Search results
Search for "tetrahydrofuran" in Full Text gives 324 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Multiple threading of a triple-calix[6]arene host
Beilstein J. Org. Chem. 2019, 15, 2092–2104, doi:10.3762/bjoc.15.207

- mass spectra were calibrated externally, and a linear calibration was applied. All chemicals were reagent grade and were used without further purification. Tetrahydrofuran was dried by heating under reflux over sodium wire in the presence of benzophenone as indicator while dimethylformamide was dried
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
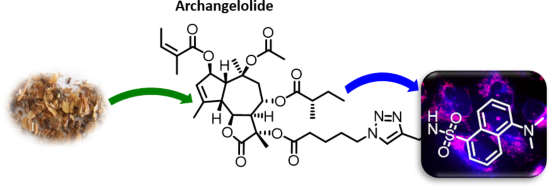
- , dicyclohexylurea; Dns, dansyl amide; DMAP, 4-dimethylaminopyridine; DTB, 8-O-(dodecanoyl-8-O-debutanoyltrilobolid); IL-6, interleukin 6; IL-1β, interleukin 1β; INF-γ, interferon gamma; TBTA, tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; TEA, triethylamine; THF, tetrahydrofuran; TLC, thin-layer chromatography
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- methyl-substituted derivative H2 were prepared according to previously reported methods [30]. Starting from helic[6]arene H1, helic[6]arene derivatives H3 and H4 were conveniently synthesized by etherification of H1 with bromobutane or 2-bromoethyl methyl ether, respectively, in tetrahydrofuran in the
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- both pairs of ethyl esters [26]. When a mixture of tert-butyl esters (2R,1'S)-5d and (2S,1'S)-5d was subjected to kinetic resolution in the presence of potassium tert-butoxide in tetrahydrofuran (2R,1'S)-5d was produced with low 40% de [27]. The absolute configuration at C2 in esters 5 was established
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

Synthesis, enantioseparation and photophysical properties of planar-chiral pillar[5]arene derivatives bearing fluorophore fragments
Beilstein J. Org. Chem. 2019, 15, 1601–1611, doi:10.3762/bjoc.15.164

- toluene (40 mL), tetrahydrofuran (60 mL) and water (10 mL). After bubbling argon through the mixture for 15 min, Pd(PPh3)4 (300 mg, 0.3 mmol) was added, and the mixture was stirred and refluxed for 8 h. Then, the reaction mixture was extracted with dichloromethane, and the organic layer was dried over
Transient and intermediate carbocations in ruthenium tetroxide oxidation of saturated rings
Beilstein J. Org. Chem. 2019, 15, 1552–1562, doi:10.3762/bjoc.15.158

- tetroxide-mediated oxidation of cyclopentane, tetrahydrofuran, tetrahydrothiophene and N-substituted pyrrolidines has been studied computationally by DFT and topological (analysis of the electron localization function, ELF) methods. In agreement with experimental observations and previous DFT calculations
- oxidation of cyclopentane, tetrahydrofuran, tetrahydrothiophene, and N-methyl- and N-benzylpyrrolidine to evaluate the extension in which transient carbocations can be formed (and whether they can become energy minima) during the rate-limiting step (Scheme 3). The RuO4 oxidation of cyclopentane [44] and
- tetrahydrofuran [45] have been experimentally reported as well as the oxidation of N-acylpyrrolidines to the corresponding lactams [46]. Admittedly, the oxidation of tetrahydrothiophene has been approached only computationally since in that case the sulfur atom would be more easily oxidized. Since the general
A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: a mild, catalyst-free approach towards O-thiocarbamates and dithiocarbamates
Beilstein J. Org. Chem. 2019, 15, 1523–1533, doi:10.3762/bjoc.15.155
- and HPLC–MS. Based on preliminary experiments in our laboratory, the reaction was performed in tetrahydrofuran (THF) at 40 °C for 1 h using a 1.5 equiv excess of sodium hydride as the base, S8 and the alcohol component (Table 1, entry 1) resulting in the desired thiocarbamate 3a in 58% yield. During
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- 1,1’-bis(diphenylphosphino)ferrocene (dppf) from Frontier Scientific. Absolute tetrahydrofuran, dichloromethane, and toluene were provided from Sigma-Aldrich and purified using a Büchi MB SPS-800. Dimethyl sulfoxide, acetonitrile, and acetone were purchased from Merck and Sigma-Aldrich, purified, and
Stereo- and regioselective hydroboration of 1-exo-methylene pyranoses: discovery of aryltriazolylmethyl C-galactopyranosides as selective galectin-1 inhibitors
Beilstein J. Org. Chem. 2019, 15, 1046–1060, doi:10.3762/bjoc.15.102
- -galactoheptulose (3): Compound 2 (3.66 g, 6.82 mmol) was dissolved in dry tetrahydrofuran (130 mL) under nitrogen and cooled to 0 °C. Borane dimethyl sulfide complex in dry tetrahydrofuran (4.78 mL, 9.55 mmol, 2 M) was added slowly, and the reaction was kept at 0 °C for 2 h. The reaction mixture went from light
- , 577.2566. 3,4,5,7-Tetra-O-benzyl-2-deoxy-β-D-mannoheptulose (5): Compound 4 (35 mg, 0.065 mmol) was dissolved in dry tetrahydrofuran (3 mL) under nitrogen and cooled to 0 °C. Borane dimethyl sulfide complex in dry tetrahydrofuran (40 µL, 0.078 mmol, 2 M) was added slowly and the reaction was kept at 0 °C
- , 129.86, 129.49, 129.45, 129.2, 129.1, 128.99, 128.95, 86.7, 81.4, 81.3, 77.4, 76.5, 76.4, 74.8, 73.3, 71.8, 62.9; HRMS (m/z): [M + H]+ calcd for 555.2743; found, 555.2747. 3,4,5,7-Tetra-O-benzyl-2-deoxy-β-D-glucoheptulose (7): Compound 6 (67 mg, 0.125 mmol) was dissolved in dry tetrahydrofuran (3 mL
Fabrication, characterization and adsorption properties of cucurbit[7]uril-functionalized polycaprolactone electrospun nanofibrous membranes
Beilstein J. Org. Chem. 2019, 15, 992–999, doi:10.3762/bjoc.15.97

