Search results
Search for "reaction mechanisms" in Full Text gives 135 result(s) in Beilstein Journal of Organic Chemistry.
On the mass spectrometric fragmentations of the bacterial sesterterpenes sestermobaraenes A–C
Beilstein J. Org. Chem. 2020, 16, 2807–2819, doi:10.3762/bjoc.16.231
- investigation of the electron impact mass spectrometry (EIMS) fragmentation reactions of these sesterterpene hydrocarbons. Keywords: isotopes; mass spectrometry; reaction mechanisms; sesterterpenes; Streptomyces mobaraensis; Introduction The sestermobaraenes A–F (1–6) and sestermobaraol (7) are a series of
- terpene fragmentations in EIMS in the future by the strategy applied in this work to learn more about the underlying reaction mechanisms. Experimental Preparation of 13C-labelled compounds 1–3 and GC–MS analysis The 25 isotopomers of (13C)-1, (13C)-2, and (13C)-3 were prepared enzymatically with SmTS1
Photosensitized direct C–H fluorination and trifluoromethylation in organic synthesis
Beilstein J. Org. Chem. 2020, 16, 2151–2192, doi:10.3762/bjoc.16.183
Synthesis, docking study and biological evaluation of ᴅ-fructofuranosyl and ᴅ-tagatofuranosyl sulfones as potential inhibitors of the mycobacterial galactan synthesis targeting the galactofuranosyltransferase GlfT2
Beilstein J. Org. Chem. 2020, 16, 1853–1862, doi:10.3762/bjoc.16.152
- mechanism studies using computational chemistry methods. The probable reaction mechanisms were studied by hybrid DFT QM/MM molecular dynamics simulations [11] where the possible transition state (TS) structures were localized. The observation of the possible TS structure opens the opportunities for the in
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
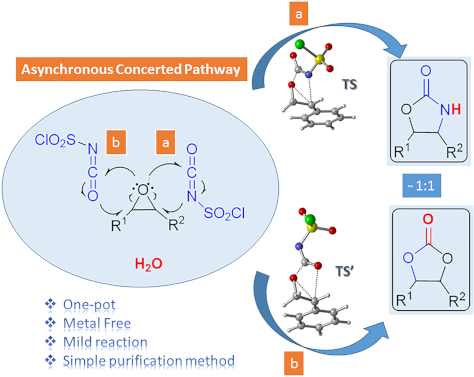
- likely to be rate-determining for both reaction mechanisms. Besides, explicit inclusion of water molecules is crucial for lowering the energy barrier making the process plausible without changing the nature of the rate determining step of the formation of 9f. Our computational results adequately explain
In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions
Beilstein J. Org. Chem. 2020, 16, 1465–1475, doi:10.3762/bjoc.16.122
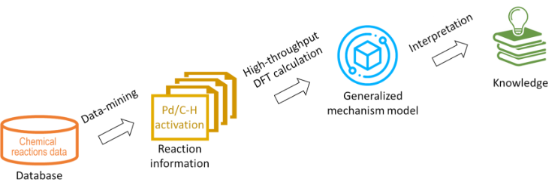
- computational data, a threshold to distinguish between two possible reaction mechanisms was established. Computational Methods The NWChem, an open source software package, was used for the DFT calculations. It is easily scalable and designed to solve large scientific computational problems efficiently employing
Preparation of 2-phospholene oxides by the isomerization of 3-phospholene oxides
Beilstein J. Org. Chem. 2020, 16, 818–832, doi:10.3762/bjoc.16.75
- oxide, while the corresponding 2-phospholene oxides 4a or 7 are the preferred isomers for the unsubstituted or methyl-substituted phospholene oxide derivatives (Table 5, entries 1–3). According to the thermodynamic data without the consideration of the reaction mechanisms and transition states (Table 5
- 10: 44:56. dRatio of trans–cis isomers of 10: 45:55. eRatio of trans–cis isomers of 10: 40:60. Three possible reaction mechanisms considered in the theoretical studies for the isomerization of 3-phospholene oxides 1 under thermal conditions. *The calculated ΔG values for mechanism B and C were
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- . A variety of reaction mechanisms are initiated, which, aiming to the photocleaver, may lead to DNA damage. A requirement for nucleic acid’s and most protein’s “transparency” is irradiating at wavelengths longer than 310 nm [15]. “Transparency” means lack of damage due to irradiation itself and
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates
Beilstein J. Org. Chem. 2020, 16, 159–167, doi:10.3762/bjoc.16.18
- hypothesis. Computational studies on the proposed reaction mechanism Several possible reaction mechanisms were considered to account for the unexpected products obtained. Ultimately, we employed quantum chemical calculations to shed light on the most probable reaction pathway for the observed products, as
[1,3]/[1,4]-Sulfur atom migration in β-hydroxyalkylphosphine sulfides
Beilstein J. Org. Chem. 2020, 16, 88–105, doi:10.3762/bjoc.16.11
- conditions clearly showed that distinct reaction mechanisms were responsible for the substrate’s transformation under Lewis and Brønsted acid catalysis, but both seemed to include the preliminary formation of alkenylphosphine sulfide. This was shown for the Brønsted acid-mediated reaction (Table 4) but
Emission and biosynthesis of volatile terpenoids from the plasmodial slime mold Physarum polycephalum
Beilstein J. Org. Chem. 2019, 15, 2872–2880, doi:10.3762/bjoc.15.281
- similarities occurred between PpolyTPS1 and PpolyTPS4 (72%) and between PpolyTPS2 and PpolyTPS3 (64%). PpolyTPS1/4 and PpolyTPS2/3, however, showed only ≈30% sequence similarity to each other. Terpene synthases can be classified into class I and class II, based on the reaction mechanisms they catalyze. These
Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series
Beilstein J. Org. Chem. 2019, 15, 2655–2663, doi:10.3762/bjoc.15.258
- displays such a complex and fascinating collection of molecular rearrangements. Building on a long history, new synthetic applications [1][2] and explanations of carbocation reaction mechanisms [3][4][5][6] continue to be discovered. Chemistry in superacid solutions has played a major role in this field [7
Click chemistry towards thermally reversible photochromic 4,5-bisthiazolyl-1,2,3-triazoles
Beilstein J. Org. Chem. 2019, 15, 2161–2169, doi:10.3762/bjoc.15.213

