Search results
Search for "quinoline" in Full Text gives 190 result(s) in Beilstein Journal of Organic Chemistry.
Microwave-assisted multicomponent reactions in heterocyclic chemistry and mechanistic aspects
- Shivani Gulati,
- Stephy Elza John and
- Nagula Shankaraiah
Beilstein J. Org. Chem. 2021, 17, 819–865, doi:10.3762/bjoc.17.71

- ). Abonia and co-workers [85] established a catalyst-free construction of quinoline-based pyridopyridines 97 by employing a microwave-assisted three-component reaction of 3-formyl-2-oxoquinoline derivatives 95, 2,4,6-triaminopyrimidine (96) and a cyclic 1,3-diketone such as dimedone (6a) in DMF. The
- opening resulting in imine intermediate E. A consecutive intramolecular cyclization and tautomerization yields azepino[5,4,3-cd]indoles 143b. 9 Quinolines Quinolines are bicyclic aromatic heterocycles consisting of a fused pyridine and benzene ring. Quinoline and its derivatives are important both from

Graphical Abstract

Figure 1: Marketed drugs with acridine moiety.

Scheme 1: Synthesis of 4-arylacridinediones.

Scheme 2: Proposed mechanism for acridinedione synthesis.

Scheme 3: Synthesis of tetrahydrodibenzoacridinones.

Scheme 4: Synthesis of naphthoacridines.

Scheme 5: Plausible mechanism for naphthoacridines.

Figure 2: Benzoazepines based potent molecules.

Scheme 6: Synthesis of azepinone.

Scheme 7: Proposed mechanism for azepinone formation.

Scheme 8: Synthesis of benzoazulenen-1-one derivatives.

Scheme 9: Proposed mechanism for benzoazulene-1-one synthesis.

Figure 3: Indole-containing pharmacologically active molecules.

Scheme 10: Synthesis of functionalized indoles.

Scheme 11: Plausible mechanism for the synthesis of functionalized indoles.

Scheme 12: Synthesis of spirooxindoles.

Scheme 13: Synthesis of substituted spirooxindoles.

Scheme 14: Plausible mechanism for the synthesis of substituted spirooxindoles.

Scheme 15: Synthesis of pyrrolidinyl spirooxindoles.

Scheme 16: Proposed mechanism for pyrrolidinyl spirooxindoles.

Figure 4: Pyran-containing biologically active molecules.

Scheme 17: Synthesis of functionalized benzopyrans.

Scheme 18: Plausible mechanism for synthesis of benzopyran.

Scheme 19: Synthesis of indoline-spiro-fused pyran derivatives.

Scheme 20: Proposed mechanism for indoline-spiro-fused pyran.

Scheme 21: Synthesis of substituted naphthopyrans.

Figure 5: Marketed drugs with pyrrole ring.

Scheme 22: Synthesis of tetra-substituted pyrroles.

Scheme 23: Mechanism for silica-supported PPA-SiO2-catalyzed pyrrole synthesis.

Scheme 24: Synthesis of pyrrolo[1,10]-phenanthrolines.

Scheme 25: Proposed mechanism for pyrrolo[1,10]-phenanthrolines.

Figure 6: Marketed drugs and molecules containing pyrimidine and pyrimidinones skeletons.

Scheme 26: MWA-MCR pyrimidinone synthesis.

Scheme 27: Two proposed mechanisms for pyrimidinone synthesis.

Scheme 28: MWA multicomponent synthesis of dihydropyrimidinones.

Scheme 29: Proposed mechanism for dihydropyrimidinones.

Figure 7: Biologically active fused pyrimidines.

Scheme 30: MWA- MCR for the synthesis of pyrrolo[2,3-d]pyrimidines.

Scheme 31: Proposed mechanism for pyrrolo[2,3-d]pyrimidines.

Scheme 32: Synthesis of substituted pyrrolo[2,3-d]pyrimidine-2,4-diones.

Scheme 33: Probable pathway for pyrrolo[2,3-d]pyrimidine-2,4-diones.

Scheme 34: Synthesis of pyridopyrimidines.

Scheme 35: Plausible mechanism for the synthesis of pyridopyrimidines.

Scheme 36: Synthesis of dihydropyridopyrimidine and dihydropyrazolopyridine.

Scheme 37: Proposed mechanism for the formation of dihydropyridopyrimidine.

Scheme 38: Synthesis of thiopyrano[4,3-d]pyrimidines.

Scheme 39: Plausible mechanism for the synthesis of thiopyrano[4,3-d]pyrimidines.

Scheme 40: Synthesis of decorated imidazopyrimidines.

Scheme 41: Proposed mechanism for imidazopyrimidine synthesis.

Figure 8: Pharmacologically active molecules containing purine bases.

Scheme 42: Synthesis of aza-adenines.

Scheme 43: Synthesis of 5-aza-7-deazapurines.

Scheme 44: Proposed mechanism for deazapurines synthesis.

Figure 9: Biologically active molecules containing pyridine moiety.

Scheme 45: Synthesis of steroidal pyridines.

Scheme 46: Proposed mechanism for steroidal pyridine.

Scheme 47: Synthesis of N-alkylated 2-pyridones.

Scheme 48: Two possible mechanisms for pyridone synthesis.

Scheme 49: Synthesis of pyridone derivatives.

Scheme 50: Postulated mechanism for synthesis of pyridone.

Figure 10: Biologically active fused pyridines.

Scheme 51: Benzimidazole-imidazo[1,2-a]pyridines synthesis.

Scheme 52: Mechanism for the synthesis of benzimidazole-imidazo[1,2-a]pyridines.

Scheme 53: Synthesis of pyrazolo[3,4-b]pyridine-5-spirocycloalkanedione derivatives.

Scheme 54: Proposed mechanism for spiro-pyridines.

Scheme 55: Functionalized macrocyclane-fused pyrazolo[3,4-b]pyridine derivatives.

Scheme 56: Mechanism postulated for macrocyclane-fused pyrazolo[3,4-b]pyridine.

Scheme 57: Generation of pyrazolo[3,4-b]pyridines.

Scheme 58: Proposed mechanism for the synthesis of pyrazolo[3,4-b]pyridines.

Scheme 59: Proposed mechanism for the synthesis of azepinoindole.

Figure 11: Pharmaceutically important molecules with quinoline moiety.

Scheme 60: Povarov-mediated quinoline synthesis.

Scheme 61: Proposed mechanism for Povarov reaction.

Scheme 62: Synthesis of pyrazoloquinoline.

Scheme 63: Plausible mechanism for pyrazoloquinoline synthesis.

Figure 12: Quinazolinones as pharmacologically significant scaffolds.

Scheme 64: Four-component reaction for dihydroquinazolinone.

Scheme 65: Proposed mechanism for dihydroquinazolinones.

Scheme 66: Synthesis purine quinazolinone and PI3K-δ inhibitor.

Scheme 67: Synthesis of fused benzothiazolo/benzoimidazoloquinazolinones.

Scheme 68: Proposed mechanism for fused benzothiazolo/benzoimidazoloquinazolinones.

Scheme 69: On-water reaction for synthesis of thiazoloquinazolinone.

Scheme 70: Proposed mechanism for the thiazoloquinazolinone synthesis.

Scheme 71: β-Cyclodextrin-mediated synthesis of indoloquinazolinediones.

Scheme 72: Proposed mechanism for synthesis of indoloquinazolinediones.

Figure 13: Triazoles-containing marketted drugs and pharmacologically active molecules.

Scheme 73: Cu(I) DAPTA-catalyzed 1,2,3-triazole formation.

Scheme 74: Mechanism for Cu(I) DAPTA-catalyzed triazole formation.

Scheme 75: Synthesis of β-hydroxy-1,2,3-triazole.

Scheme 76: Proposed mechanism for synthesis of β-hydroxy-1,2,3-triazoles.

Scheme 77: Synthesis of bis-1,2,4-triazoles.

Scheme 78: Proposed mechanism for bis-1,2,4-triazoles synthesis.

Figure 14: Thiazole containing drugs.

Scheme 79: Synthesis of a substituted thiazole ring.

Scheme 80: Synthesis of pyrazolothiazoles.

Figure 15: Chromene containing drugs.

Scheme 81: Magnetic nanocatalyst-mediated aminochromene synthesis.

Scheme 82: Proposed mechanism for the synthesis of chromenes.
Synthetic reactions driven by electron-donor–acceptor (EDA) complexes
- Zhonglie Yang,
- Yutong Liu,
- Kun Cao,
- Xiaobin Zhang,
- Hezhong Jiang and
- Jiahong Li
Beilstein J. Org. Chem. 2021, 17, 771–799, doi:10.3762/bjoc.17.67
- phenanthridine 38 (also including quinoline products, Scheme 12). In this way, a nitrogen-centered radical was given via the EDA complex that was initiated by single-electron transfer, accomplishing the synthesis of a variety of highly functionalized nitrogen-containing aromatics with excellent yield. In 2019

Graphical Abstract

Scheme 1: The electron transfer process in EDA complexes.

Scheme 2: Synthesis of benzo[b]phosphorus oxide 3 initiated by an EDA complex.

Scheme 3: Mechanism of the synthesis of quinoxaline derivative 7.

Scheme 4: Synthesis of imidazole derivative 10 initiated by an EDA complex.

Scheme 5: Synthesis of sulfamoylation product 12 initiated by an EDA complex.

Scheme 6: Mechanism of the synthesis of sulfamoylation product 12.

Scheme 7: Synthesis of indole derivative 22 initiated by an EDA complex.

Scheme 8: Synthesis of perfluoroalkylated pyrimidines 26 initiated by an EDA complex.

Scheme 9: Synthesis of phenanthridine derivative 29 initiated by an EDA complex.

