Search results
Search for "potassium" in Full Text gives 642 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Synthesis of 3-substituted isoxazolidin-4-ols using hydroboration–oxidation reactions of 4,5-unsubstituted 2,3-dihydroisoxazoles
Beilstein J. Org. Chem. 2020, 16, 1313–1319, doi:10.3762/bjoc.16.112
- , respectively, according to our procedure [7][10]. The compounds 5a and 5b were first converted into the isoxazolidine-4,5-diols by the treatment with potassium osmate/N-methylmorpholine N-oxide (NMO). The dihydroxylation reactions proceeded with an excellent trans selectivity with respect to the substituent at
Photocatalytic trifluoromethoxylation of arenes and heteroarenes in continuous-flow
Beilstein J. Org. Chem. 2020, 16, 1305–1312, doi:10.3762/bjoc.16.111
- soluble bases such as amines and tetrabutylammonium salts (Table 2, entries 1–5) proved unfortunately not successful, neither in batch nor flow, so we extended the investigation to inorganic bases. Among the bases tested, potassium hydrogenphosphates K2HPO4 and KH2PO4 led to increased yields, reaching up
Activated carbon as catalyst support: precursors, preparation, modification and characterization
Beilstein J. Org. Chem. 2020, 16, 1188–1202, doi:10.3762/bjoc.16.104

- . investigated the chemical activation with sodium or potassium hydroxide by the use of an anthracite precursor [8][13][14]. Wood Non-fossil precursors for the preparation of activated carbons are of great interest due to an increasing demand of these materials. Wood and the lignocellulosic wastes from forestry
- solutions Xu et al. used energy-rich carbon precursors for the spherical carbon preparation via ultrasonic spray pyrolysis. Lithium, sodium or potassium propiolates are one class of such energy rich materials and exhibit leaving groups such as CO, CO2 or C2H2 eliminated by decarbonylation or decarboxylation
- found that KOH plays a crucial role in the formation of porosity. Metallic potassium is formed during the carbonization of the carbon precursor, which intercalates in the carbon structure and is responsible for further release of carbon dioxide, carbon monoxide and hydrogen [71]. According to Marsh and
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- salt 6.1 was promoted by the acridinium photocatalyst OD2. The resulting alkyl radical can be intercepted by a protonated heteroarene 6.2. The addition of potassium persulfate leads to the regeneration of the photocatalyst and the rearomatization of the intermediate, delivering the desired alkylated
Synthesis of novel multifunctional carbazole-based molecules and their thermal, electrochemical and optical properties
Beilstein J. Org. Chem. 2020, 16, 1066–1074, doi:10.3762/bjoc.16.93
- potassium acetate (KOAc) and dichlorobis(triphenylphosphine)palladium(II) in 1,4-dioxane [36][37]. Upon chromatography (9-hexylcarbazole-3-yl)boronic acid pinacol ester (5) was obtained as a liquid in good yield. (9-Hexylcarbazole-3-yl)boronic acid pinacol ester (5) was subjected to Suzuki–Miyaura reaction
- either with 2-bromopyridine-4-carbaldehyde (6a) or 2-bromopyridine-5-carbaldehyde (6b) in the presence of potassium carbonate and bis(triphenylphosphine)palladium(II) dichloride in tetrahydrofuran [2]. Upon repeated purification by chromatography, compounds 7a and 7b were obtained as liquids in good
- ), 0.86 (t, J = 7.0 Hz, 4H). (9-Hexylcarbazole-3-yl)boronic acid pinacol ester (5): 5 was synthesised as reported previously [36][37]. Bis(pinacolato)diboron (423 mg, 1.7 mmol), potassium acetate (446 mg, 4.5 mmol) and dichlorobis(triphenylphosphine)palladium(II) (35 mg, 0.05 mmol) catalyst were added to
Fluorinated phenylalanines: synthesis and pharmaceutical applications
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- the free amines gave amides 28a,b. Treatment of the latter with aqueous potassium hydroxide finally afforded (S)-2-amino-3-(2,3,4,5-tetrafluorophenyl)propionic acid (29a) and (S)-2-amino-3-(2,3,5,6-tetrafluorophenyl)propionic acid (29b) [42] (Scheme 6). Alternatively, phenylalanines 29a,b were also
Suzuki–Miyaura cross coupling is not an informative reaction to demonstrate the performance of new solvents
Beilstein J. Org. Chem. 2020, 16, 1001–1005, doi:10.3762/bjoc.16.89
- 1b) [13]. Here, palladium acetate without an auxiliary ligand was used for a pre-catalyst and the base changed to potassium carbonate. Reaction conditions of 20 hours at 65 °C were decided after observing 11% conversion after 2 hours and 33% after 20 hours in NMP at room temperature. The majority of
