Search results
Search for "condensation" in Full Text gives 770 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- reported a visible light-promoted photochemical reductive decarboxylation/alkylation of carboxylic acid analogs (Scheme 4) [45]. In this protocol, the carboxylic acids are converted into the corresponding RAE 4.1 by a condensation with N-hydroxyphthalimide. The organic dye eosin Y (OD13, E(PC/PC−) ≈ −1.1 V
A simple and easy to perform synthetic route to functionalized thienyl bicyclo[3.2.1]octadienes
Beilstein J. Org. Chem. 2020, 16, 1092–1099, doi:10.3762/bjoc.16.96
- 54, 10000 Zagreb, Croatia NMR Centre, Ruđer Bošković Institute, Bijenička cesta 54, 10000 Zagreb, Croatia 10.3762/bjoc.16.96 Abstract In order to prepare novel polycyclic derivatives of bicyclo[3.2.1]octadiene systems fused with a thiophene ring, photochemical cyclization and aldol condensation
- purification, the aldehydes 1 and 2 were obtained in very good yields (1: 79%; 2: 68%), and subsequently used as novel starting substrates for further addition/condensation reactions. The Wittig reaction of the prepared aldehydes with the corresponding triphenylphosphonium salts provided five new styryl
- of the substituent could be seen through a shift of the aromatic singlet, which is, in the case of methoxy-substituted derivative 9, shifted downfield, due to the electronic and anisotropic effect of the methoxy group. In continuation of the study herein presented, the aldol condensation reaction of
Fluorinated phenylalanines: synthesis and pharmaceutical applications
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- . Thus, a three-component condensation of a series of fluorinated benzaldehydes 50a–h, N-acetyl- or N-benzoylglycine 51a or 51b, respectively, and an excess of acetic anhydride in the presence of sodium acetate afforded the oxazolones 52a–h. The subsequent reductive ring cleavage of 52a–h without
- improve protein stability, natural hydrocarbon amino acids were replaced with Pff 77a. The effect of enhanced protein stability upon this replacement is referred as to ‘fluoro-stabilization effect’ [56]. 1.7. Knoevenagel condensation of methyl isocyanoacetate Three isomers of fluorinated phenylalanines
- 53a,b and 81 were synthesized by Knoevenagel condensation of methyl isocyanoacetate (79) and the corresponding fluorinated benzaldehyde derivatives 50a,b, and m-fluorobenzaldehyde (78) as electrophiles in the presence of catalytic amounts of Cu(I) and base. The cinnamate derivatives 80a–c obtained
Fabclavine diversity in Xenorhabdus bacteria
Beilstein J. Org. Chem. 2020, 16, 956–965, doi:10.3762/bjoc.16.84
- reductase, Nit: nitrilase, A: adenylation, C: condensation, E: epimerization, TP: transport. Comparison of the fcl BGCs in Xenorhabdus and Photorhabdus strains responsible for the fabclavine biosynthesis. a: X. szentirmaii, b: X. budapestensis, c: X. cabanillasii, d: X. indica, e: X. hominickii, f: X
- , A: adenylation, C: condensation, E: epimerization, TP: transport. Compound list of the fabclavine derivatives identified in this work. The structures are based on MALDI–HRMS and MALDI–MS2 analyses using the known structure of 1 as a reference [20]. The derivatives 1–4 and 17–22 were described
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- ), which were obtained by condensation between meso-tetrakis(4-formylphenyl)porphyrin (p-Por-CHO) and benzene-1,4-diamine, and 1,4-phenylenediacetonitrile, for Por-COF-1 and Por-COF-2, respectively (Scheme 54) [103]. The sp2 carbon-linked COF conferred high chemical stability to the material (Por-COF-2
Synthesis of new asparagine-based glycopeptides for future scanning tunneling microscopy investigations
Beilstein J. Org. Chem. 2020, 16, 888–894, doi:10.3762/bjoc.16.80