Scheme 10: Synthesis of cis-tetrahydroquinoline derivative 32 initiated by an EDA complex.

Scheme 11: Mechanism of the synthesis of cis-tetrahydroquinoline derivative 32.

Scheme 12: Synthesis of phenanthridine derivative 38 initiated by an EDA complex.

Scheme 13: Synthesis of spiropyrroline derivative 40 initiated by an EDA complex.

Scheme 14: Synthesis of benzothiazole derivative 43 initiated by an EDA complex.

Scheme 15: Synthesis of perfluoroalkyl-s-triazine derivative 45 initiated by an EDA complex.

Scheme 16: Synthesis of indoline derivative 47 initiated by an EDA complex.

Scheme 17: Mechanism of the synthesis of spirocyclic indoline derivative 47.

Scheme 18: Synthesis of cyclobutane product 50 initiated by an EDA complex.

Scheme 19: Mechanism of the synthesis of spirocyclic indoline derivative 50.

Scheme 20: Synthesis of 1,3-oxazolidine compound 59 initiated by an EDA complex.

Scheme 21: Synthesis of trifluoromethylated product 61 initiated by an EDA complex.

Scheme 22: Synthesis of indole alkylation product 64 initiated by an EDA complex.

Scheme 23: Synthesis of perfluoroalkylation product 67 initiated by an EDA complex.

Scheme 24: Synthesis of hydrotrifluoromethylated product 70 initiated by an EDA complex.

Scheme 25: Synthesis of β-trifluoromethylated alkyne product 71 initiated by an EDA complex.

Scheme 26: Mechanism of the synthesis of 2-phenylthiophene derivative 74.

Scheme 27: Synthesis of allylated product 80 initiated by an EDA complex.

Scheme 28: Synthesis of trifluoromethyl-substituted alkynyl product 84 initiated by an EDA complex.

Scheme 29: Synthesis of dearomatized fluoroalkylation product 86 initiated by an EDA complex.

Scheme 30: Mechanism of the synthesis of dearomatized fluoroalkylation product 86.

Scheme 31: Synthesis of C(sp3)–H allylation product 91 initiated by an EDA complex.

Scheme 32: Synthesis of perfluoroalkylation product 93 initiated by an EDA complex.

Scheme 33: Synthesis of spirocyclic indolene derivative 95 initiated by an EDA complex.

Scheme 34: Synthesis of perfluoroalkylation product 97 initiated by an EDA complex.

Scheme 35: Synthesis of alkylated indole derivative 100 initiated by an EDA complex.

Scheme 36: Mechanism of the synthesis of alkylated indole derivative 100.

Scheme 37: Synthesis of arylated oxidized indole derivative 108 initiated by an EDA complex.

Scheme 38: Synthesis of 4-ketoaldehyde derivative 111 initiated by an EDA complex.

Scheme 39: Mechanism of the synthesis of 4-ketoaldehyde derivative 111.

Scheme 40: Synthesis of perfluoroalkylated olefin 118 initiated by an EDA complex.

Scheme 41: Synthesis of alkylation product 121 initiated by an EDA complex.

Scheme 42: Synthesis of acylation product 123 initiated by an EDA complex.

Scheme 43: Mechanism of the synthesis of acylation product 123.

Scheme 44: Synthesis of trifluoromethylation product 126 initiated by an EDA complex.

Scheme 45: Synthesis of unnatural α-amino acid 129 initiated by an EDA complex.

Scheme 46: Synthesis of thioether derivative 132 initiated by an EDA complex.

Scheme 47: Synthesis of S-aryl dithiocarbamate product 135 initiated by an EDA complex.

Scheme 48: Mechanism of the synthesis of S-aryl dithiocarbamate product 135.

Scheme 49: Synthesis of thioether product 141 initiated by an EDA complex.

Scheme 50: Mechanism of the synthesis of borate product 144.

Scheme 51: Synthesis of boronation product 148 initiated by an EDA complex.

Scheme 52: Synthesis of boration product 151 initiated by an EDA complex.

Scheme 53: Synthesis of boronic acid ester derivative 154 initiated by an EDA complex.

Scheme 54: Synthesis of β-azide product 157 initiated by an EDA complex.

Scheme 55: Decarboxylation reaction initiated by an EDA complex.

Scheme 56: Synthesis of amidated product 162 initiated by an EDA complex.

Scheme 57: Synthesis of diethyl phenylphosphonate 165 initiated by an EDA complex.

Scheme 58: Mechanism of the synthesis of diethyl phenylphosphonate derivative 165.

Scheme 59: Synthesis of (Z)-2-iodovinyl phenyl ether 168 initiated by an EDA complex.

Scheme 60: Mechanism of the synthesis of (Z)-2-iodovinyl phenyl ether derivative 168.

Scheme 61: Dehalogenation reaction initiated by an EDA complex.
Total synthesis of pyrrolo[2,3-c]quinoline alkaloid: trigonoine B
- Takashi Nishiyama,
- Erina Hamada,
- Daishi Ishii,
- Yuuto Kihara,
- Nanase Choshi,
- Natsumi Nakanishi,
- Mari Murakami,
- Kimiko Taninaka,
- Noriyuki Hatae and
- Tominari Choshi
Beilstein J. Org. Chem. 2021, 17, 730–736, doi:10.3762/bjoc.17.62
- , Yokohama University of Pharmacy, 601 Matano, Totsuka-ku, Yokohama 245-0066, Japan 10.3762/bjoc.17.62 Abstract The first total synthesis of the pyrrolo[2,3-c]quinoline alkaloid trigonoine B (1) was accomplished via a six-step sequence involving the construction of an N-substituted 4-aminopyrrolo[2,3-c
- ]quinoline framework via electrocyclization of 2-(pyrrol-3-yl)benzene containing a carbodiimide moiety as a 2-azahexatriene system. The employed six-step sequence afforded trigonoine B (1) in 9.2% overall yield. The described route could be employed for the preparation of various N-substituted 4
- -aminopyrroloquinolines with various biological activities. Keywords: 2-azahexatiriene system; carbodiimide; electrocyclization; pyrrolo[2,3-c]quinoline; trigonoine B; Introduction In 2011, two novel alkaloids, namely trigonoine A and B, were isolated from the leaves of Trigonostemon lii by Hao and co-workers [1]. The

Graphical Abstract

Figure 1: Natural products possessing the pyrrolo[2,3-c]quinoline skeleton.

Scheme 1: Total synthesis of marinoquinolines and the failure of the introduction of a tetrahydroquinoline mo...

Scheme 2: Retrosynthetic analysis of the pyrrolo[2,3-c]quinoline ring construction.

Scheme 3: Synthesis of N-substituted 4-aminopyrrolo[3,2-c]quinoline 18.

Scheme 4: Synthesis of the tetrahydroquinoline moiety through cycloamination.

Scheme 5: Synthesis of trigonoine B (1).
Deoxygenative C2-heteroarylation of quinoline N-oxides: facile access to α-triazolylquinolines
- Geetanjali S. Sontakke,
- Rahul K. Shukla and
- Chandra M. R. Volla
Beilstein J. Org. Chem. 2021, 17, 485–493, doi:10.3762/bjoc.17.42
- simplicity of the developed protocol. The current transformation was also found to be compatible for the late-stage modification of natural products. Keywords: amination; heteroarylation; quinoline N-oxides; regioselective; triazoles; Introduction Quinoline is a key heterocyclic moiety found in many
- ][37][38][39][40][41][42][43][44][45]. For example, Yin and co-workers developed a protocol for the deoxygenative C2-amination of pyridine/quinoline N-oxides using t-BuNH2 and Ts2O/TFA in 2007 (Scheme 1a) [48]. Later, Londregan and co-workers were successful in achieving C2-amination employing
- derivatives is highly desired. The continuous interest and efforts of our group for the derivatization of quinoline moieties [57] and use of N-sulfonyl-1,2,3-triazoles as heterocyclic precursors encouraged us to develop a new strategy for the regioselective C2-triazolylation of quinoline N-oxide under mild

Graphical Abstract

Figure 1: Bioactive molecules containing the 2-aminoquinoline motif.

Scheme 1: C2-selective C–N bond formation of N-oxides.

Scheme 2: Substrate scope of N-sulfonyl-1,2,3-triazoles. Reaction conditions: 1a (0.2 mmol), 2 (0.24 mmol) an...

Scheme 3: Substrate scope of quinoline N-oxides. Reaction conditions: 1 (0.2 mmol), 2a (0.24 mmol) and DCE (2...

Scheme 4: Late-stage modification of natural products.

Scheme 5: Substrate scope for the reaction of substituted triazoles with isoquinoline N-oxide.

Scheme 6: Gram-scale and one-pot synthesis.

Scheme 7: Proposed mechanism.
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- Anthony J. Fernandes,
- Armen Panossian,
- Bastien Michelet,
- Agnès Martin-Mingot,
- Frédéric R. Leroux and
- Sébastien Thibaudeau
Beilstein J. Org. Chem. 2021, 17, 343–378, doi:10.3762/bjoc.17.32

- , affording biaryl species 161. Using this strategy, several trifluoromethyl ketones 162 and alcohols 163 bearing heteroaryl substituents (i.e., benzothiazole, quinoline, isoquinoline, benzimidazole, or imidazole) prone to be protonated were elegantly converted into the corresponding alcohols 163 and biphenyl

Graphical Abstract

Figure 1: Stabilizing interaction in the CF3CH2+ carbenium ion (top) and structure of the first observable fl...

Scheme 1: Isodesmic equations accounting for the destabilizing effect of the CF3 group. ΔE in kcal⋅mol−1, cal...

Scheme 2: Stabilizing effect of fluorine atoms by resonance electron donation in carbenium ions (δ in ppm).

