in the presence of a macrocyclic pyridine-tetraaza complex of Ag(I) as a catalyst
Photochromic diarylethene ligands featuring 2-(imidazol-2-yl)pyridine coordination site and their iron(II) complexes
Beilstein J. Org. Chem. 2019, 15, 2428–2437, doi:10.3762/bjoc.15.235
- for realization of light-triggered guest uptake/release [52] and light-controlled interconversion between distinct supramolecular assemblies [53]. Ligand 6 featuring two coordination sites in the heteroaryl moiety and bridge provides unique opportunities to construct novel macrocyclic systems. Our
Effect of ring size on photoisomerization properties of stiff stilbene macrocycles
Beilstein J. Org. Chem. 2019, 15, 2408–2418, doi:10.3762/bjoc.15.233

- of the macrocyclic stiff stilbene diethers, a conformational analysis was undertaken (Figure 6). According to X-ray crystallography, in compound (E)-7 (Scheme 4) the aromatic rings of the two indane units are in the same plane (dihedral angle 180°), whereas in (Z)-7 this angle is 9.1° [21]. In the
- macrocyclic diethers 1a–d, all Z-isomers have a dihedral angle of 12–14°, roughly similar to the one in the crystal structure of (Z)-7. The deviation of this angle from 0° is due to steric interaction between two aromatic protons in position 4 (Figure 9). In the E-isomers, an increasing distortion of the
- stated differently, the obtained yellow oil was purified by CC (pentane/DCM 1:0 to 1:1). The obtained product was dried under high vacuum overnight. Synthesis of macrocyclic stiff stilbene diether (Z)-1a The synthesis followed general procedure B with compound 6a (0.279 g, 0.7 mmol) as starting material
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- photochromism in the spiropyran switch [18]. Other recent studies of photochromic systems within macrocyclic and supramolecular hosts [19] include dihydroazulene switches [20] and red-shifted azobenzenes [21][22] inside cucurbiturils and cyclodextrins. The behavior of light-responsive compounds can also be
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- polymers etc. [14][15]. Noncovalent interactions play a dynamic role in the binding mechanism of triazoles as macrocyclic receptors. It has been reported that the combined effects of both an electron lone pair on the nitrogen of the heterocycle and the acidic C5–H proton make 1,2,3-triazoles interesting
- of 1,4-disubstituted 1,2,3- triazole units [19][20][21][22][23][24]. Macrocyclic ring closure can be achieved by the CuAAC of building blocks functionalized with both azide and alkyne, using [1 + 1], [2 + 2], [n + n] strategies depending on how much triazoles are needed to be included in the
- to be very difficult to further proceed. However, the anion binding property of the corresponding bistriazolium macrocyclic part 5·(BF4)2 (Figure 5) has successfully been investigated using 1H NMR titration experiments in CD3CN. The highest binding affinity was found to be almost similar for both BzO
Multiple threading of a triple-calix[6]arene host
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- , daisy-chain pseudorotaxanes, olympiadane, Janus rotaxanes [5]) is generally obtained through a template-approach [9] exploiting the threading process between linear (axle) and macrocyclic (wheel) components. In order to synthesize high-order interpenetrated architectures, much attention has been









































































































































































































































































