- ™, GVL, and 2-MeTHF were poor solvents. Replacing potassium carbonate with triethylamine, the conversion in Cyrene™ rose slightly to 10%. Despite the variation between experiments no discernible correlation between any solvent properties and the observed conversions was found. The results achieved in DMI
Aryl-substituted acridanes as hosts for TADF-based OLEDs
Beilstein J. Org. Chem. 2020, 16, 989–1000, doi:10.3762/bjoc.16.88

- bromide (0.06 g, 0.1 mmol) and potassium hydroxide (0.31 g, 5.7 mmol) were added and the mixture stirred for 30 min. Then, bromoethane (0.31 g, 2.85 mmol) was added dropwise to the reaction mixture with constant stirring and the mixture refluxed for 1 h. The reaction mixture was then poured into ice water
Bipyrrole boomerangs via Pd-mediated tandem cyclization–oxygenation. Controlling reaction selectivity and electronic properties
Beilstein J. Org. Chem. 2020, 16, 895–903, doi:10.3762/bjoc.16.81

- of NDAnH and NMInH carried out in the presence of 6 equiv of potassium acetate produced the corresponding dilactams in higher yields (53–73%) with complete conversion (Table 1, entries 2, 7, 12, and 18). Under these conditions, the cNMI2H and cNMI3H intermediates were not isolated. When the same
Photocatalytic deaminative benzylation and alkylation of tetrahydroisoquinolines with N-alkylpyrydinium salts
Beilstein J. Org. Chem. 2020, 16, 809–817, doi:10.3762/bjoc.16.74
- ). Eosin Y was inefficient under these conditions (Table 1, entry 20). Since [Ru(bpy)3]Cl2 is significantly cheaper than the iridium catalyst, the former was used in further reactions. Regarding the addition of different bases, the yield remained unchanged, when lutidine was added. Potassium carbonate and
Reaction of indoles with aromatic fluoromethyl ketones: an efficient synthesis of trifluoromethyl(indolyl)phenylmethanols using K2CO3/n-Bu4PBr in water
Beilstein J. Org. Chem. 2020, 16, 778–790, doi:10.3762/bjoc.16.71
- of potassium carbonate and tetrabutylphophonium bromide, which mediate the reaction in water through the formation of the interface between organic and aqueous phases. The advantageous of this reaction include high yields, no column chromatography, broad substrate scope, multiscale synthesis, and
Efficient synthesis of dipeptide analogues of α-fluorinated β-aminophosphonates
Beilstein J. Org. Chem. 2020, 16, 756–762, doi:10.3762/bjoc.16.69
- developing systems, and products were detected by inspection under UV light (254 nm) and with a solution of potassium permanganate. Merck Kieselgel 60 (0.063–0.200 μm), Merck Kieselgel 60 (0.040–0.063 μm), and Merck Kieselgel 60 (0.015–0.004 μm), were used for column chromatography. X-ray diffraction data
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- ) as atom-economical boron sources leading to the desired products 432–435 in moderate to good ees (Scheme 69) [132]. In addition, the reaction of potassium N-(4-methoxyphenyl)-3-(trifluoroborato)butanamide (436) as the nucleophilic coupling partner was investigated with heteroarylchlorides 437 leading
Synthesis of organic liquid crystals containing selectively fluorinated cyclopropanes
Beilstein J. Org. Chem. 2020, 16, 674–680, doi:10.3762/bjoc.16.65
- to generate 13a and 13b as a mixture of regioisomers in a ratio of 4:1. There was no requirement to separate these isomers at this stage. Subsequent addition of potassium tert-butoxide into a mixture of 13a and 13b in dichloromethane (DCM) resulted in an efficient elimination to generate vinyl
Towards the total synthesis of chondrochloren A: synthesis of the (Z)-enamide fragment
Beilstein J. Org. Chem. 2020, 16, 670–673, doi:10.3762/bjoc.16.64
- choice in this type of coupling reaction. Potassium carbonate was chosen due to the sensitivity of the amide 3 towards harsh basic conditions. With these conditions we were able to couple (Z)-bromide 4 with amide 3 selectively to yield (Z)-enamide 16. The obtained double bond geometry was confirmed by
- the indicative NMR coupling constants of 9.6 Hz. Moreover, we observed a concentration dependent formation of the undesired desilylated (Z)-enamide 17 (Table 1). The best results were achieved using a 65 mM solution of the amide 3. Using dry potassium carbonate, purified copper(I) iodide provided the