Scheme 3: Direct in situ NMR observation of α-(trifluoromethyl)carbenium ion or protonated alcohols. Δδ = δ19...

Scheme 4: Reported 13C NMR chemical shifts for the α-(trifluoromethyl)carbenium ion 10c (δ in ppm).

Scheme 5: Direct NMR observation of α-(trifluoromethyl)carbenium ions in situ (δ in ppm).

Scheme 6: Illustration of the ion pair solvolysis mechanism for sulfonate 13f. YOH = solvent.

Figure 2: Solvolysis rate for 13a–i and 17.

Figure 3: Structures of allyl triflates 18 and 19 and allyl brosylate 20. Bs = p-BrC6H4SO2.

Figure 4: Structure of tosylate derivatives 21.

Figure 5: a) Structure of triflate derivatives 22. b) Stereochemistry outcomes of the reaction starting from (...

Scheme 7: Solvolysis reaction of naphthalene and anthracenyl derivatives 26 and 29.

Figure 6: Structure of bisarylated derivatives 34.

Figure 7: Structure of bisarylated derivatives 36.

Scheme 8: Reactivity of 9c in the presence of a Brønsted acid.

Scheme 9: Cationic electrocyclization of 38a–c under strongly acidic conditions.

Scheme 10: Brønsted acid-catalyzed synthesis of indenes 42 and indanes 43.

Scheme 11: Reactivity of sulfurane 44 in triflic acid.

Scheme 12: Solvolysis of triflate 45f in alcoholic solvents.

Scheme 13: Synthesis of labeled 18O-52.

Scheme 14: Reactivity of sulfurane 53 in triflic acid.

Figure 8: Structure of tosylates 56 and 21f.

Scheme 15: Resonance forms in benzylic carbenium ions.

Figure 9: Structure of pyrrole derivatives 58 and 59.

Scheme 16: Resonance structure 60↔60’.

Scheme 17: Ga(OTf)3-catalyzed synthesis of 3,3’- and 3,6’-bis(indolyl)methane from trifluoromethylated 3-indol...

Scheme 18: Proposed reaction mechanism.

Scheme 19: Metal-free 1,2-phosphorylation of 3-indolylmethanols.

Scheme 20: Superacid-mediated arylation of thiophene derivatives.

Scheme 21: In situ mechanistic NMR investigations.

Scheme 22: Proposed mechanisms for the prenyltransferase-catalyzed condensation.

Scheme 23: Influence of a CF3 group on the allylic SN1- and SN2-mechanism-based reactions.

Scheme 24: Influence of the CF3 group on the condensation reaction.

Scheme 25: Solvolysis of 90 in TFE.

Scheme 26: Solvolysis of allyl triflates 94 and 97 and isomerization attempt of 96.

Scheme 27: Proposed mechanism for the formation of 95.

Scheme 28: Formation of α-(trifluoromethyl)allylcarbenium ion 100 in a superacid.

Scheme 29: Lewis acid activation of CF3-substituted allylic alcohols.

Scheme 30: Bimetallic-cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 31: Reactivity of cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 32: α-(Trifluoromethyl)propargylium ion 122↔122’ generated from silyl ether 120 in a superacid.

Scheme 33: Formation of α-(trifluoromethyl)propargylium ions from CF3-substituted propargyl alcohols.

Scheme 34: Direct NMR observation of the protonation of some trifluoromethyl ketones in situ and the correspon...

Scheme 35: Selected resonance forms in protonated fluoroketone derivatives.

Scheme 36: Acid-catalyzed Friedel–Crafts reactions of trifluoromethyl ketones 143a,b and 147a–c.

Scheme 37: Enantioselective hydroarylation of CF3-substituted ketones.

Scheme 38: Acid-catalyzed arylation of ketones 152a–c.

Scheme 39: Reactivity of 156 in a superacid.

Scheme 40: Reactivity of α-CF3-substituted heteroaromatic ketones and alcohols as well as 1,3-diketones.

Scheme 41: Reactivity of 168 with benzene in the presence of a Lewis or Brønsted acid.

Scheme 42: Acid-catalyzed three-component asymmetric reaction.

Scheme 43: Anodic oxidation of amines 178a–c and proposed mechanism.

Scheme 44: Reactivity of 179b in the presence of a strong Lewis acid.

Scheme 45: Trifluoromethylated derivatives as precursors of trifluoromethylated iminium ions.

Scheme 46: Mannich reaction with trifluoromethylated hemiaminal 189.

Scheme 47: Suitable nucleophiles reacting with 192 after Lewis acid activation.

Scheme 48: Strecker reaction involving the trifluoromethylated iminium ion 187.

Scheme 49: Reactivity of 199 toward nucleophiles.

Scheme 50: Reactivity of 204a with benzene in the presence of a Lewis acid.

Scheme 51: Reactivity of α-(trifluoromethyl)-α-chloro sulfides in the presence of strong Lewis acids.

Scheme 52: Anodic oxidation of sulfides 213a–h and Pummerer rearrangement.

Scheme 53: Mechanism for the electrochemical oxidation of the sulfide 213a.

Scheme 54: Reactivity of (trifluoromethyl)diazomethane (217a) in HSO3F.

Figure 10: a) Structure of diazoalkanes 217a–c and b) rate-limiting steps of their decomposition.

Scheme 55: Deamination reaction of racemic 221 and enantioenriched (S)-221.

Scheme 56: Deamination reaction of labeled 221-d2. Elimination products were formed in this reaction, the yiel...

Scheme 57: Deamination reaction of 225-d2. Elimination products were also formed in this reaction in undetermi...

Scheme 58: Formation of 229 from 228 via 1,2-H-shift.

Scheme 59: Deamination reaction of 230. Elimination products were formed in this reaction, the yield of which ...

Scheme 60: Deamination of several diazonium ions. Elimination products were formed in these reactions, the yie...

Scheme 61: Solvolysis reaction mechanism of alkyl tosylates.

Scheme 62: Solvolysis outcome for the tosylates 248 and 249 in HSO3FSbF5.

Figure 11: Solvolysis rate of 248, 249, 252, and 253 in 91% H2SO4.

Scheme 63: Illustration of the reaction pathways. TsCl, pyridine, −5 °C (A); 98% H2SO4, 30 °C (B); 98% H2SO4, ...

Scheme 64: Proposed solvolysis mechanism for the aliphatic tosylate 248.

Scheme 65: Solvolysis of the derivatives 259 and 260.

Scheme 66: Solvolysis of triflate 261. SOH = solvent.

Scheme 67: Intramolecular Friedel–Crafts alkylations upon the solvolysis of triflates 264 and 267.

Scheme 68: α-CF3-enhanced γ-silyl elimination of cyclobutyltosylates 270a,b.

Scheme 69: γ-Silyl elimination in the synthesis of a large variety of CF3-substituted cyclopropanes. Pf = pent...

Scheme 70: Synthetic pathways to 281. aNMR yields.

Scheme 71: The cyclopropyl-substituted homoallylcyclobutylcarbenium ion manifold.

Scheme 72: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 287a–c. LG = leaving group.

Scheme 73: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 291a–c.

Scheme 74: Superacid-promoted dimerization or TFP.

Scheme 75: Reactivity of TFP in a superacid.

Scheme 76: gem-Difluorination of α-fluoroalkyl styrenes via the formation of a “hidden” α-RF-substituted carbe...

Scheme 77: Solvolysis of CF3-substituted pentyne 307.

Scheme 78: Photochemical rearrangement of 313.

Figure 12: Structure of 2-norbornylcarbenium ion 318 and argued model for the stabilization of this cation.

Figure 13: Structures and solvolysis rate (TFE, 25 °C) of the sulfonates 319–321. Mos = p-MeOC6H4SO2.

Scheme 79: Mechanism for the solvolysis of 323. SOH = solvent.

Scheme 80: Products formed by the hydrolysis of 328.

Scheme 81: Proposed carbenium ion intermediates in an equilibrium during the solvolysis of tosylates 328, 333,...
Decarboxylative trifluoromethylthiolation of pyridylacetates
- Ryouta Kawanishi,
- Kosuke Nakada and
- Kazutaka Shibatomi
Beilstein J. Org. Chem. 2021, 17, 229–233, doi:10.3762/bjoc.17.23
- gave the desired products 2e–g in moderate yields. The method could also be applied to substrates with quinoline and isoquinoline backbones to afford the corresponding products 2h and 2i. In addition, the reaction of α-monosubstituted 2-pyridylacetate 8 was performed to yield the corresponding mono

Graphical Abstract

Scheme 1: Electrophilic decarboxylative functionalization of 2-pyridylacetates.

Scheme 2: One-pot procedure for the synthesis of 2a.

Scheme 3: Substrate scope. aSaponification was carried out with 2.5 equiv of LiOH, and 2.5 equiv of 6 was use...

Scheme 4: Reaction of α-monosubstituted 2-pyridylacetates.

Scheme 5: Proposed reaction pathway.

Scheme 6: Reaction of 3- and 4-pyridylacetates.
Synthesis of imidazo[1,5-a]pyridines via cyclocondensation of 2-(aminomethyl)pyridines with electrophilically activated nitroalkanes
- Dmitrii A. Aksenov,
- Nikolai A. Arutiunov,
- Vladimir V. Maliuga,
- Alexander V. Aksenov and
- Michael Rubin
Beilstein J. Org. Chem. 2020, 16, 2903–2910, doi:10.3762/bjoc.16.239
- -(bromomethyl)quinoline (21c) was prepared from commercially available 6-bromo-2-methylquinoline (22c, via radical bromination in the presence of NBS and dibenzoyl peroxide [51]. To this end, the methylquinoline 22c (3.33 g, 15 mmol) was dissolved in carbon tetrachloride (30 mL), and N-bromosuccinimide (2.94 g
- was concentrated in vacuum. The crude product was purified by flash column chromatography eluting with EtOAc/petroleum ether 1:6. Then, the amine was afforded via a Gabriel synthesis using a modified protocol described in the literature [52]. To a stirred solution of 6-bromo-2-(bromomethyl)quinoline
- , 118.5, 113.5; ATR-FTIR (ZnSe) νmax: 3066, 1749, 1676, 1603, 1473, 1458, 1433, 1358, 1305, 1251, 1267, 1120 cm−1; HRESIMS (TOF) m/z: [M + H]+ calcd for C13H11N2, 195.0917; found, 195.0918. 6-Bromo-7-methoxyimidazo[1,5-a]quinoline (19de) The title compound was obtained via the typical procedure 2

Graphical Abstract

Figure 1: Biologically active imidazo[1,5-a]pyridines.

