Search results
Search for "transition states" in Full Text gives 183 result(s) in Beilstein Journal of Organic Chemistry.
Ring-closing metathesis of prochiral oxaenediynes to racemic 4-alkenyl-2-alkynyl-3,6-dihydro-2H-pyrans
Beilstein J. Org. Chem. 2020, 16, 2757–2768, doi:10.3762/bjoc.16.226
- ). The search for the saddle points (starting complexes of both unsaturated compounds 12b and 13, transition states 15A–D and the corresponding Diels–Alder products 14A–D (Figure 4)) showed that the reaction is strongly exergonic and irreversible with an activation free Gibbs energy in the range between
- of other conformations, a lower accuracy of pure functional used, and an incompleteness of the double zeta basis set. The structures of the corresponding transition states 15B, 15C are depicted in Figure 7, and the relative free Gibbs energies of all saddle points for all four stereoisomers are shown
- . Conformations of dihydropyran 12b. The two most stable s-trans (left) and s-cis (right) conformations of dihydropyran 12b. The two most stable transition states endo-trans 15B and exo-cis 15C (hydrogens are omitted for clarity). PES of the Diels–Alder reaction of dihydropyran 12b and maleimide 13. RCEYM with Ru
Asymmetric Mannich reactions of (S)-N-tert-butylsulfinyl-3,3,3-trifluoroacetaldimines with yne nucleophiles
Beilstein J. Org. Chem. 2020, 16, 2671–2678, doi:10.3762/bjoc.16.217
- the tert-butyl group but, as a major factor, the corresponding chelated transition states. As all diastereomers 3 can be easily isolated in optical pure form by routine column chromatography, this reaction should be of certain value for biological studies. In this regard, we decided to demonstrate the
Photosensitized direct C–H fluorination and trifluoromethylation in organic synthesis
Beilstein J. Org. Chem. 2020, 16, 2151–2192, doi:10.3762/bjoc.16.183
- ). Ideally, to achieve the best selectivity, the β- and the γ-position must be distinctive based on their geometric constraints. We note that the nature of the transition states in this ketone-directed C(sp3)–H fluorination (and the subsequent carbonyl-directed C–H fluorinations) is not well-characterized
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
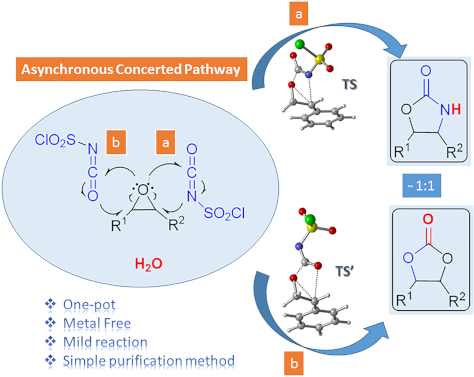
- performed. Formation of oxazolidinone 9f There are two possible channels for the cyclization reaction of epoxide 7f with CSI to form oxazolidinone intermediates 10 and 11 as shown in Figure 1. In both transition states it is found that the ring-opening reaction of the epoxide, a nucleophilic attack of N4
- observed oxazolidinone 9f since its precursor intermediate 10 has a remarkably lower activation barrier compared to 11. Methodology All calculations have been carried with the Gaussian 09 program package [58]. Geometry optimizations of all the minima and transition states involved have been performed using
- stationary points to verify whether they are minima (no imaginary frequencies) or transition states (a single imaginary frequency). Thermodynamic calculations have been performed at 25 °C and 1 atm. The same level of intrinsic reaction coordinate (IRC) [62][63] calculations have been performed to check the
Pauson–Khand reaction of fluorinated compounds
Beilstein J. Org. Chem. 2020, 16, 1662–1682, doi:10.3762/bjoc.16.138

- equiv of NMO, yielding bicyclic derivatives 19 in moderate yields and high diastereoselectivity (de > 95%). The observed diastereoselectivity was rationalized considering two transition states of the PKR, and assuming the CF3 group occupies an axial position due to the steric and electrostatic
Models of necessity
Beilstein J. Org. Chem. 2020, 16, 1649–1661, doi:10.3762/bjoc.16.137
- become apparent [40]. This is because non-equilibrium structures such as transition states that cannot be described by the conventional Lewis model are passed through during reactions. Quantum chemical methods, often in combination with molecular dynamics, can predict the course of a specific reaction
Rearrangement of o-(pivaloylaminomethyl)benzaldehydes: an experimental and computational study
Beilstein J. Org. Chem. 2020, 16, 1636–1648, doi:10.3762/bjoc.16.136