- the condensation with HBTU and HATU for the preparation of the glycosyl amino acids 3a–f since previous studies showed HATU to be more efficient than HBTU in similar cases [33][34][35]. Table 1 summarizes the obtained yields for conversions 2→3, 3→4, 3→5, 4→6, and 5→7 (Scheme 2). As can be seen in
Direct borylation of terrylene and quaterrylene
Beilstein J. Org. Chem. 2020, 16, 621–627, doi:10.3762/bjoc.16.58
- photovoltaic components, the scalable synthesis of pure soluble compounds is essential. Recently, facile preparation methods of terrylene [8] and quaterrylene [9] were reported, after 50 years from the first reports, respectively [10][11]. Friedel–Crafts ring condensation reaction of 3-(1-naphthyl)perylene
- calculations showed a good agreement with the observed absorption spectra. Taking the successful result of the terrylene borylation, next we tried to perform the same reaction to quaterrylene (Scheme 2). The quaterrylene was prepared by the oxidative condensation reaction of perylene with TfOH and DDQ [9
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52
- ) and Cu(II)@IPSi (1b) was investigated in the one-pot three-component condensation of various benzyl bromides, various phenylacetylenes, and sodium azide (Scheme 1). The authors stated that benzyl azide was formed in situ by the nucleophilic addition of the benzyl bromide and sodium azide. Subsequently
- synthesize benzimidazole–triazole derivatives through a one‐pot sequence of activation of the alkyne, [3 + 2] condensation, cyclization, and aromatization under mild reaction conditions (Scheme 2). Moreover, the BS–Cu(II)@SiO2 (11) catalyst could be recovered and recycled for seven cycles without a notable
- , compound 36 could be recycled and used in seven subsequent cycles. A further example of a silica-supported CuAAC catalyst was reported by Moghadam et al. (Scheme 7) [29]. In this study, the bis(benzothiazole)-substituted pyridine ligand BTP (41) was synthesized through the condensation of 2-aminothiophenol
Synthesis of 4-amino-5-fluoropyrimidines and 5-amino-4-fluoropyrazoles from a β-fluoroenolate salt
Beilstein J. Org. Chem. 2020, 16, 445–450, doi:10.3762/bjoc.16.41
- (5). First, a Finkelstein halogen exchange reaction and a dehydration reaction were combined to obtain fluoroacetonitrile (7) in 82% yield [36]. The latter then underwent a Claisen condensation with ethyl formate to obtain 8 in 77% yield with a purity of 90%. The product contained about 10% (1H NMR
Architecture and synthesis of P,N-heterocyclic phosphine ligands
Beilstein J. Org. Chem. 2020, 16, 362–383, doi:10.3762/bjoc.16.35

- chiral acetal ligands have been reported by Lyle et al. where the fluorine–metal exchange was achieved by treatment with potassium tert-butoxide for a relatively long period (24 h) (Scheme 4) [65]. Acid-catalyzed condensation of compound 20 with enantiomerically pure C2-symmetric 1,2-tosylate analogs 21
- pyrrolylphosphine ligands 40 and 43 was reported by Kumar et al. [68] (Scheme 7). The condensation of pyrrole (38) with formaldehyde and the amine gave the bis(diaminomethyl)pyrrole 39, which on reaction with Ph2PH gave diphosphine pyrrole ligand 40 in good yield (90%). Following a different route, condensation of
- pyrrole (38) with diphenylketone gave diphenyl(dipyrrolyl)methane 41. Subsequent Mannich condensation reaction resulted in the pyrrole diamine 42. Refluxing a toluene solution of intermediate 42 and diphenylphosphine gave dipyrrolyldiphosphine ligand 43 in very good yield (92%). Generally, high
Ultrasonic-assisted unusual four-component synthesis of 7-azolylamino-4,5,6,7-tetrahydroazolo[1,5-a]pyrimidines
Beilstein J. Org. Chem. 2020, 16, 281–289, doi:10.3762/bjoc.16.27
- , which had been obtained earlier [5] by the three-component reaction of starting materials 1c, 2a–f, and 3a (HOAc, 65 °C, 48 h). This instability complicated the isolation and characterization of the heterocycles 4p–u. The condensation of compounds 1c, 2a–f, and 3a in dry DMF under otherwise identical