Scheme 1: Activation of nitroalkanes towards nucleophilic attack by amines.

Scheme 2: Mechanistic rationale.

Scheme 3: Reaction of the N-tosylate 17 with electrophilic nitroalkanes.

Scheme 4: Reaction of 2-(aminomethyl)pyridine (12) with electrophilic nitroalkanes.

Scheme 5: Reaction of the 2-(aminomethyl)quinolines 18 with electrophilic nitroalkanes.

Scheme 6: Reactivity of α-nitroacetophenone (1h) and α-nitroacetic ester (1i).
Synthesis and characterization of S,N-heterotetracenes
- Astrid Vogt,
- Florian Henne,
- Christoph Wetzel,
- Elena Mena-Osteritz and
- Peter Bäuerle
Beilstein J. Org. Chem. 2020, 16, 2636–2644, doi:10.3762/bjoc.16.214
- -substituted thienopyrrole 26, saponification to carbonic acid 27, and subsequent Cu-mediated decarboxylation in quinoline resulted in thienopyrrole 28 in more than 80% yield over three steps. Lithiation of 28 with n-BuLi and reaction with TIPS chloride selectively occurred at the thiophene α-position to give

Graphical Abstract

Figure 1: Heteroacenes: tetrathienoacene (TTA), S,N-heteroacenes SN4, SN4', and SN4''.

Scheme 1: Synthesis of fused S,N-heterotetracene SN4 9 starting from thieno[3,2-b]thiophene (1).

Scheme 2: Synthesis of parent H-SN4 13 via the azide route.

Scheme 3: Synthesis of tetracyclic H-SN4 13 via the Cadogan route.

Scheme 4: Synthesis of tetracyclic indole derivative 19 via the Cadogan route.

Scheme 5: Synthesis of hexacyclic heteroacene SN4' 22 via the Cadogan route.

Scheme 6: Synthesis of heterotetracene SN4'' 33 via the azide and Buchwald–Hartwig amination route.

Figure 2: UV–vis absorption spectra of TTA, Hex-SN4 9, Pr-SN4'' 33 and fluorescence spectrum of 33 in THF at ...

Figure 3: Energy diagram of the frontier molecular orbitals of heterotetracenes TTA, 9, 13, 19, 22, and 33, a...
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Copper-based fluorinated reagents for the synthesis of CF2R-containing molecules (R ≠ F)
- Louise Ruyet and
- Tatiana Besset
Beilstein J. Org. Chem. 2020, 16, 1051–1065, doi:10.3762/bjoc.16.92

- ). The reaction was efficient (65 examples, up to 97% yield), showed a good functional group tolerance (i.e., cyano, ester, ketone) and even heteroarenes such as pyridine, quinoline and quinoxaline were functionalized with the four fluorinated moieties (Scheme 17). In situ-generated copper-based

Graphical Abstract

Scheme 1: Synthesis of the first isolable (NHC)CuCF2H complexes from TMSCF2H and their application for the sy...

Scheme 2: Pioneer works for the in situ generation of CuCF2H from TMSCF2H and from n-Bu3SnCF2H. Phen = 1,10-p...

Scheme 3: A Sandmeyer-type difluoromethylation reaction via the in situ generation of CuCF2H from TMSCF2H. a ...

Scheme 4: A one pot, two-step sequence for the difluoromethylthiolation of various classes of compounds via t...

Scheme 5: A copper-mediated oxidative difluoromethylation of terminal alkynes via the in situ generation of a...

Scheme 6: A copper-mediated oxidative difluoromethylation of heteroarenes.

Scheme 7: Synthesis of difluoromethylphosphonate-containing molecules using the in situ-generated CuCF2PO(OEt)...

Scheme 8: Synthesis of difluoromethylphosphonate-containing molecules using in situ-generated CuCF2PO(OEt)2 s...

Scheme 9: Synthesis of difluoromethylphosphonate-containing molecules using in situ-generated CuCF2PO(OEt)2 s...

Scheme 10: Synthesis of (diethylphosphono)difluoromethylthiolated molecules using in situ-generated CuCF2PO(OE...

Scheme 11: Access to (diethylphosphono)difluoromethylthiolated molecules via the in situ generation of CuCF2PO...

Scheme 12: Synthesis of (phenylsulfonyl)difluoromethyl-containing molecules via the in situ generation of CuCF2...

Scheme 13: Copper-mediated 1,1-difluoroethylation of diaryliodonium salts by using the in situ-generated CuCF2...

Scheme 14: Pioneer works for the pentafluoroethylation and heptafluoropropylation using a copper-based reagent...

Scheme 15: Pentafluoroethylation of (hetero)aryl bromides using the (Phen)CuCF2CF3 complex. 19F NMR yields wer...

Scheme 16: Synthesis of pentafluoroethyl ketones using the (Ph3P)Cu(phen)CF2CF3 reagent. 19F NMR yields were g...

Scheme 17: Synthesis of (Phen)2Cu(O2CCF2RF) and functionalization of (hetero)aryl iodides.

Scheme 18: Pentafluoroethylation of arylboronic acids and (hetero)aryl bromides via the in situ-generated CuCF2...

Scheme 19: In situ generation of CuCF2CF3 species from a cyclic-protected hexafluoroacetone and KCu(Ot-Bu)2. 19...

Scheme 20: Pentafluoroethylation of bromo- and iodoalkenes. Only examples of isolated compounds were depicted.

Scheme 21: Fluoroalkylation of aryl halides via a RCF2CF2Cu species.

Scheme 22: Synthesis of perfluoroorganolithium copper species or perfluroalkylcopper derivatives from iodoperf...

Scheme 23: Formation of the PhenCuCF2CF3 reagent by means of TFE and pentafluoroethylation of iodoarenes and a...

Scheme 24: Generation of a CuCF2CF3 reagent from TMSCF3 and applications.
Fluorinated phenylalanines: synthesis and pharmaceutical applications
- Laila F. Awad and
- Mohammed Salah Ayoup
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- other hand, when the quinoline-based ligand 162 was used, it was shown to promote the palladium-catalyzed direct electrophilic fluorination of β-methylene C(sp3)–H bonds. Thus, fluorinations of ʟ-phenylalanine 4-trifluoromethylphenylamides 161a–l carrying a range of functional groups such as fluoro
- -fluorinated Phe derivatives using quinoline-based ligand 162 in the Pd-catalyzed direct fluorination of β-methylene C(sp3)–H bonds. Synthesis of β,β-difluorophenylalanine derivatives from 2,2-difluoroacetaldehyde derivatives 164a,b. Synthesis of β,β-difluorophenylalanine derivatives via an imine chiral

Graphical Abstract

Figure 1: Categories I–V of fluorinated phenylalanines.

Scheme 1: Synthesis of fluorinated phenylalanines via Jackson’s method.

Scheme 2: Synthesis of all-cis-tetrafluorocyclohexylphenylalanines.

Scheme 3: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine (nPt: neopentyl, TCE: trichloroethyl).

Scheme 4: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine derivatives 17.

Scheme 5: Synthesis of fluorinated Phe analogues from Cbz-protected aminomalonates.

Scheme 6: Synthesis of tetrafluorophenylalanine analogues via the 3-methyl-4-imidazolidinone auxiliary 25.

Scheme 7: Synthesis of tetrafluoro-Phe derivatives via chiral auxiliary 31.

Scheme 8: Synthesis of 2,5-difluoro-Phe and 2,4,5-trifluoro-Phe via Schöllkopf reagent 34.

Scheme 9: Synthesis of 2-fluoro- and 2,6-difluoro Fmoc-Phe derivatives starting from chiral auxiliary 39.

Scheme 10: Synthesis of 2-[18F]FPhe via chiral auxiliary 43.

Scheme 11: Synthesis of FPhe 49a via photooxidative cyanation.

Scheme 12: Synthesis of FPhe derivatives via Erlenmeyer azalactone synthesis.

Scheme 13: Synthesis of (R)- and (S)-2,5-difluoro Phe via the azalactone method.

Scheme 14: Synthesis of 3-bromo-4-fluoro-(S)-Phe (65).

Scheme 15: Synthesis of [18F]FPhe via radiofluorination of phenylalanine with [18F]F2 or [18F]AcOF.

Scheme 16: Synthesis of 4-borono-2-[18F]FPhe.

Scheme 17: Synthesis of protected 4-[18F]FPhe via arylstannane derivatives.

Scheme 18: Synthesis of FPhe derivatives via intermediate imine formation.

Scheme 19: Synthesis of FPhe derivatives via Knoevenagel condensation.

Scheme 20: Synthesis of FPhe derivatives 88a,b from aspartic acid derivatives.

Scheme 21: Synthesis of 2-(2-fluoroethyl)phenylalanine derivatives 93 and 95.

Scheme 22: Synthesis of FPhe derivatives via Zn2+ complexes.

Scheme 23: Synthesis of FPhe derivatives via Ni2+ complexes.

