Search results
Search for "inhibitor" in Full Text gives 419 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Reversible photoswitching of the DNA-binding properties of styrylquinolizinium derivatives through photochromic [2 + 2] cycloaddition and cycloreversion
Beilstein J. Org. Chem. 2020, 16, 111–124, doi:10.3762/bjoc.16.13
- deactivation of a stilbene tyrosine kinase inhibitor by a [2 + 2] photocycloaddition [59]. As the quinolizinium ion has been established as a versatile platform for the development of DNA intercalators [60], we identified styryl-substituted quinolizinium derivatives as a promising basis for the search for
Pigmentosins from Gibellula sp. as antibiofilm agents and a new glycosylated asperfuran from Cordyceps javanica
Beilstein J. Org. Chem. 2019, 15, 2968–2981, doi:10.3762/bjoc.15.293

- leave target microbes vulnerable to antibiotics [6]. A complementary approach of using a combination of an antibiotic with a biofilm inhibitor appears to be a promising solution to control biofilm-associated pathogens, as based on the evidence that traditional antibiotics were more effective when used
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein J. Org. Chem. 2019, 15, 2830–2839, doi:10.3762/bjoc.15.276
- nucleotide pyrophosphatase enzyme h-NPP-3. In case of 5e, an inhibitory value IC50 ± SEM = 0.26 ± 0.01 µM was observed which, thus, might be considered as potential inhibitor of h-NPP-3. Compound 5c with an inhibitory value for h-NPP-1 of IC50 ± SEM = 1.29 ± 0.07 µM was more active against NPP1 than against
- . However, the zinc–metal interaction was exhibited by the substituted phenyl moiety on the indole ring. The oxygen atom of the furan ring of dual inhibitor 6e showed a single hydrogen bond with Phe548. Asp423 was found to be involved in two π–cation bindings with the indole ring, whereas π–π stacked, π–π T
- -shaped and π–alkyl related bindings were noticed, connecting Ile235, Phe220, Ala546, Trp322 and Ile419. The fluorine atom was perceived interacting with Ile419. Docking studies of h-ENPP3 inhibitors The binding of ENPP3 was studied for selective inhibitor 5e. A dual affinity was observed for 5h and 6e
An improved, scalable synthesis of Notum inhibitor LP-922056 using 1-chloro-1,2-benziodoxol-3-one as a superior electrophilic chlorinating agent
Beilstein J. Org. Chem. 2019, 15, 2790–2797, doi:10.3762/bjoc.15.271
- , Kings Cross, London NW1 1AT, UK 10.3762/bjoc.15.271 Abstract Background: The carboxylesterase Notum has been shown to act as a key negative regulator of the Wnt signalling pathway by mediating the depalmitoleoylation of Wnt proteins. LP-922056 (1) is an orally active inhibitor of Notum. We are
- 1 (or 17) which combines Notum inhibition with CNS penetration would be a valuable chemical probe for investigating the role of Notum in disease models. Keywords: brain penetration; 1-chloro-1,2-benziodoxol-3-one; electrophilic chlorination; LP-922056; Notum inhibitor; Introduction The Wnt
- , Figure 1) is an orally active inhibitor of Notum recently reported by Lexicon Pharmaceuticals [5][6]. Their research with 1 has shown that Notum is a potential drug target for stimulating bone formation and treating osteoporosis [7]. However, although 1 demonstrates low plasma clearance, the structure
Skeletocutins M–Q: biologically active compounds from the fruiting bodies of the basidiomycete Skeletocutis sp. collected in Africa
Beilstein J. Org. Chem. 2019, 15, 2782–2789, doi:10.3762/bjoc.15.270
- inhibition activity against S. aureus, they showed only weak activity with 20 and 56% inhibition of the biofilm, respectively, at a concentration 256 µg/mL. Tyromycin A (6) was previously reported to be an inhibitor of leucine aminopeptidase in HeLa S3 cells [6]. Accordingly, all compounds 1–5 were tested
Fluorinated maleimide-substituted porphyrins and chlorins: synthesis and characterization
Beilstein J. Org. Chem. 2019, 15, 2704–2709, doi:10.3762/bjoc.15.263