- investigated in this work (Scheme 1). Results and Discussion As a common biodegradable polymer, PCL has been frequently reported as the fiber template material for the fabrication of electrospun nanofibers. Various common solvents such as acetone, dichloromethane, chloroform, tetrahydrofuran, DMF or their
Mechanochemical synthesis of poly(trimethylene carbonate)s: an example of rate acceleration
Beilstein J. Org. Chem. 2019, 15, 963–970, doi:10.3762/bjoc.15.93
- polymerization usually requires long reaction times to achieve high conversions (Table 1) [27]. The DBU-catalyzed polymerization of trimethylene carbonate in chloroform, tetrahydrofuran, toluene, and methylene chloride converted less than 5% monomer into poly(trimethylene carbonate) (PTMC) within 1 h (Table 1
- ). The RI measurements were carried out using an instrument set composed of a Waters 1515 isocratic pump, a 2414 differential refractive index detector, and a column-heating module with Shodex KF-804, KF-803, and KF-802.5 columns in series. The columns were eluted with tetrahydrofuran (preservative-free
Hoveyda–Grubbs catalysts with an N→Ru coordinate bond in a six-membered ring. Synthesis of stable, industrially scalable, highly efficient ruthenium metathesis catalysts and 2-vinylbenzylamine ligands as their precursors
Beilstein J. Org. Chem. 2019, 15, 769–779, doi:10.3762/bjoc.15.73
- in the presence of air. Boiling in tetrahydrofuran or acetonitrile even under an argon atmosphere leads to a rapid decomposition of the catalysts within 5–10 minutes (the solutions turn black). Therefore, THF and MeCN were excluded from further studies. In the test reactions, three concentrations of
Synthesis of the aglycon of scorzodihydrostilbenes B and D
Beilstein J. Org. Chem. 2019, 15, 610–616, doi:10.3762/bjoc.15.56
- TraceGC Ultra (GC–MS). Column chromatography was performed with Fluka silica gel 60 (230–400 mesh) and thin-layer chromatography was carried out by using Merck TLC Silicagel 60F254 aluminium sheets. Tetrahydrofuran (THF) and toluene were refluxed under nitrogen over sodium wire and a small amount of
Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines
Beilstein J. Org. Chem. 2019, 15, 474–489, doi:10.3762/bjoc.15.41

- chromatographically and spectroscopically homogeneous materials. Anhydrous methylene chloride, dimethylformamide and acetonitrile were obtained by distillation over CaH2, tetrahydrofuran was obtained by distillation over sodium. Commercial reagents were used as received. NMR spectra were recorded on Bruker Avance 300
Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives
Beilstein J. Org. Chem. 2019, 15, 401–430, doi:10.3762/bjoc.15.36