- the thermal back reaction after 313-nm light irradiation to 1o–3o in MeCN at 28 °C. Concentration of compounds are the same as in Figure 1. (a) 1c. (b) 2c. (c) 3c. Reaction mechanisms of Huisgen cyclization catalyzed by Cu(I) and Ru(I). Synthesis and photochromism of bisthiazolyltriazoles. Wavelengths
Synthesis of benzo[d]imidazo[2,1-b]benzoselenoazoles: Cs2CO3-mediated cyclization of 1-(2-bromoaryl)benzimidazoles with selenium
Beilstein J. Org. Chem. 2019, 15, 2029–2035, doi:10.3762/bjoc.15.199
- reaction mechanisms for these syntheses have not been reported. Therefore, we carried out several control experiments to clarify the reaction mechanism (Scheme 2). However, the reaction of 1-phenylbenzimidazole (11) without bromine at the phenyl group with diphenyl diselenide (12a) did not afford the
Synthesis and anion binding properties of phthalimide-containing corona[6]arenes
Beilstein J. Org. Chem. 2019, 15, 1976–1983, doi:10.3762/bjoc.15.193
- [1][2][11][12]. Moreover, the designed macrocycles are useful molecular tools in the investigation of supramolecular catalysis and reaction mechanisms [1][2][13][14][15][16]. Heteracalixaromatics or heteroatom-bridged calix(het)arenes [17][18][19][20][21] are synthetic macrocycles composed of
Reactions of 2-carbonyl- and 2-hydroxy(or methoxy)alkyl-substituted benzimidazoles with arenes in the superacid CF3SO3H. NMR and DFT studies of dicationic electrophilic species
Beilstein J. Org. Chem. 2019, 15, 1962–1973, doi:10.3762/bjoc.15.191
- -arylmethyl-substituted benzimidazoles, in yields up to 90%. The reaction intermediates, protonated species derived from starting benzimidazoles in TfOH, were thoroughly studied by means of NMR and DFT calculations and plausible reaction mechanisms are discussed. Keywords: benzimidazoles; cations; Friedel
- ). Summarizing all data obtained by DFT calculations (Table 1) and NMR studies (Table 2) of intermediate cations generated from benzimidazoles 1–8 in TfOH, and their reactions with arenes (Tables 3–5, Scheme 1, and Scheme 2), the following reaction mechanisms are proposed (Scheme 3 and Scheme 4). 2-Carbonyl
Inherent atomic mobility changes in carbocation intermediates during the sesterterpene cyclization cascade
Beilstein J. Org. Chem. 2019, 15, 1890–1897, doi:10.3762/bjoc.15.184

- diphosphate, IM: intermediate. Quiannulatene is formed by the deprotonation of IM11. Phase (I): 5/12/5 tricycle formation is highlighted in blue. Phase (II): conformational changes and hydrogen shifts are highlighted in orange. Phase (III): ring rearrangements are highlighted in yellow. Reaction mechanisms of
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- of the common reactants with 2-aminopyridines/2-aminoquinolines/1-aminoisoquinolines 3, 75, 76 to give the desired products, respectively (Scheme 27). Both EWGs and EDGs on the reactants resulted in good to excellent yields of the product. This process followed two types of coupling reaction
- mechanisms, i.e., a Chan–Lam coupling and an Ullmann coupling. The Chan–Lam coupling involved a C–N bond formation (intermediate I, 84) which then entered into the Ullmann coupling to undergo intramolecular cyclization to form final product 78 and release Cu(III) to Cu(I) by reductive elimination. In this
Different reactivity of phosphorylallenes under the action of Brønsted or Lewis acids: a crucial role of involvement of the P=O group in intra- or intermolecular interactions at the formation of cationic intermediates
Beilstein J. Org. Chem. 2019, 15, 1491–1504, doi:10.3762/bjoc.15.151
- intermolecular hydroarylation of allenes bearing electron-withdrawing substituents. Plausible reaction mechanisms have been proposed on the basis of the investigated reactions, and NMR analysis and DFT studies of the intermediate cationic species. Keywords: aluminum chloride; cation; intermediate
- achieved. This reaction gave rise to phosphoryl-substituted alkenes and indanes. The intermediates of these reactions were investigated by means of NMR and DFT calculations, that shed light on the reaction mechanisms. Allenes 1a–j used in this study. 31P NMR monitoring of the progress of transformation of
Unexpected polymorphism during a catalyzed mechanochemical Knoevenagel condensation
Beilstein J. Org. Chem. 2019, 15, 1141–1148, doi:10.3762/bjoc.15.110