- ” printed in a Gaussian 09 vibrational frequency calculation. Standard state correction was taken into account. The transition states were optimized with the QST3 or the TS (berny) method. Transition states were identified by having one imaginary frequency in the Hessian matrix, and IRC calculations were
- performed in order to prove that the transition states connect two corresponding minima. General procedure I for the synthesis of compounds 2a,b, 3a,b, 8a,b, and 23a. TFA (0.1 equiv) was added to a solution of 1a–e in DCM (20 mL). After stirring for 24 h at room temperature, an aqueous sodium carbonate
- to the transformations. Relative Gibbs free energy values corresponding to the transition states. Supporting Information Detailed NMR studies (1H NMR, 13C NMR, DeptQ, Dept-135, edHSQC, selective HSQC, HMBC, selective HMBC, NOESY, 1H,1H-COSY, one-dimensional selective NOE, selective TOCSY spectra) of
In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions
Beilstein J. Org. Chem. 2020, 16, 1465–1475, doi:10.3762/bjoc.16.122
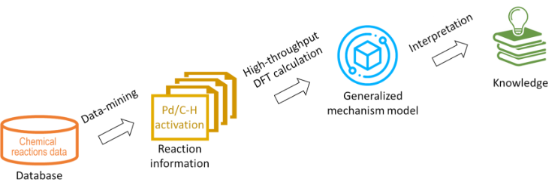
- on the reaction path. In this case, the intermediates are likely to be close to, and resemble, transition states. Due to that, their relative energy of formation can be translated to relative reaction kinetic barriers and thus be used, as the first approximation, to predict distributions of the final
One-step route to tricyclic fused 1,2,3,4-tetrahydroisoquinoline systems via the Castagnoli–Cushman protocol
Beilstein J. Org. Chem. 2020, 16, 1456–1464, doi:10.3762/bjoc.16.121
- observed different reactivity of the anhydrides 5–7 towards 1-methyl-3,4-dihydroisoquinoline 19 needs additional explanation. Work in this field based on the theoretical treatment of the possible stabilities of the intermediates and the hypothetic transition states of the steps is in progress. Conclusion
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- aromatization of the polycyclic intermediates provides the corresponding polycyclic pyrrolo-isoindoles and isoindolo-pyrrolo-indoles. A theoretical study on the stereoselective Diels–Alder reactions, carried out by calculating the endo/exo transition states, revealed the assistance of non-covalent interactions
Preparation of 2-phospholene oxides by the isomerization of 3-phospholene oxides
Beilstein J. Org. Chem. 2020, 16, 818–832, doi:10.3762/bjoc.16.75
- oxide, while the corresponding 2-phospholene oxides 4a or 7 are the preferred isomers for the unsubstituted or methyl-substituted phospholene oxide derivatives (Table 5, entries 1–3). According to the thermodynamic data without the consideration of the reaction mechanisms and transition states (Table 5
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates
Beilstein J. Org. Chem. 2020, 16, 159–167, doi:10.3762/bjoc.16.18
- shown in Scheme 2. For simplicity, the reaction of benzyl isocyanide (2a) with O-benzyl S-methyl dithiocarbonate (1a) was chosen for the calculations, forming the intermediates Int1–3 via the most probable transition states TS1–3, respectively, which, after hydrolysis, formed the observed product 4a. To
- with Gaussian 09 [36]. The HF/6-31G(d) level of theory in the gas phase was only used to locate the transition state geometries. An intrinsic reaction coordinate (IRC) analysis was conducted for each transition state studied in this work to confirm that the transition states were associated with the
- respective minima. The final IRC structures were further optimized (Figure S14, Supporting Information File 1). The geometries of all reactants, transition states, and intermediates were then fully optimized at the B3LYP/6-311++G(d,p) level of theory in the DMF solvent phase using the polarized continuum
Synthesis of C-glycosyl phosphonate derivatives of 4-amino-4-deoxy-α-ʟ-arabinose
Beilstein J. Org. Chem. 2020, 16, 9–14, doi:10.3762/bjoc.16.2
- -benzyl-protected gluco and galacto derivatives [20][27][30][31]. In addition to exocyclic glycals mimicking putative planar transition states of substrates involved in enzymatic reactions, such as glycosyl transfer, mutase, and epimerization, endo-glycals are also of interest [21][22][32][33][34][35
Why do thioureas and squaramides slow down the Ireland–Claisen rearrangement?