Regio- and stereoselective synthesis of new ensembles of diversely functionalized 1,3-thiaselenol-2-ylmethyl selenides by a double rearrangement reaction
Beilstein J. Org. Chem. 2020, 16, 515–523, doi:10.3762/bjoc.16.47
- potassium selenocyanate proceeded via a rearrangement with ring expansion, leading to a six-membered 2,3-dihydro-1,4-thiaselenin-2-yl selenocyanate (kinetic product) which in turn underwent rearrangement with ring contraction to a 1,3-thiaselenol-2-ylmethyl selenocyanate (thermodynamic product). These
- 1 with potassium selenocyanate was studied in detail. We found that the reaction of thiaselenole 1 with KSeCN at room temperature afforded a five-membered heterocycle, selenocyanate 4, in a quantitative yield. This result was unusual since previously studied substitution reactions of thiaselenole 1
- potassium selenocyanate led to the six-membered thiaselenine 5 (kinetic product), which underwent a rearrangement to a five-membered thiaselenole selenocyanate 4 (thermodynamic product). These rearrangements proceeded by a nucleophilic attack of the selenocyanate anion on two different carbon atoms of the
KOt-Bu-promoted selective ring-opening N-alkylation of 2-oxazolines to access 2-aminoethyl acetates and N-substituted thiazolidinones
Beilstein J. Org. Chem. 2020, 16, 492–501, doi:10.3762/bjoc.16.44
- important role to promote this ring-opening N-alkylation, but also acts as an oxygen donor. Keywords: N-alkylation; oxazolines; potassium tert-butoxide; ring opening; thiazolidinones; Introduction 2-Oxazolines are important structural units in pharmaceutical applications and efficient ligands in
- to synthesize thiazolidinone derivatives through a Barton–McCombie pathway in 2015 [17] (Scheme 1b). Recently, potassium tert-butoxide has been shown to be an efficient promoter for C–C-bond formation reactions [18][19][20][21][22]. However, only few reports described C–N-bond cross-coupling
- reactions using potassium tert-butoxide as promoter. Wu developed an efficient protocol for the KOt-Bu-promoted synthesis of 1-aminoisoquinolines from 2-methylbenzonitriles and benzonitriles [23], and the carbonylative cyclization of propargylic amines with selenium under CO gas-free conditions [24]. Based
Synthesis of 4-amino-5-fluoropyrimidines and 5-amino-4-fluoropyrazoles from a β-fluoroenolate salt
Beilstein J. Org. Chem. 2020, 16, 445–450, doi:10.3762/bjoc.16.41
- potassium 2-cyano-2-fluoroethenolate was developed. A broad substrate scope was tested and mostly excellent yields were obtained. The synthesis of fluorinated aminopyrazoles from the same fluorinated precursor could be demonstrated but proceeded with lower efficiency. Keywords: cyclization; fluorinated
- fluorinated C3 building block recently described and used by our laboratory for the synthesis of 2 [36]. Herein, we present the synthesis of fluorinated pyrimidines and pyrazoles from the same precursor. Potassium (Z)-2-cyano-2-fluoroethenolate (8) was synthesized in three steps starting from chloroacetamide
- ) of potassium formate, which could not easily be removed without risking the decomposition of the enolate salt (Scheme 1). As the fluorine atom is part of a building block, harsh conditions for a late-stage fluorination can be avoided, and even products with sensitive functionalities are accessible
Architecture and synthesis of P,N-heterocyclic phosphine ligands
Beilstein J. Org. Chem. 2020, 16, 362–383, doi:10.3762/bjoc.16.35

- the red phosphorus with 2-bromopyridine in potassium hydroxide/dimethyl sulfoxide emulsion, pyridylphosphine was obtained in moderate yields. Traces of phosphine oxide were present as evidenced by the observation of two phosphorus peaks in the 15P NMR spectrum. An optimized method via Grignard
- chiral acetal ligands have been reported by Lyle et al. where the fluorine–metal exchange was achieved by treatment with potassium tert-butoxide for a relatively long period (24 h) (Scheme 4) [65]. Acid-catalyzed condensation of compound 20 with enantiomerically pure C2-symmetric 1,2-tosylate analogs 21
- phosphorylation with diphenylphosphine in the presence of potassium tert-butoxide and 18-crown-6. The use of silyl and dialkylamine as reagents Organosilyl, silylphosphine derivatives, along with dialkylamines can also be used as alternative substrates to halogen-based reagents. These compounds are more stable