- reaction environment. It was subsequently demonstrated how a combination of different in situ methods can provide more thorough investigation of mechanochemical reaction mechanisms [14][15][16]. Of particular benefit to synthetic reactions, such as C–C bond formation [17][18], the use of Raman spectroscopy
SO2F2-mediated transformation of 2'-hydroxyacetophenones to benzo-oxetes
Beilstein J. Org. Chem. 2019, 15, 976–980, doi:10.3762/bjoc.15.95
- proposed to be E-configured. However, the exact configuration was not confirmed [40]. Two possible reaction mechanisms were proposed for this SO2F2-mediated transformation of 2'-hydroxyacetophenones to benzo-oxetes (Scheme 3). The first mechanism commences with the deprotonation of 2'-hydroxyacetophenone 1
- mmol, 3.0 equiv) in DMSO (2.0 mL) was stirred at 90 ° C under a SO2F2 atmosphere (balloon) for 20 h. Yields refer to isolated yields. aReaction performed at 50 °C. Two proposed reaction mechanisms. B = base. Screening and optimization of the reaction conditions.a Supporting Information Supporting
Homo- and hetero-difunctionalized β-cyclodextrins: Short direct synthesis in gram scale and analysis of regiochemistry
Beilstein J. Org. Chem. 2019, 15, 710–720, doi:10.3762/bjoc.15.66
- react only with the more distant glucose units, therefore only AC and AD disubstitution takes place. The detailed study of the reaction mechanisms is out of the scope of this paper, however, the different mechanisms and reaction intermediates are likely responsible for the distinct outcome of the two
Chemical structure of cichorinotoxin, a cyclic lipodepsipeptide that is produced by Pseudomonas cichorii and causes varnish spots on lettuce
Beilstein J. Org. Chem. 2019, 15, 299–309, doi:10.3762/bjoc.15.27
- , as shown in Figure 9. Consequently, the overall structures of compounds A and B are depicted in Figure S18 (Supporting Information File 1). Figure 10 shows the reaction mechanisms for generating compounds A and B from cichorinotoxin by treatment with dilute KOH. The proton at α-position of D
Computational characterization of enzyme-bound thiamin diphosphate reveals a surprisingly stable tricyclic state: implications for catalysis
Beilstein J. Org. Chem. 2019, 15, 145–159, doi:10.3762/bjoc.15.15

- , computational studies have been used to investigate full reaction mechanisms of ThDP enzymes, including pyruvate decarboxylase (PDC) [25][26][27][28], benzoylformate decarboxylase (BFDC) [29][30], acetohydroxy acid synthase [24][31][32][33][34][35], pyruvate dehydrogenase (PDH) [36], benzaldehyde lyase [37
Mechanistic studies of an L-proline-catalyzed pyridazine formation involving a Diels–Alder reaction with inverse electron demand
Beilstein J. Org. Chem. 2019, 15, 30–43, doi:10.3762/bjoc.15.3

- ; L-proline; reaction mechanism; Introduction Electrospray (ESI) mass spectrometry (MS) [1] is well suited for studying reaction mechanisms as it is a soft ionization method leaving most species intact [1][2][3]. In addition, it is a fast analytical method [3] making it possible to study transient
Generation of 1,2-oxathiolium ions from (arysulfonyl)- and (arylsulfinyl)allenes in Brønsted acids. NMR and DFT study of these cations and their reactions
Beilstein J. Org. Chem. 2018, 14, 2897–2906, doi:10.3762/bjoc.14.268
- mixtures, (arylsulfinyl)allenes give allyl alcohols (ArSO2–CR1=CH–C(OH)R2R3). Plausible reaction mechanisms have been proposed for all studied reactions. Keywords: (arylsulfinyl)allenes; (arylsulfonyl)allenes; butadienes; 1,2-oxathiolium ions; thiochromene 1,1-dioxides; Introduction Allenes are widely
- (CCDC 1843239); ellipsoid contours of probability levels are 50%. (Arylsulfinyl)allenes 1 and (arylsulfonyl)allenes 2 used in this study. Plausible reaction mechanisms of transformations of allene 2a in Brønsted acids. Selective formation of butadienes 3a–h from allenes 2a–h. Reactions of allenes 2 in






























































































































































































































































































































































































































































