Beilstein J. Org. Chem. 2019, 15, 2948–2957, doi:10.3762/bjoc.15.290
- difficult due to their rather nonpolar transition states, which are difficult to be addressed by catalysts [29]. Several stereoselective [3,3]-sigmatropic rearrangements are realized with chiral Brønsted acids [30][31][32][33][34]. Jacobsen reported guanidinium-catalyzed enantioselective Claisen
- activate the enolates or the corresponding silyl ketene acetals or stabilize the corresponding transition states. In addition, chiral organocatalysts could induce diastereo- as well as enantioselectivity. Therefore, we examined the Ireland–Claisen rearrangement of ester 1c in the presence of a range of
- rearrangements proceed via isopolar transition states and, therefore solvent effects are rather small. For this reason and due to the complete lack of enantioselectivity, at this stage, we did not investigate other solvents with the catalysts collected in Figure 1. In order to gain further insight into the
A combinatorial approach to improving the performance of azoarene photoswitches
Beilstein J. Org. Chem. 2019, 15, 2753–2764, doi:10.3762/bjoc.15.266

- derivatives are predicted to occur through a transition state in which the N atom next to the benzene ring linearizes in an inversion mechanism (see B-type transition states in Figure 2 for 4pzH-F2 and 4pzMe-F2, and Figure S1 in Supporting Information File 1 for the rest of di-ortho-substituted photoswitches
- the possible conformers both in the Z- and E-forms were fully optimized by using the hybrid exchange-correlation PBE0 functional [37] including the Grimme’s dispersion correction in its latest version (D3) [38]. The split-valence Pople’s basis set 6-31G** was used throughout [39]. Transition states
- were optimized by using the Berny algorithm at the same level of theory [40]. Theoretical calculations were carried out in the gas phase. Theoretical kinetic studies were carried out at the PBE0-D3/6-31G** level of theory by considering all possible transition states and minimum-energy conformers for
Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series
Beilstein J. Org. Chem. 2019, 15, 2655–2663, doi:10.3762/bjoc.15.258
- computations on carbocation intermediates and transition states were carried out with the B3LYP functional and 6-31+G(d,p) basis set, using the polarizable continuum model in dichloroethane to model solvation [42]. Each stationary point was characterized as a minimum or transition state by vibrational
Thermal stability of N-heterocycle-stabilized iodanes – a systematic investigation
Beilstein J. Org. Chem. 2019, 15, 2311–2318, doi:10.3762/bjoc.15.223

- electrophilic hypervalent iodine atom in its ground state or directly influences its reactivity by stabilizing reactive intermediates or transition states. In recent years, a plethora of cyclic and pseudocyclic iodanes have been developed with covalently attached stabilizing ligands L2 and applied in a variety
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- (2a’) derivatives would lead to higher energy anti-aromatic transition states [1][2][9][15]. Additionally, upon conversion to the dipole forms (Scheme 1), the fulvene loses total planarity through the exocyclic carbon sp2 → sp3 hybridisation, allowing some loss of energy (and gain in stability
The cyclopropylcarbinyl route to γ-silyl carbocations
Beilstein J. Org. Chem. 2019, 15, 1769–1780, doi:10.3762/bjoc.15.170
- spectra of new compounds. Supporting Information File 74: M062X/6-611+G** calculated structures, energies, and Cartesian coordinates for carbocations and transition states.