- conditions was a way to avoid the rapid conversion of triazolyl derivatives 4p–u into 7-hydroxytriazolo[1,5-a]pyrimidines 5a–f and to isolate the heterocycles 4p–u, but in lower yields in comparison to the reaction in acetic acid. We consequently screened various condensation conditions, specifically by
Synthesis of 4-(2-fluorophenyl)-7-methoxycoumarin: experimental and computational evidence for intramolecular and intermolecular C–F···H–C bonds
Beilstein J. Org. Chem. 2020, 16, 190–199, doi:10.3762/bjoc.16.22
- , numerous methods for the synthesis of these compounds have been developed, examples are the Pechmann condensation [10][11], Stille coupling reaction [12], Knoevenagel condensation [13], Heck coupling reaction [14], Kostanecki reaction, Baylis–Hillman reaction [15], Michael reaction [16], Suzuki–Miyaura
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
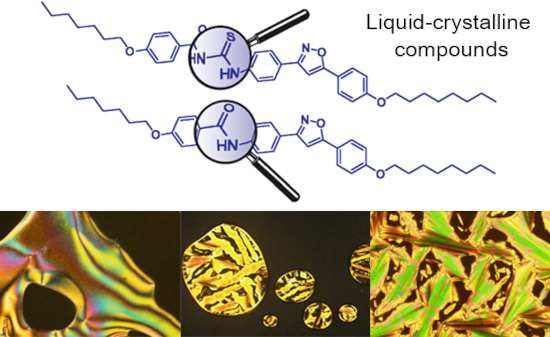
- were synthetized and the liquid crystal properties studied. Thioureas were obtained using a condensation reaction of benzoyl chlorides, arylamines and ammonium thiocyanate. The amides, on the other hand, were the byproduct of a quantitative reaction which used potassium cyanate as the starting material
- ]+ during the condensation reaction of thiocyanate salt, a reaction test was performed to confirm the reactivity between the amines and acyl chloride. This involved the formation and isolation of amide 24, as a result of the reaction between the reactive intermediate nonyloxybenzoyl chloride from acid 23
The reaction of arylmethyl isocyanides and arylmethylamines with xanthate esters: a facile and unexpected synthesis of carbamothioates
Beilstein J. Org. Chem. 2020, 16, 159–167, doi:10.3762/bjoc.16.18
- hydride-mediated reaction of arylmethyl isocyanides with xanthate esters in DMF is reported. The products thus obtained were compared with the carbamothioates obtained by the sodium hydride-mediated condensation of the corresponding benzylamines and xanthate esters in DMF. To account for these unexpected
- , the condensation reaction of O-benzyl S-methyl dithiocarbonate (1a) with benzylamine 5a in the presence of sodium hydride as the base of choice was evaluated using different solvents, DMF, THF, acetonitrile, DMSO, and toluene (Table 1, method B, entries 1–5). DMF was found to be the best solvent
- give O-(2-methylbenzyl) benzylcarbamothioate (4d) in 81% yield. The xanthate ester O-(3-methoxybenzyl) S-methyl carbonodithioate (1c) underwent condensation with 4-fluorobenzylamine (5c) and 4-chlorobenzylamine (5d) to afford the corresponding O-(3-methoxybenzyl) (4-fluorobenzyl)carbamothioate and O-(3
Synthesis of 3-alkenylindoles through regioselective C–H alkenylation of indoles by a ruthenium nanocatalyst
Beilstein J. Org. Chem. 2020, 16, 140–148, doi:10.3762/bjoc.16.16
- groups have used Wittig reactions for the synthesis of 3-alkenylindoles [13][14][15]. Another variant that uses the Doebner condensation was reported by Singh and co-worker, who condensed indole-3-carbaldehyde with phenylacetic acid in the presence of pyridine as the solvent/base and piperidine as the
Potent hemithioindigo-based antimitotics photocontrol the microtubule cytoskeleton in cellulo
Beilstein J. Org. Chem. 2020, 16, 125–134, doi:10.3762/bjoc.16.14

- condensation of benzaldehyde onto thioindoxyls. However, the key step is the formation (and where necessary, isolation) of the thioindoxyl species. In our studies, the electron-rich dimethoxy- and trimethoxy-substituted thioindoxyls were noted to be unstable to air, base, and silica gel during chromatography
Starazo triple switches – synthesis of unsymmetrical 1,3,5-tris(arylazo)benzenes
Beilstein J. Org. Chem. 2020, 16, 22–31, doi:10.3762/bjoc.16.4