Scheme 24: Synthesis of 3,4,5-trifluorophenylalanine hydrochloride (109).

Scheme 25: Synthesis of FPhe derivatives via phenylalanine aminomutase (PAM).

Scheme 26: Synthesis of (R)-2,5-difluorophenylalanine 115.

Scheme 27: Synthesis of β-fluorophenylalanine via 2-amino-1,3-diol derivatives.

Scheme 28: Synthesis of β-fluorophenylalanine derivatives via the oxazolidinone chiral auxiliary 122.

Scheme 29: Synthesis of β-fluorophenylalanine from pyruvate hemiketal 130.

Scheme 30: Synthesis of β-fluorophenylalanine (136) via fluorination of β-hydroxyphenylalanine (137).

Scheme 31: Synthesis of β-fluorophenylalanine from aziridine derivatives.

Scheme 32: Synthesis of β-fluorophenylalanine 136 via direct fluorination of pyruvate esters.

Scheme 33: Synthesis of β-fluorophenylalanine via fluorination of ethyl 3-phenylpyruvate enol using DAST.

Scheme 34: Synthesis of β-fluorophenylalanine derivatives using photosensitizer TCB.

Scheme 35: Synthesis of β-fluorophenylalanine derivatives using Selectflour and dibenzosuberenone.

Scheme 36: Synthesis of protected β-fluorophenylalanine via aziridinium intermediate 150.

Scheme 37: Synthesis of β-fluorophenylalanine derivatives via fluorination of α-hydroxy-β-aminophenylalanine d...

Scheme 38: Synthesis of β-fluorophenylalanine derivatives from α- or β-hydroxy esters 152a and 155.

Scheme 39: Synthesis of a series of β-fluoro-Phe derivatives via Pd-catalyzed direct fluorination of β-methyle...

Scheme 40: Synthesis of series of β-fluorinated Phe derivatives using quinoline-based ligand 162 in the Pd-cat...

Scheme 41: Synthesis of β,β-difluorophenylalanine derivatives from 2,2-difluoroacetaldehyde derivatives 164a,b....

Scheme 42: Synthesis of β,β-difluorophenylalanine derivatives via an imine chiral auxiliary.

Scheme 43: Synthesis of α-fluorophenylalanine derivatives via direct fluorination of protected Phe 174.

Figure 2: Structures of PET radiotracers of 18FPhe derivatives.

Figure 3: Structures of melfufen (179) and melphalan (180) anticancer drugs.

Figure 4: Structure of gastrazole (JB95008, 181), a CCK2 receptor antagonist.

Figure 5: Dual CCK1/CCK2 antagonist 182.

Figure 6: Structure of sitagliptin (183), an antidiabetic drug.

Figure 7: Structure of retaglpitin (184) and antidiabetic drug.

Figure 8: Structure of evogliptin (185), an antidiabetic drug.

Figure 9: Structure of LY2497282 (186) a DPP-4 inhibitor for the treatment of type II diabetes.

Figure 10: Structure of ulimorelin (187).

Figure 11: Structure of GLP1R (188).

Figure 12: Structures of Nav1.7 blockers 189 and 190.
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
- Valerian Dragutan,
- Ileana Dragutan,
- Albert Demonceau and
- Lionel Delaude
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- aminohydroxylation. For this purpose, they first prepared the properly functionalized (–)-quinines from quinoline derivatives in six steps. These chiral intermediates were then submitted to an enyne metathesis reaction with the Grubbs first-generation Ru catalyst (10 mol %), under an ethylene atmosphere, to generate

Graphical Abstract

Scheme 1: Intramolecular (A) and intermolecular (B) enyne metathesis reactions.

Scheme 2: Ene–yne and yne–ene mechanisms for intramolecular enyne metathesis reactions.

Scheme 3: Metallacarbene mechanism in intermolecular enyne metathesis.

Scheme 4: The Oguri strategy for accessing artemisinin analogs 1a–c through enyne metathesis.

Scheme 5: Access to the tetracyclic core of nanolobatolide (2) via tandem enyne metathesis followed by an Eu(...

Scheme 6: Synthesis of (−)-amphidinolide E (3) using an intermolecular enyne metathesis as the key step.

Scheme 7: Synthesis of amphidinolide K (4) by an enyne metathesis route.

Scheme 8: Trost synthesis of des-epoxy-amphidinolide N (5) [72].

Scheme 9: Enyne metathesis between the propargylic derivative and the allylic alcohol in the synthesis of the...

Scheme 10: Synthetic route to amphidinolide N (6a).

Scheme 11: Synthesis of the stereoisomeric precursors of amphidinolide V (7a and 7b) through alkyne ring-closi...

Scheme 12: Synthesis of the anthramycin precursor 8 from ʟ-methionine by a tandem enyne metathesis–cross metat...

Scheme 13: Synthesis of (−)‐clavukerin A (9) and (−)‐isoclavukerin A (10) by an enyne metathesis route startin...

Scheme 14: Synthesis of (−)-isoguaiene (11) through an enyne metathesis as the key step.

Scheme 15: Synthesis of erogorgiaene (12) by a tandem enyne metathesis/cross metathesis sequence using the sec...

Scheme 16: Synthesis of (−)-galanthamine (13) from isovanilin by an enyne metathesis.

Scheme 17: Application of enyne metathesis for the synthesis of kempene diterpenes 14a–c.

Scheme 18: Synthesis of the alkaloid (+)-lycoflexine (15) through enyne metathesis.

Scheme 19: Synthesis of the AB subunits of manzamine A (16a) and E (16b) by enyne metathesis.

Scheme 20: Jung's synthesis of rhodexin A (17) by enyne metathesis/cross metathesis reactions.

Scheme 21: Total synthesis of (−)-flueggine A (18) and (+)-virosaine B (19) from Weinreb amide by enyne metath...

Scheme 22: Access to virgidivarine (20) and virgiboidine (21) by an enyne metathesis route.

Scheme 23: Enyne metathesis approach to (−)-zenkequinone B (22).

Scheme 24: Access to C-aryl glycoside 23 by an intermolecular enyne metathesis/Diels–Alder cycloaddition.

Scheme 25: Synthesis of spiro-C-aryl glycoside 24 by a tandem intramolecular enyne metathesis/Diels–Alder reac...

Scheme 26: Pathways to (−)-exiguolide (25) by Trost’s Ru-catalyzed enyne cross-coupling and cross-metathesis [94].
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
- Pezhman Shiri and
- Jasem Aboonajmi
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52

Graphical Abstract

Scheme 1: Chemical structure of the catalysts 1a and 1b and their catalytic application in CuAAC reactions.

Scheme 2: Synthetic route to the catalyst 11 and its catalytic application in CuAAC reactions.

Scheme 3: Synthetic route of dendrons, illustrated using G2-AMP 23.

Scheme 4: The catalytic application of CuYAu–Gx-AAA–SBA-15 in a CuAAC reaction.

Scheme 5: Synthetic route to the catalyst 36.

Scheme 6: Application of the catalyst 36 in CuAAC reactions.

Scheme 7: The synthetic route to the catalyst 45 and catalytic application of 45 in “click” reactions.

Scheme 8: Synthetic route to the catalyst 48 and catalytic application of 48 in “click” reactions.

Scheme 9: Synthetic route to the catalyst 58 and catalytic application of 58 in “click” reactions.

Scheme 10: Synthetic route to the catalyst 64 and catalytic application of 64 in “click” reactions.

Scheme 11: Chemical structure of the catalyst 68 and catalytic application of 68 in “click” reactions.

Scheme 12: Chemical structure of the catalyst 69 and catalytic application of 69 in “click” reactions.

Scheme 13: Synthetic route to, and chemical structure of the catalyst 74.

Scheme 14: Application of the cayalyst 74 in “click” reactions.

Scheme 15: Synthetic route to, and chemical structure of the catalyst 78 and catalytic application of 78 in “c...

Scheme 16: Synthetic route to the catalyst 85.

Scheme 17: Application of the catalyst 85 in “click” reactions.

Scheme 18: Synthetic route to the catalyst 87 and catalytic application of 87 in “click” reactions.

Scheme 19: Chemical structure of the catalyst 88 and catalytic application of 88 in “click” reactions.

Scheme 20: Synthetic route to the catalyst 90 and catalytic application of 90 in “click” reactions.

Scheme 21: Synthetic route to the catalyst 96 and catalytic application of 96 in “click” reactions.

Scheme 22: Synthetic route to the catalyst 100 and catalytic application of 100 in “click” reactions.

Scheme 23: Synthetic route to the catalyst 102 and catalytic application of 23 in “click” reactions.

Scheme 24: Synthetic route to the catalysts 108–111.

Scheme 25: Catalytic application of 108–111 in “click” reactions.

Scheme 26: Synthetic route to the catalyst 121 and catalytic application of 121 in “click” reactions.

Scheme 27: Synthetic route to 125 and application of 125 in “click” reactions.

Scheme 28: Synthetic route to the catalyst 131 and catalytic application of 131 in “click” reactions.

Scheme 29: Synthetic route to the catalyst 136.

Scheme 30: Application of the catalyst 136 in “click” reactions.

Scheme 31: Synthetic route to the catalyst 141 and catalytic application of 141 in “click” reactions.

Scheme 32: Synthetic route to the catalyst 144 and catalytic application of 144 in “click” reactions.

Scheme 33: Synthetic route to the catalyst 149 and catalytic application of 149 in “click” reactions.

Scheme 34: Synthetic route to the catalyst 153 and catalytic application of 153 in “click” reactions.

Scheme 35: Synthetic route to the catalyst 155 and catalytic application of 155 in “click” reactions.

Scheme 36: Synthetic route to the catalyst 157 and catalytic application of 157 in “click” reactions.