Transient and intermediate carbocations in ruthenium tetroxide oxidation of saturated rings
Beilstein J. Org. Chem. 2019, 15, 1552–1562, doi:10.3762/bjoc.15.158

- can lead to different products in a ratio that depends on reaction dynamics [31][32][33]. The study of molecular dynamics trajectories has allowed characterization of ambimodal transition states in reactions involving carbocations [34][35]. We have demonstrated computationally the presence of
Anomeric sugar boronic acid analogues as potential agents for boron neutron capture therapy
Beilstein J. Org. Chem. 2019, 15, 1355–1359, doi:10.3762/bjoc.15.135
- stereoselectivity in the hydroboration reaction we hypothesized the transition states bearing to the two epimers. We assumed that borane would react firstly with the free hydroxy group generating an intermediate alkoxyborane, and that the hydroboration reaction occurs intramolecularly on such intermediate. Based on
- these premises, two transition states can be identified (Figure 4) for the hydroboration reaction on each of the two diastereotopic faces of the double bond. The transition state deriving from the attack on the si face, which leads to the arabino-configurated product, contains two destabilizing
- conserved into the analogues). Structures of boron analogues. Synthetic strategy. Postulated transition states for the hydroboration reaction. Synthesis of 2-deoxy analogue 8. Synthesis of 2,3-dideoxy analogue 11. Supporting Information Supporting Information File 488: Experimental procedures and
Enantioselective Diels–Alder reaction of anthracene by chiral tritylium catalysis
Beilstein J. Org. Chem. 2019, 15, 1304–1312, doi:10.3762/bjoc.15.129
- sulfonylhydrazine 6 (1.2 equiv) in CH2Cl2 led to the desired N-tosylhydrazone 7 in 83% yield and with 82% ee (Scheme 3b). The absolute configuration was assigned on the basis of the structure of 7, which was confirmed unambiguously by an X-ray crystallographic study [39]. Tentative transition states to account for
Mechanistic investigations on multiproduct β-himachalene synthase from Cryptosporangium arvum
Beilstein J. Org. Chem. 2019, 15, 1008–1019, doi:10.3762/bjoc.15.99
- vanished crosspeak. Combining the information deduced from the extensive incubation experiments stated above, a structural model for the reactive conformation of cation D is proposed (Figure S19, Supporting Information File 1). This intermediate, or structurally related transition states for the
An anomalous addition of chlorosulfonyl isocyanate to a carbonyl group: the synthesis of ((3aS,7aR,E)-2-ethyl-3-oxo-2,3,3a,4,7,7a-hexahydro-1H-isoindol-1-ylidene)sulfamoyl chloride
Beilstein J. Org. Chem. 2019, 15, 931–936, doi:10.3762/bjoc.15.89
- and B, we use the notation A/B throughout the article. Figure 3 shows the relative energy profile for the reaction mechanism shown in Scheme 4. The rate-determining steps for the formation of 10, 11, and 12 are the transition states 9/14, 9/11, and 9/12, respectively. The difference between the
Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives
Beilstein J. Org. Chem. 2019, 15, 401–430, doi:10.3762/bjoc.15.36































































































































































































































































































































































