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- uncorrected. FTIR spectra were obtained in a Perkin–Elmer 1310 spectrometer using potassium bromide pellets. NMR spectra were recorded on an Agilent 500/54 (500 MHz and 125 MHz for 1H and 13C, respectively) spectrometer using CDCl3, and/or DMSO-d6 as solvent. Chemical shifts are given in ppm and J values in
Copper-promoted/copper-catalyzed trifluoromethylselenolation reactions
Beilstein J. Org. Chem. 2020, 16, 305–316, doi:10.3762/bjoc.16.30
- form C(sp)–SeCF3 bonds (Scheme 3) [18]. Therein, Dess–Martin periodinane (DMP) was used as the oxidant and potassium fluoride as the base, and the reactions were performed at room temperature in DMF as the solvent. The desired compounds were obtained in moderate to very good yields. Both electron
- tandem formation of C–Se and Se–fluoroalkyl bonds have emerged in the last five years. In 2014, Hor and Weng reported the trifluoromethylselenolation of (hetero)aryl iodides and alkyl bromides with the Ruppert–Prakash reagent, TMSCF3, elemental selenium, potassium fluoride, and silver carbonate under
Synthesis and herbicidal activities of aryloxyacetic acid derivatives as HPPD inhibitors
Beilstein J. Org. Chem. 2020, 16, 233–247, doi:10.3762/bjoc.16.25
- shown in Scheme 2, Scheme 3 and Scheme 4. The synthesis of compounds I and III was depicted in Scheme 2 and Scheme 3. The commercially available starting materials reacted with methyl chloroacetate in CH3CN and anhydrous potassium carbonate (K2CO3) as the base, and the corresponding products C and K
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
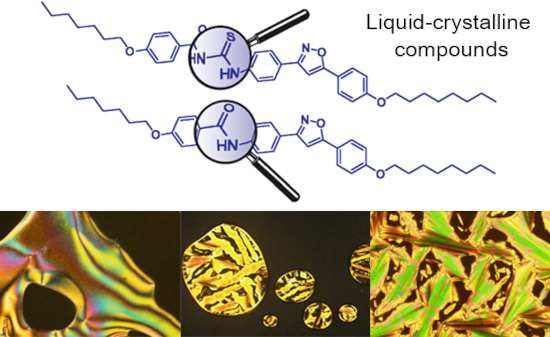
- were synthetized and the liquid crystal properties studied. Thioureas were obtained using a condensation reaction of benzoyl chlorides, arylamines and ammonium thiocyanate. The amides, on the other hand, were the byproduct of a quantitative reaction which used potassium cyanate as the starting material
- between the acyl isothiocyanate 16 and amines 3, 4, 8, 9, 12 and 13 yielded the title compounds 17a–c and 18a–c in 56–62% yields (17a 57%; 17b 56%; 17c 58%; 18a 61%; 18b 60%; 18c 62%). In an attempt to improve the structural diversity of thioureas, potassium cyanate was used as a nucleophile instead of
- and the amine 4 (Scheme 3, part B). Attempts to improve the reactivity of potassium cyanate by adding crown ethers did not result in the formation of ureas as expected. Thus, five new amides 19–22 and 24 were obtained in yield range of 42–71% (19 42%; 20 53%; 21 58%; 22 53%; 24 71%). Mesomorphic
Efficient method for propargylation of aldehydes promoted by allenylboron compounds under microwave irradiation
Beilstein J. Org. Chem. 2020, 16, 168–174, doi:10.3762/bjoc.16.19
- were obtained in short reaction time, high yield and purity without the need of any solvent when allenylboronic acid pinacol ester was used, or using a minimal amount of acetone when potassium allenyltrifluoroborate was used. Keywords: boron compounds; microwave; propargylation; regioselectivity
- small proportion of the corresponding regioisomer in some cases, in the development of new synthetic methods, it is desirable that it gives the corresponding product as a single compound. Our group described the synthesis of homopropargylic alcohols using potassium allenyltrifluoroborate as the
- NMR [39] (Scheme 2). Potassium allenyltrifluoroborate (4) is a crystalline solid and despite several microwave promoted reactions can be conducted without the use of solvents, the propargylation reaction using 2-naphthaldehyde and 4 under the previously optimized conditions gave the desired product in
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates
Beilstein J. Org. Chem. 2020, 16, 159–167, doi:10.3762/bjoc.16.18
- of xanthate esters with amines [18]. Furthermore, many methods have also been reported for the synthesis of cyclic thiocarbamates, and these include reactions of isothiocyanates with aldehydes in the presence of organocatalysts [19][20], reactions of vicinal diols with potassium thiocyanate [21









































































































































































































































































































































































