- then adjusted to 4 and then to 6–7 and the aqueous solution containing the nicotinamide riboside was mixed with an aqueous saturated sodium chloride solution and extracted with a considerable volume of tetrahydrofuran to remove excess of Nam and sodium trifluoromethanesulfonate. After multiple cycles
Synthesis of C3-symmetric star-shaped molecules containing α-amino acids and dipeptides via Negishi coupling as a key step
Beilstein J. Org. Chem. 2019, 15, 371–377, doi:10.3762/bjoc.15.33
- tetrahydrofuran (THF) to obtain the N-Boc-serine methyl ester (5) in 93% yield [42]. Afterwards, the protected methyl ester 5 was subjected to iodination in the presence of iodine (I2), triphenylphosphane (PPh3) and imidazole in CH2Cl2 at 0 °C to deliver the iodo derivative 6 in 63% yield [43][44]. Finally, the
A novel and efficient synthesis of phenanthrene derivatives via palladium/norbornadiene-catalyzed domino one-pot reaction
Beilstein J. Org. Chem. 2019, 15, 291–298, doi:10.3762/bjoc.15.26
- this reaction (Table 1, entries 10 and 11). Furthermore, different solvents were then tested, including MeCN, toluene, 1,4-dioxane, tetrahydrofuran (THF), and dimethylacetamide (DMAC), and we found the yield of y-1 was inferior to that done by DMF (Table 1, entries 12–16). With the optimum reaction
Synthesis of unnatural α-amino esters using ethyl nitroacetate and condensation or cycloaddition reactions
Beilstein J. Org. Chem. 2018, 14, 2846–2852, doi:10.3762/bjoc.14.263
- 16. A second [2 + 3] cycloaddition-based approach is described in Scheme 4. It started with the preparation of the methylene-bearing dipolarophile 25 from propargylamide 24 using gold(I) chemistry, which turned out to be tolerant to a wide variety of dry solvents (dichloromethane, tetrahydrofuran
Synthesis of pyrrolidine-based hamamelitannin analogues as quorum sensing inhibitors in Staphylococcus aureus
Beilstein J. Org. Chem. 2018, 14, 2822–2828, doi:10.3762/bjoc.14.260
- optimal side chain substituents are an o-chlorobenzamide on the 5-position and a non-substituted benzamide on the 2’-position. In absence of any structural information of the inhibitor–target interaction, we were interested in replacing the core tetrahydrofuran scaffold by a pyrrolidine ring in order to
Synthesis of mono-functionalized S-diazocines via intramolecular Baeyer–Mills reactions
Beilstein J. Org. Chem. 2018, 14, 2799–2804, doi:10.3762/bjoc.14.257

- precursor 16 was reduced using a borane tetrahydrofuran complex and immediately reacted in the Baeyer–Mills reaction without further purification. In Table 2 the yields using the Baeyer–Mills reaction are compared with the yields using the reductive azo condensation with lead [17]. Compared to the reductive
Quinolines from the cyclocondensation of isatoic anhydride with ethyl acetoacetate: preparation of ethyl 4-hydroxy-2-methylquinoline-3-carboxylate and derivatives
Beilstein J. Org. Chem. 2018, 14, 2529–2536, doi:10.3762/bjoc.14.229
- special safety precautions. Results and Discussion The anhydrides 9a–h were readily accessible by treatment of anthranilic acids AA with one equivalent of triphosgene in refluxing tetrahydrofuran at 0.36 molar concentration (Scheme 3). This procedure is an alternative to the existing published protocol
- ]oxazine-2,4(1H)-dione (9b). A 500 mL single neck round-bottomed flask equipped with a football-shaped PTFE stirring bar (16 mm × 37 mm) was charged with 2-amino-5-bromobenzoic acid (10.0 g, 46.3 mmol, 1.0 equiv) followed by the addition of tetrahydrofuran (230 mL, 0.2 molar) and solid triphosgene (13.7 g
Novel photochemical reactions of carbocyclic diazodiketones without elimination of nitrogen – a suitable way to N-hydrazonation of C–H-bonds
Beilstein J. Org. Chem. 2018, 14, 2250–2258, doi:10.3762/bjoc.14.200
- with CH2- and O-bridges in their structure, diazoindandione 1f, diazocyclopentenedione 1g, and as a С–Н donor tetrahydrofuran was employed in the study (Figure 1). To determine the most efficient conditions for this reaction with diazodiketones 1, three sensitizers, acetophenone, benzophenone, and
- other hand, the sensitized photoexcitation of diazodiketones 1 gives rise to generation of triplet excited states of diazo compounds 3(1)1 which interact with the H-donor (tetrahydrofuran) producing N-alkyl-substituted hydrazones 2 (Scheme 2, reaction II). Based on the comparison study of the
- workup procedure hydrazone 2a and unreacted diazoketone 1a were isolated. 2-(2-(Tetrahydrofuran-2-yl)hydrazono)cyclopentane-1,3-dione (2a). Yield 168 mg (42%, calculated on the reacted diazodiketone 1a), bright-yellow oil; 1H NMR (400 MHz, CDCl3, δ) 12.93 (s, 1H), 5.61–5.53 (m, 1H), 4.08–3.99 (m, 1H
Synthesis and supramolecular self-assembly of glutamic acid-based squaramides
Beilstein J. Org. Chem. 2018, 14, 2065–2073, doi:10.3762/bjoc.14.180

- , chloroform, tetrahydrofuran, xylene, toluene, benzene, and chlorobenzene, whereas it was insoluble in water even after heating. In contrast, gel materials that did not flow upon inversion of the vial upside-down were obtained in 12 solvents with critical gelation concentration (CGC) values ranging from 16
Cationic cobalt-catalyzed [1,3]-rearrangement of N-alkoxycarbonyloxyanilines
Beilstein J. Org. Chem. 2018, 14, 1972–1979, doi:10.3762/bjoc.14.172
- solvents, such as CHCl3, CH2Cl2, and PhCl, ethereal solvent, such as Et2O and tert-butyl methyl ether (MTBE), and toluene were less efficient (Table 2, entries 2–7), while the use of polar solvents, such as tetrahydrofuran (THF), acetonitrile, and N,N-dimethylformamide (DMF), resulted in quantitative




































































































































































































































































































































































































































































