- . Maleimides are considered as a biologically important scaffold that possess almost all types of biological activities including antibacterial and antifungal activity [24], anticancer activity [25], cox-2 inhibitor and anti-inflammatory, antidiabetic activity [26] and photodynamic activity [27]. Attaching of
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- activity against human and murine tumour cell lines. This suggests that the regioisomeric lactone moiety could play an important role in the observed cytotoxicity. Interestingly, 3 (SF002-96-1 [4]) was previously shown to inhibit survivin, which is a member of the inhibitor of apoptosis (IAP) family and a
α,ß-Didehydrosuberoylanilide hydroxamic acid (DDSAHA) as precursor and possible analogue of the anticancer drug SAHA
Beilstein J. Org. Chem. 2019, 15, 2524–2533, doi:10.3762/bjoc.15.245
- refractory cutaneous T-cell lymphoma (CTCL) [3]. Moreover, it also shows anticancer activity against a large number of hematological and solid malignancies [4][5]. Its anticancer activity is related to inhibition of histone deacetylase inhibitor (HDACi) at nanomolar concentrations (IC50 < 86 nM). Although
- , originally recognized as a pan-inhibitor, recent studies have established that it is unable to inhibit Class IIA lysine deacetylases (HDAC/4/5/79), thus showing some selectivity profile [6][7]. Moreover, pan-inhibition is also a cause of increased concern due to adverse side effects [8]. The closely related
- hydroxamic acid derivative belinostat (2) is also approved for the treatment of peripheral T-cell lymphoma (PTCL) [9]. On the other hand, trichostatin A (3), containing an α,ß-unsaturated hydroxamic acid unit is the best known HDAC inhibitor which shows antifungal activities [10][11]. Because of the
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- -crystallized with AHD, a compound that mimics the diphosphate group of GGDP and acts as a suicide inhibitor. The crystal structure of CotB2wt·Mg2+3·AHD (PDB-ID 6GGJ; [37]) confirmed the active site architecture as observed in the structure of CotB2wt·Mg2+3·F-Dola and more importantly, that the folding of the C
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- bioactivity, allows the probing of pharmacologically relevant systems with spatiotemporal resolution. A hetero-stilbene lead resulting from the screening of a compound that was originally designed as kinase inhibitor served as a starting point for the design of photoswitchable sirtuin inhibitors. Because the
- selective Sirt1 inhibitor, passed phase II clinical trials as a disease-modifying therapeutic for Huntington’s disease (HD) and was acquainted by AOP Orphan Pharmaceuticals AG for phase III trials in 2017 [9][10]. Its structure comprises a carboxamide moiety, which mimics the amide group of the endogenous
- pan-sirtuin inhibitor nicotinamide (Figure 1). Likewise Sirt2 inhibition was shown to have beneficial effects in animal and cell models of neurodegenerative diseases like HD and Parkinson’s disease [11][12]. Sirt3 activity recently was found to play an important role in cardiovascular diseases and
Characterization of two new degradation products of atorvastatin calcium formed upon treatment with strong acids
Beilstein J. Org. Chem. 2019, 15, 2085–2091, doi:10.3762/bjoc.15.206

- , Germany 10.3762/bjoc.15.206 Abstract Atorvastatin calcium (Lipitor®, Sortis®) is a well-established cholesterol synthesis enzyme (CSE) inhibitor commonly used in the therapy of hypercholesterolemia. This drug is known to be sensitive to acid treatment, but only little data has been published on the
Regioselective Pd-catalyzed direct C1- and C2-arylations of lilolidine for the access to 5,6-dihydropyrrolo[3,2,1-ij]quinoline derivatives
Beilstein J. Org. Chem. 2019, 15, 2069–2075, doi:10.3762/bjoc.15.204

- ; Introduction Lilolidine (Figure 1, left), which is a commercially available compound, contains a 5,6-dihydropyrrolo[3,2,1-ij]quinoline skeleton found in several bioactive molecules. For example, tivantinib (Figure 1, middle) exhibits MET inhibitor properties [1]; whereas tarazepide (Figure 1, right) is being
Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
Beilstein J. Org. Chem. 2019, 15, 2020–2028, doi:10.3762/bjoc.15.198
- effects on cells infected with respiratory syncytial virus [22][23], induction of aerial mycelium formation in Fusarium culmorum [24], and as an inhibitor of ubiquinol-cytochrome c reductase binding protein, blocking mitochondrial ROS-mediated vascular endothelial growth factor receptor type 2 signalling
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- pathways involved in the regulation of reactive oxygen species (ROS) and/or nitric oxide (NO) [9], histone methyltransferase [10], HSP70 [11], Jak2, Bcl-2/Bax [12], caspase 8 [13], NF-κB [14], X-linked inhibitor of apoptosis protein (XIAP) [15], MAPK, PI3K [16], and MPK1, ERK-1/2, and JNK-1/2 [17]. The
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
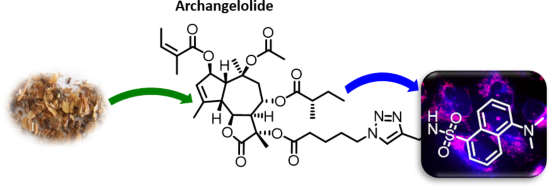
- evokes the synthesis of IL-6, INF-γ and TNF-α (see section “Abbreviations” at the end of the text). Furthermore, compound 2 has a very similar structure to the well-described SL thapsigargin, which is the best-known inhibitor of sarcoplasmic/endoplasmic reticular calcium ATPase (SERCA) with Ki values in
- reticulum and mitochondrial markers were performed. As it is apparent from Figure 3 and Figure S20 (Supporting Information File 1), compound 5 localized in the endoplasmic reticulum, which is in agreement with the site of localization of another SL, the SERCA inhibitor compound 2 [8]. However, we observed
- that this SL does not act as SERCA inhibitor. Therefore, we proceeded to confirm this hypothesis by a molecular dynamics simulation study. Molecular dynamics simulation of compounds 1 and 2 with SERCA The binding cavity for thapsigargin and compound 2 in the SERCA protein lies in the transmembrane
Installation of -SO2F groups onto primary amides
Beilstein J. Org. Chem. 2019, 15, 1907–1912, doi:10.3762/bjoc.15.186
- binding sites (Figure 1) [20]. The smallest member of this family, methyl sulfonyl fluoride (MSF), is known as a selective and irreversible inhibitor of acetylcholinesterase (AChE) [21][22]. The sulfonyl fluoride inhibitors NSC 127755 was found for specifically modifying tyrosine-31 of DHFR in chicken
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- )-7 (Scheme 6) which was found to be an effective inhibitor of the mitotic kinesin. The biologically active enantiomer of mexiletine (R)-24 was efficiently synthesized from the alcohol (2R,1'R)-7 (Scheme 7) [45]. When the respective tosylate (2R,1'R)-25 was treated with 2,6-dimethylphenoxide two
- protected diamine (R)-80a. Removal of the chiral auxiliary yielded the diamine (R)-80b which after acylation provided analogues (R)-76. Compound (R)-76 (R = C9H19) appeared inactive as inhibitor of human sphingosine kinases 1 and 2. 1,2-Diamino-3-hydroxy derivatives The interest in sphingoid analogues stems
- from the involvement of sphingolipid metabolites in an array of important cell processes. ᴅ-threo-PDMP (1R,2R)-81 is a ceramide analogue identified as an inhibitor of glucosylceramide synthase (GCS) at micromolar concentrations [66][67]. It was efficiently synthesized [68][69] employing the alcohol (2R
Synthesis of 9-O-arylated berberines via copper-catalyzed CAr–O coupling reactions
Beilstein J. Org. Chem. 2019, 15, 1575–1580, doi:10.3762/bjoc.15.161
- elaborate motifs such as heterocycles [10][11][12], glucose [13], nitric oxide [14], and the multidrug-resistance pump inhibitor 5-nitro-2-phenylindole (INF55) [15] have been covalently attached to BBR, further widening its pharmacological spectrum. Aromatic rings are another family of substituents having
Synthesis and biological evaluation of truncated derivatives of abyssomicin C as antibacterial agents
Beilstein J. Org. Chem. 2019, 15, 1468–1474, doi:10.3762/bjoc.15.147