Scheme 37: Synthetic route to the catalyst 162.

Scheme 38: Application of the catalyst 162 in “click” reactions.

Scheme 39: Synthetic route to the catalyst 167 and catalytic application of 167 in “click” reactions.

Scheme 40: Synthetic route to the catalyst 169 and catalytic application of 169 in “click” reactions.

Scheme 41: Synthetic route to the catalyst 172.

Scheme 42: Application of the catalyst 172 in “click” reactions.
Recent advances in photocatalyzed reactions using well-defined copper(I) complexes
- Mingbing Zhong,
- Xavier Pannecoucke,
- Philippe Jubault and
- Thomas Poisson
Beilstein J. Org. Chem. 2020, 16, 451–481, doi:10.3762/bjoc.16.42
- -a]pyrrolo[3,4-c]quinoline derivatives were obtained for the first time. This transformation starts with the oxidation of the excited photocatalyst with O2. The aniline is then oxidized into an N-centered radical cation, which further gives the α-amino radical. The latter reacts with the maleimide to

Graphical Abstract

Scheme 1: [Cu(I)(dap)2]Cl-catalyzed ATRA reaction under green light irradiation.

Scheme 2: Photocatalytic allylation of α-haloketones.

Scheme 3: [Cu(I)(dap)2]Cl-photocatalyzed chlorosulfonylation and chlorotrifluoromethylation of alkenes.

Scheme 4: Photocatalytic perfluoroalkylchlorination of electron-deficient alkenes using the Sauvage catalyst.

Scheme 5: Photocatalytic synthesis of fluorinated sultones.

Scheme 6: Photocatalyzed haloperfluoroalkylation of alkenes and alkynes.

Scheme 7: Chlorosulfonylation of alkenes catalyzed by [Cu(I)(dap)2]Cl. aNo Na2CO3 was added. b1 equiv of Na2CO...

Scheme 8: Copper-photocatalyzed reductive allylation of diaryliodonium salts.

Scheme 9: Copper-photocatalyzed azidomethoxylation of olefins.

Scheme 10: Benzylic azidation initiated by [Cu(I)(dap)2]Cl.

Scheme 11: Trifluoromethyl methoxylation of styryl derivatives using [Cu(I)(dap)2]PF6. All redox potentials ar...

Scheme 12: Trifluoromethylation of silyl enol ethers.

Scheme 13: Synthesis of annulated heterocycles upon oxidation with the Sauvage catalyst.

Scheme 14: Oxoazidation of styrene derivatives using [Cu(dap)2]Cl as a precatalyst.

Scheme 15: [Cu(I)(dpp)(binc)]PF6-catalyzed ATRA reaction.

Scheme 16: Allylation reaction of α-bromomalonate catalyzed by [Cu(I)(dpp)(binc)]PF6 following an ATRA mechani...

Scheme 17: Bromo/tribromomethylation reaction using [Cu(I)(dmp)(BINAP)]PF6.

Scheme 18: Chlorotrifluoromethylation of alkenes catalyzed by [Cu(I)(N^N)(xantphos)]PF6.

Scheme 19: Chlorosulfonylation of styrene and alkyne derivatives by ATRA reactions.

Scheme 20: Reduction of aryl and alkyl halides with the complex [Cu(I)(bcp)(DPEPhos)]PF6. aIrradiation was car...

Scheme 21: Meerwein arylation of electron-rich aromatic derivatives and 5-exo-trig cyclization catalyzed by th...

Scheme 22: [Cu(I)(bcp)(DPEPhos)]PF6-photocatalyzed synthesis of alkaloids. aYield over two steps (cyclization ...

Scheme 23: Copper-photocatalyzed decarboxylative amination of NHP esters.

Scheme 24: Photocatalytic decarboxylative alkynylation using [Cu(I)(dq)(binap)]BF4.

Scheme 25: Copper-photocatalyzed alkylation of glycine esters.

Scheme 26: Copper-photocatalyzed borylation of organic halides. aUnder continuous flow conditions.

Scheme 27: Copper-photocatalyzed α-functionalization of alcohols with glycine ester derivatives.

Scheme 28: δ-Functionalization of alcohols using [Cu(I)(dmp)(xantphos)]BF4.

Scheme 29: Photocatalytic synthesis of [5]helicene and phenanthrene.

Scheme 30: Oxidative carbazole synthesis using in situ-formed [Cu(I)(dmp)(xantphos)]BF4.

Scheme 31: Copper-photocatalyzed functionalization of N-aryl tetrahydroisoquinolines.

Scheme 32: Bicyclic lactone synthesis using a copper-photocatalyzed PCET reaction.

Scheme 33: Photocatalytic Pinacol coupling reaction catalyzed by [Cu(I)(pypzs)(BINAP)]BF4. The ligands of the ...

Scheme 34: Azide photosensitization using a Cu-based photocatalyst.
Architecture and synthesis of P,N-heterocyclic phosphine ligands
- Wisdom A. Munzeiwa,
- Bernard Omondi and
- Vincent O. Nyamori
Beilstein J. Org. Chem. 2020, 16, 362–383, doi:10.3762/bjoc.16.35

- -quinolyhydrazine (112) at a molar ratio of 2:1 in the presence of a base gave compound 113 in very good yield. Heating of compound 113 in toluene induced isomerization where the pendant arm is shifted to the quinoline ring via a P–C migration to obtain compound 114. The corresponding P–N and P–P rearrangements

Graphical Abstract

Scheme 1: Synthesis of pyridylphosphine ligands.

Figure 1: Pyridylphosphine ligands.

Scheme 2: Synthesis of piperidyl- and oxazinylphosphine ligands.

Scheme 3: Synthesis of linear multi-chelate pyridylphosphine ligands.

Scheme 4: Synthesis of chiral acetal pyridylphosphine ligands.

Scheme 5: Synthesis of diphenylphosphine-substituted triazine ligands.

Scheme 6: Synthesis of (pyridine-2-ylmethyl)phosphine ligands.

Scheme 7: Synthesis of diphosphine pyrrole ligands.

Scheme 8: Synthesis of 4,5-diazafluorenylphosphine ligands.

Scheme 9: Synthesis of thioether-containing pyridyldiphosphine ligands starting from ethylene sulfide and dip...

Scheme 10: Synthesis of monoterpene-derived phosphine pyridine ligands.

Scheme 11: Synthesis of N-phenylphosphine-substituted imidazole ligands.

Scheme 12: Synthesis of triazol-4-ylphosphine ligands.

Scheme 13: Synthesis of phosphanyltriazolopyridines and product selectivity depending on the substituents’ eff...

Scheme 14: Synthesis of PTA-phosphine ligands.

Scheme 15: Synthesis of isomeric phosphine dipyrazole ligands by varying the reaction temperature.

Scheme 16: Synthesis of N-tethered phosphine imidazolium ligands (route A) and diphosphine imidazolium ligands...

Scheme 17: Synthesis of {1-[2-(pyridin-2-yl)- (R = CH) and {1-[2-(pyrazin-2-yl)quinazolin-4-yl]naphthalen-2-yl...

Scheme 18: Synthesis of oxazolylindolylphosphine ligands 102.

Scheme 19: Synthesis of pyrrolylphosphine ligands.

Scheme 20: Synthesis of phosphine guanidinium ligands.

Scheme 21: Synthesis of a polydentate aminophosphine ligand.

Scheme 22: Synthesis of quinolylphosphine ligands.

Scheme 23: Synthesis of N-(triazolylmethyl)phosphanamine ligands.

Figure 2: Triazolylphosphanamine ligands synthesized by Wassenaar’s method [22].

Scheme 24: Synthesis of oxazaphosphorines.

Scheme 25: Synthesis of paracyclophane pyridylphosphine ligands.

Scheme 26: Synthesis of triazolylphosphine ligands.

Figure 3: Click-phosphine ligands.

Scheme 27: Ferrocenyl pyridylphosphine imine ligands.

Scheme 28: Synthesis of phosphinooxazolines (PHOX).

Scheme 29: Synthesis of ferrocenylphosphine oxazoles.
Experimental and computational electrochemistry of quinazolinespirohexadienone molecular switches – differential electrochromic vs photochromic behavior
- Eric W. Webb,
- Jonathan P. Moerdyk,
- Kyndra B. Sluiter,
- Benjamin J. Pollock,
- Amy L. Speelman,
- Eugene J. Lynch,
- William F. Polik and
- Jason G. Gillmore
Beilstein J. Org. Chem. 2019, 15, 2473–2485, doi:10.3762/bjoc.15.240
- photochemistry of two closely related and more reducible quinazolinespirohexadienones (QSHDs), wherein the naphthalene of the PSHD is replaced with a quinoline. In the present work, we report our investigation of the electrochemistry of these asymmetric QSHDs. In addition to the short wavelength and photochromic
- substrates [11][12][13][14][15], or vinylcarbazole or alkoxystyrene derivatives for radical cation cylcloaddition and polymerization reactions [16][17][18][19][20]). We thus proposed the replacement of the naphthalene in 1a with a more electron-deficient quinoline ring. Due to the saturated spirocyclic
- carbon insulating the dienone electrophore from the quinoline moiety in the SW form, we expected minimal change in the SW reduction potential relative to the PSHDs, but a significant difference for the completely conjugated LW isomer(s). Previously we reported the detailed synthesis of two novel

Graphical Abstract

Scheme 1: PSHD photochromism [10].

Figure 1: Proposed gating of sensitivity to photoinduced charge transfer by a photochromic photooxidant in wh...

Scheme 2: QSHD photochromism [21].

Figure 2: Cyclic voltammograms of a) 1b before irradiation or electrolysis (solid blue), b) 1b/2b after 25 sc...

Figure 3: Cyclic voltammograms of a) 3b (with trace 5b) before irradiation or electrolysis (solid blue), b) 3b...

Figure 4: Cyclic voltammograms of a) 3a before irradiation or electrolysis (solid blue), b) 3a + 5a after 25 ...

Figure 5: 1H NMR distinction between SW 3a, thermal/eLW 5a, and pLW 4a, in acetone-d6, as observed a) before ...

Figure 6: HOMO (MO 105, red and blue) and LUMO (MO 106, green and yellow) computed for 3a in its ground (S0) ...

Scheme 3: Proposed mechanism for differential formation of pLW (4) and eLW (5) from SW (3).

Figure 7: Frontier orbital occupancies of relevant electronic states of 3a. Note: the photochemical excited s...
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
- Zhen Cao,
- Aline Lacoudre,
- Cybille Rossy and
- Brigitte Bibal
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
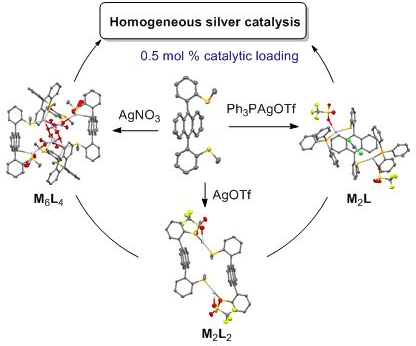
- /cycloisomerization was previously described in high yields (>95%) using 5 mol % catalyst loadings starting from 2-(alkynyl)quinoline-3-carbaldehyde [60][61] with AgOTf catalyst and starting from 2-alkynylbenzaldehyde derivatives [62] in the presence of a macrocyclic pyridine-tetraaza complex of Ag(I) as a catalyst

Graphical Abstract

Scheme 1: Synthesis of ligand 1, as its syn-atropisomer.

Figure 1: X-ray structures of complex 1a, as two diastereoisomeric macrocycles (R,S-1)2·(AgOTf)2 with ligands...

Figure 2: X-ray structure of complex 1c, as a (R,S-1)4·(AgNO3)6 cage with three nitrate anions as coordinatin...

Figure 3: X-ray structure of complex 1d, as a racemic mixture of (R,R)- and (S,S)-(syn-1)·(PPh3AgOTf)2.

Figure 4: Variable temperature 1H NMR of complex 1a in CDCl3 (7 mM) from −30 °C to 60 °C.
Cyclopropanation–ring expansion of 3-chloroindoles with α-halodiazoacetates: novel synthesis of 4-quinolone-3-carboxylic acid and norfloxacin
- Sara Peeters,
- Linn Neerbye Berntsen,
- Pål Rongved and
- Tore Bonge-Hansen
Beilstein J. Org. Chem. 2019, 15, 2156–2160, doi:10.3762/bjoc.15.212
- : catalysis; cyclopropanation; indole; norfloxacin quinoline; quinolone; Rh(II); ring expansion; Introduction The development and use of metal carbenes occupy a central part in the field of the C–H functionalization [1]. Among the metal carbenes, the transient Rh carbenes, usually made by Rh-catalyzed
- . These halo-acceptor carbenoids undergo cyclopropanation of N–H indoles with high selectivity, and only traces of C–H or N–H insertion products were observed. The yield of ethyl quinoline-3-carboxylate is dependent on the halogen in X-EDA (Cl: 90%, Br: 84%, I: 70%). The reaction works well for
- substituted indoles with R-groups in positions 3–7. When R at position 8 = Cl, the reaction was sluggish, and when R at position 2 = Me there was no quinoline formed. The overall transformation is formally a cyclopropanation of the indole C2–C3 double bond followed by a spontaneous ring expansion of the

Graphical Abstract

Scheme 1: The effect of indole substituents on the yields of ethyl quinoline-3-carboxylates [15]. Green = good, o...

Figure 1: Quinolone 3-carboxylate scaffold, norfloxacin (1) and ciprofloxacin (2).

Scheme 2: Retrosynthetic outline for the synthesis of quinolone-3-carboxlates from indole derivatives.

Scheme 3: Synthesis of ethyl 4-quinolone-3-carboxylate (6) and proposed mechanism. a: Rh2(esp)2 (1 mol %), CH2...

Scheme 4: Synthesis of norfloxacin. a: Cl-EDA (1.3 equiv), Rh2(esp)2 (1 mol %), toluene, rt, Cs2CO3, 75%. b: ...
Regioselective Pd-catalyzed direct C1- and C2-arylations of lilolidine for the access to 5,6-dihydropyrrolo[3,2,1-ij]quinoline derivatives
- Hai-Yun Huang,
- Haoran Li,
- Thierry Roisnel,
- Jean-François Soulé and
- Henri Doucet
Beilstein J. Org. Chem. 2019, 15, 2069–2075, doi:10.3762/bjoc.15.204

- ; Introduction Lilolidine (Figure 1, left), which is a commercially available compound, contains a 5,6-dihydropyrrolo[3,2,1-ij]quinoline skeleton found in several bioactive molecules. For example, tivantinib (Figure 1, middle) exhibits MET inhibitor properties [1]; whereas tarazepide (Figure 1, right) is being
- of β-arylated 5,6-dihydropyrrolo[3,2,1-ij]quinoline derivatives [6] (Scheme 1a). Three α-arylated 5,6-dihydropyrrolo[3,2,1-ij]quinoline derivatives have been prepared by Pal et al. via the cyclization of 8-arylethynyl-1,2,3,4-tetrahydroquinolines [9] (Scheme 1b). The best results were obtained using
- 38% yield; whereas the use of 2-chlorobenzonitrile afforded 12 in 62% regioselectivity and in 34% yield. In both cases, partial conversions of the aryl chlorides were observed. Pyridines and quinoline heterocycles are very important structures in pharmaceutical chemistry as more than 100 currently

Graphical Abstract

Figure 1: Structures of lilolidine, tivantinib and tarazepide.

Scheme 1: Access to α- and β-arylated lilolidine derivatives.

Scheme 2: Synthesis of α-arylated lilolidine derivatives.

Scheme 3: Synthesis of α,β-di(hetero)arylated lilolidine derivatives.

Scheme 4: Synthesis of α,β-diarylated lilolidine derivatives via successive direct arylations.

Scheme 5: Synthesis of 5,6-dihydrodibenzo[a,c]pyrido[3,2,1-jk]carbazoles via successive direct arylations.
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
- Arnault Heynderickx,
- Sébastien Nénon,
- Olivier Siri,
- Vladimir Lokshin and
- Vladimir Khodorkovsky
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- the formation of hydrogen bonds, an approach known as ‘crystal engineering’ (see, for instance, [13][14] and references therein). Indeed, whereas several two-component molecular systems cocrystallizing in a special layered way involving pyrazine, quinoline and phenazine as the H-bond acceptor and
- kcal/mol. For comparison, we calculated the proton affinities of quinoline: 217.0 and 4-dimethylaminopyridine: 240.1 kcal/mol at the same level of theory (the experimental values for these two derivatives are 214.4 and 235.7 kcal/mol, respectively [25]). Thus, the basicity of 3 is close to that of 4

Graphical Abstract

Figure 1: Quinoxaline derivatives 1–3.

Scheme 1: Synthesis of THTTA (3).

Scheme 2: Protonation, alkylation and acylation of 3.

Figure 2: The ORTEP view of 3. Torsion angles within the benzene ring are given in red.

Figure 3: The ORTEP view of 6a. Torsion angles within the benzene ring are given in red.

Figure 4: The ORTEP view of 7a (the iodine counter anion has been omitted for clarity). Torsion angles within...

Scheme 3: Charge transfer and delocalization within 3 and its diprotonated (6a) and monomethylated (7a) deriv...

Figure 5: Normalized absorption spectra of 3 in: toluene (black), acetone (blue), ethanol (green), water (red...

Figure 6: Protonation of 3 in ethanol. Two isosbestic points (IBP) are indicated by arrows.

Figure 7: Absorption spectra of 7a (blue) and 8 (red) in ethanol.

Figure 8: Absorption spectra of 10a (black), 10b (red). Solid curves: in toluene, dashed curves: in acetone.

Figure 9: View of the supramolecular array generated by 3 in the solid state. Color coding: nitrogen, blue; c...

Figure 10: View of the supramolecular array generated by 6a in the solid state. Color coding: nitrogen, blue; ...

Figure 11: View of the supramolecular array generated by 7a in the solid state. Color coding: nitrogen, blue; ...
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- carbonate as a base under aerobic conditions. Along with the synthesis of pyrido[1,2-a]benzimidazoles 78, they have reported the synthesis of benzimidazo[1,2-a]quinoline 79 and benzimidazo[1,2-a]isoquinoline 80 in good to excellent yields. They have used differently substituted arylboronic acids 77 as one

Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.
Synthesis of ([1,2,4]triazolo[4,3-a]pyridin-3-ylmethyl)phosphonates and their benzo derivatives via 5-exo-dig cyclization
- Aleksandr S. Krylov,
- Artem A. Petrosian,
- Julia L. Piterskaya,
- Nataly I. Svintsitskaya and
- Albina V. Dogadina
Beilstein J. Org. Chem. 2019, 15, 1563–1568, doi:10.3762/bjoc.15.159
- the phosphoryl unit resonating at 3.75–4.19 ppm with 2JHP = 20 Hz. At lower field, the signals of 6 protons of the (iso)quinoline rings are present at 7.1–8.7 ppm. In the 13C NMR spectra, the methylene and methine carbons of the triazole ring resonate as doublet signals with characteristic constants

Graphical Abstract

Scheme 1: Synthetic approaches to [1,2,4]triazolo[4,3-a]pyridines.

Scheme 2: Synthesis of 3-methylphosphonylated [1,2,4]triazolo[4,3-a]pyridines. Reaction conditions: 1 (1 mmol...