- covalent inhibitor of 4-amino-4-deoxychorismate (ADC) synthase, which is the enzyme that catalyzes the conversion of chorismate and glutamine into ADC and glutamate, the first step in the biosynthesis of p-aminobenzoic acid (PABA) in bacteria [6]. Specifically, AbC binds via a Michael addition between a
Synthesis of non-racemic 4-nitro-2-sulfonylbutan-1-ones via Ni(II)-catalyzed asymmetric Michael reaction of β-ketosulfones
Beilstein J. Org. Chem. 2019, 15, 1289–1297, doi:10.3762/bjoc.15.127
- frequently used in the synthesis of organic fine chemicals and natural compounds. In addition to using the sulfonyl group as an auxiliary, it is also included in some chiral bioactive molecules, such as remikiren (1, renin inhibitor for the treatment of hypertension) [5][6], eletriptan (2, Relpax®, serotonin
- 5-HT1 receptor agonist for the treatment of migraine) [7], and apremilast (3, Otezla®, inhibitor of the PDE4 for the treatment of certain types of psoriasis and psoriatic arthritis) [8] (Figure 1). Recently we have shown that racemic sulfone 4 exhibits high antiviral activity against BVDV with low
Phylogenomic analyses and distribution of terpene synthases among Streptomyces
Beilstein J. Org. Chem. 2019, 15, 1181–1193, doi:10.3762/bjoc.15.115

- the recombinant enzyme from Streptomyces malaysiensis [43]. The diterpene 7 is a precursor to the lysophospholipase inhibitor cyclooctatin (20) formed by the action of two genetically clustered cytochrome P450 monooxygenases CotB3 and CotB4 (Scheme 4) [40][44], while no derivatives from 8 are
Insertion of [1.1.1]propellane into aromatic disulfides
Beilstein J. Org. Chem. 2019, 15, 1172–1180, doi:10.3762/bjoc.15.114
- ) [1]. In their pioneering work Stepan et al. replaced a para-substituted fluorophenyl ring in the γ-secretase inhibitor BMS-708,163 with a BCP whereby the oral absorption and in vitro metabolic stability could be significantly increased [2]. BCPs are usually derived from [1.1.1]propellane (1) [7
Synthesis of aryl cyclopropyl sulfides through copper-promoted S-cyclopropylation of thiophenols using cyclopropylboronic acid
Beilstein J. Org. Chem. 2019, 15, 1162–1171, doi:10.3762/bjoc.15.113
- ]. Roniciclib, also named BAY 1000394, is a pan-cyclin-dependant kinase (CDK) inhibitor that contains an aryl cyclopropyl sulfoximine and that was developed to treat patients with untreated small cell lung cancer [6][9]. Aryl cyclopropyl sulfides 1 are also remarkable synthons in organic synthesis (Scheme 1
Stereo- and regioselective hydroboration of 1-exo-methylene pyranoses: discovery of aryltriazolylmethyl C-galactopyranosides as selective galectin-1 inhibitors
Beilstein J. Org. Chem. 2019, 15, 1046–1060, doi:10.3762/bjoc.15.102
- most potent galectin-1 inhibitor 1b has at least fiftyfold better affinity than the reference monosaccharide methyl β-D-galactoside (11) and a similar affinity as the reference disaccharide methyl β-lactoside (12). Hence, the 4-fluorophenyltriazol moiety of 1b efficiently replaces the galectin-1
- -interacting glucose unit in methyl β-lactoside (12) and at the same time induces a significantly better selectivity than that of methyl β-lactoside (12). Taken together, the 4-fluorophenyltriazole 1b represents the most potent mono-galactoside-derived galectin-1 inhibitor, albeit with an apparently lower
Novel (2-amino-4-arylimidazolyl)propanoic acids and pyrrolo[1,2-c]imidazoles via the domino reactions of 2-amino-4-arylimidazoles with carbonyl and methylene active compounds
Beilstein J. Org. Chem. 2019, 15, 1032–1045, doi:10.3762/bjoc.15.101
- -dihydro analogs are represented only by several substances [33][34]. Partially hydrogenated pyrrolo[1,2-c]imidazole is a part of (±)-axinellamines 11. 4-[(5R)-6,7-Dihydro-5H-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile (LCI-699, osilodrostat) is considered as an inhibitor of aldosterone synthase


































































































































































































































