Scheme 3: Synthesis of methylphosphonylated 6(8)-nitro-[1,2,4]triazolo[4,3-a]pyridines and 6(8)-nitro-[1,2,4]...

Scheme 4: Acid-promoted Dimroth rearrangement pathway.

Scheme 5: Synthesis of phosphonylated [1,2,4]triazolo[4,3-a]quinolines and [1,2,4]triazolo[3,4-a]isoquinoline...

Scheme 6: Plausible reaction pathway.
Synthesis of pyrrolo[1,2-a]quinolines by formal 1,3-dipolar cycloaddition reactions of quinolinium salts
- Anthony Choi,
- Rebecca M. Morley and
- Iain Coldham
Beilstein J. Org. Chem. 2019, 15, 1480–1484, doi:10.3762/bjoc.15.149
- Anthony Choi Rebecca M. Morley Iain Coldham Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, S3 7HF, UK 10.3762/bjoc.15.149 Abstract Quinolinium salts, Q+-CH2-CO2Me Br− and Q+-CH2-CONMe2 Br− (where Q = quinoline), were prepared from quinolines. Deprotonation of these salts
- with a quinoline. Pyrroloquinolines have been found to show antibacterial and antifungal activity, to be ligands for the NK1 receptor, and to be effective against the Hif hypoxia pathway in cancer cell lines [36][37][38]. Almost all of the examples of dipolar cycloaddition reactions involving
- reaction of quinolinium salts bearing electron-withdrawing groups other than ketones, we prepared ester 4 [55] and amide 5 by alkylation of quinoline. Arylidenemalononitriles such as 6a are known to undergo related chemistry [41], so we heated this compound with the quinolinium salts in the presence of

Graphical Abstract

Scheme 1: Reaction of ketone 1 with electron-deficient alkenes 2.

Scheme 2: Reactions of ester 4 and amide 5 with electron-deficient alkenes 6.

Figure 1: Single crystal X-ray structure for 7c.

Scheme 3: Reactions of ester 4 and amide 5 with N-methylmaleimide.

Figure 2: Single crystal X-ray structure for 9.

Scheme 4: Reduction and oxidation of adducts 9 and 10.

Scheme 5: Formation of amides 15a and 15b and Suzuki–Miyaura coupling to yield 16.
Multicomponent reactions (MCRs): a useful access to the synthesis of benzo-fused γ-lactams
- Edorta Martínez de Marigorta,
- Jesús M. de Los Santos,
- Ana M. Ochoa de Retana,
- Javier Vicario and
- Francisco Palacios
Beilstein J. Org. Chem. 2019, 15, 1065–1085, doi:10.3762/bjoc.15.104
- . Another aromatic aldehyde, in this case derived from a quinoline, can also be used as substrate for a multicomponent reaction, appropriate for the preparation of 3-aminoisoindolinone analogues 98 (Scheme 28) [106]. Indeed, the reaction of chloroquinolinecarbaldehydes 97 with isocyanides 42 and aromatic
- amines 2, catalysed by Pd, produced a small collection (8 examples, 75–95% yield) of quinoline derivatives 98 containing a γ-lactam moiety with different substituents at the nitrogen of the 5-membered ring and in the γ-position. Although the authors do not suggest a mechanism, this probably starts with

Graphical Abstract

Figure 1: γ-Lactam-derived structures considered in this review.

Figure 2: Alkaloids containing an isoindolinone moiety.

Figure 3: Alkaloids containing the 2-oxindole ring system.

Figure 4: Drugs and biological active compounds containing an isoindolinone moiety.

Figure 5: Drugs and biologically active compounds bearing a 2-oxindole skeleton.

Scheme 1: Three-component reaction of benzoic acid 1, amides 2 and DMSO (3).

Scheme 2: Copper-catalysed three-component reaction of 2-iodobenzoic acids 10, alkynylcarboxylic acids 11 and...

Scheme 3: Proposed mechanism for the formation of methylene isoindolinones 13.

Scheme 4: Copper-catalysed three-component reaction of 2-iodobenzamide 17, terminal alkyne 18 and pyrrole or ...

Scheme 5: Palladium-catalysed three-component reaction of ethynylbenzamides 21, secondary amines 22 and CO (23...

Scheme 6: Proposed mechanism for the formation of methyleneisoindolinones 24.

Scheme 7: Copper-catalysed three-component reaction of formyl benzoate 29, amines 2 and alkynes 18.

Scheme 8: Copper-catalysed three-component reaction of formylbenzoate 29, amines 2 and ketones 31.

Scheme 9: Non-catalysed (A) and phase-transfer catalysed (B) three-component reactions of formylbenzoic acids ...

Scheme 10: Proposed mechanism for the formation of isoindolinones 36.

Scheme 11: Three-component reaction of formylbenzoic acid 33, amines 2 and fluorinated silyl ethers 39.

Scheme 12: Three-component Ugi reaction of 2-formylbenzoic acid (33), diamines 41 and isocyanides 42.

Scheme 13: Non-catalysed (A, B) and chiral phosphoric acid promoted (C) three-component Ugi reactions of formy...

Scheme 14: Proposed mechanism for the enantioselective formation of isoindolinones 46.

Scheme 15: Three-component reaction of benzoic acids 33 or 54, amines 2 and TMSCN (52).

Scheme 16: Several variations of the three-component reaction of formylbenzoic acids 33, amines 2 and isatoic ...

Scheme 17: Proposed mechanism for the synthesis of isoindoloquinazolinones 57.

Scheme 18: Three-component reaction of isobenzofuranone 61, amines 2 and isatoic anhydrides 56.

Scheme 19: Palladium-catalysed three-component reaction of 2-aminobenzamides 59, 2-bromobenzaldehydes 62 and C...

Scheme 20: Proposed mechanism for the palladium-catalysed synthesis of isoindoloquinazolinones 57.

Scheme 21: Four-component reaction of 2-vinylbenzoic acids 67, aryldioazonium tetrafluoroborates 68, DABCO·(SO2...

Scheme 22: Plausible mechanism for the formation of isoindolinones 71.

Scheme 23: Three-component reaction of trimethylsilylaryltriflates 77, isocyanides 42 and CO2 (78).

Scheme 24: Plausible mechanism for the three-component synthesis of phthalimides 79.

Scheme 25: Copper-catalysed three-component reaction of 2-formylbenzonitriles 85, arenes 86 and diaryliodonium...

Scheme 26: Copper-catalysed three-component reaction of 2-formylbenzonitriles 85, diaryliodonium salts 87 and ...

Scheme 27: Proposed mechanism for the formation of 2,3-diarylisoindolinones 88, 89 and 92.

Scheme 28: Palladium-catalysed three-component reaction of chloroquinolinecarbaldehydes 97 with isocyanides 42...

Scheme 29: Palladium-catalysed three-component reaction of imines 99 with CO (23) and ortho-iodoarylimines 100....

Scheme 30: Palladium-catalysed three-component reaction of amines 2 with CO (23) and aryl iodide 105.

Scheme 31: Three-component reaction of 2-ethynylanilines 109, perfluoroalkyl iodides 110 and carbon monoxide (...

Scheme 32: Ultraviolet-induced three-component reaction of N-(2-iodoaryl)acrylamides 113, DABCO·(SO2)2 (69) an...

Scheme 33: Proposed mechanism for the preparation of oxindoles 115.

Scheme 34: Three-component reaction of acrylamide 113, CO (23) and 1,4-benzodiazepine 121.

Scheme 35: Multicomponent reaction of sulfonylacrylamides 123, aryldiazonium tetrafluoroborates 68 and DABCO·(...

Scheme 36: Proposed mechanism for the preparation of oxindoles 124.

Scheme 37: Three-component reaction of N-arylpropiolamides 128, aryl iodides 129 and boronic acids 130.

Scheme 38: Proposed mechanism for the formation of diarylmethylene- and diarylallylideneoxindoles 131 and 132.

Scheme 39: Three-component reaction of cyclohexa-1,3-dione (136), amines 2 and alkyl acetylenedicarboxylates 1...

Scheme 40: Proposed mechanism for the formation of 2-oxindoles 138.
New α- and β-cyclodextrin derivatives with cinchona alkaloids used in asymmetric organocatalytic reactions
- Iveta Chena Tichá,
- Simona Hybelbauerová and
- Jindřich Jindřich
Beilstein J. Org. Chem. 2019, 15, 830–839, doi:10.3762/bjoc.15.80
- with monosubstituted derivatives at position 6 on the primary rim (Figure 3). Generally, we observed four different regions with the typical signals: the first, well-resolved aromatic region belongs to the quinoline part of the cinchona alkaloid (9.00–7.55 ppm) and to the hydrogen signal of the

Graphical Abstract

Figure 1: Schematic cone-shaped (a) and structure representations (b) of α-CD (six glucopyranoside units) and...

Figure 2: Common cinchona alkaloids (cinchonine, cinchonidine, quinine, quinidine).

Scheme 1: CuAAC click reaction of propargylated cinchona alkaloids 3a–d with 6I-azido-6I-deoxy-α-CD (1) and 6I...

Scheme 2: CuAAC click reaction of per-Me-N3-α-CD (6) or per-Me-N3-β-CD (7) and propargylated cinchona alkaloi...

Scheme 3: Synthesis of difunctionalized α-CD 11 with quinine moieties.

Figure 3: Representative 1H NMR spectrum of the non-methylated quinidine–α-CD derivative 4d.

Figure 4: Representative 13C NMR spectrum and parts of the HMBC spectrum of the non-methylated quinidine–α-CD...

Scheme 4: AAA reaction of MBH carbamate 12 catalyzed by the prepared CD derivatives 4a–d, 5a–d, 8a–d, 9a–d, 11...