- evaluated for the preparation of tris(arylazo)benzenes 3. The first one relied on the consecutive condensation of anilines with nitroso compounds, Baeyer–Mills reactions [13]. With a suitable protecting group strategy, the selective construction of the individual AB branches should be achievable (Scheme 1
Chemical synthesis of tripeptide thioesters for the biotechnological incorporation into the myxobacterial secondary metabolite argyrin via mutasynthesis
Beilstein J. Org. Chem. 2019, 15, 2922–2929, doi:10.3762/bjoc.15.286
- production. As previously mentioned, one reason for this could be high specificity of the biosynthetic machinery which might require a full length phosphopantetheine moiety or even a PCP bound substrate, for condensation to take place. To verify the functionality of the truncated argyrin biosynthesis operon
One-pot synthesis of substituted pyrrolo[3,4-b]pyridine-4,5-diones based on the reaction of N-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-arylethyl)acetamide with amines
Beilstein J. Org. Chem. 2019, 15, 2840–2846, doi:10.3762/bjoc.15.277
- Federation Lomonosov Moscow State University, Moscow 119991, Russian Federation 10.3762/bjoc.15.277 Abstract The condensation of primary amines with N-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-arylethyl)acetamides was explored. Thus, a previously unknown recyclization of the starting material was
- : condensation; dihydropyrrolone derivative; one-pot synthesis; pyrrolo[3,4-b]pyridine-4,5-diones; recyclization; Introduction Derivatives of pyrrolo[3,4-b]pyridin-5-one are known for their broad-spectrum biological activity [1][2][3]. One example includes a family of compounds based on this fragment that was
- strongly desirable. In this article, we describe a convenient synthetic method for the preparation of 6-alkyl-2-methyl-7-aryl-6,7-dihydro-1H-pyrrolo[3,4-b]pyridine-4,5-diones 1. Therein, the condensation of N-(1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-2-oxo-2-arylethyl)acetamides 2 with a diverse range of
Design, synthesis and investigation of water-soluble hemi-indigo photoswitches for bioapplications
Beilstein J. Org. Chem. 2019, 15, 2822–2829, doi:10.3762/bjoc.15.275

- the phenyl ring was performed through the aldol condensation of indoxyl-3-acetate with the corresponding benzaldehydes under alkaline conditions (Scheme 1) [13]. All compounds 1a–c were obtained in good yields as pure Z-isomers as supported by the NMR data (Figures S5–S13 in Supporting Information
Diversity-oriented synthesis of spirothiazolidinediones and their biological evaluation
Beilstein J. Org. Chem. 2019, 15, 2774–2781, doi:10.3762/bjoc.15.269

- synthesis of thiazolidinedione derivatives is refluxing chloroacetic acid (2) with thiourea (1), followed by a Knoevenagel condensation with an aldehyde (Scheme 1) [25]. Results and Discussion Limited reports are available dealing with the synthesis of spiro derivatives of thiazolidine-2,4-diones [26][27
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- 115 with moderate enantioselectivity (Scheme 38). As shown in Scheme 39, the mechanism involved initial formation of radical cation 117 via anodic oxidation of enamine 116 (obtained from the condensation of 114 and 105'), which then coupled with xanthene radical 119 (Scheme 39). Finally, hydrolysis of
- ). As per the proposed mechanism, products 122 were obtained via nucleophilic addition of enamine 124' to electrophilic intermediate 127 (generated from the anodic oxidation of 120) followed by sequential hydrolysis, proton transfer and immediate intramolecular condensation (Scheme 41). Very recently
- oxidation 201 were prepared from the corresponding cinnamic acids via condensation with ʟ-proline tert-butyl ester followed by ester hydrolysis. Upon oxidative dimerization under Ronlan’s electrochemical conditions, substrates 201 were converted to bislactones 202 with good enantioselectivity. The authors
Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1H-pyridines by Michael addition–cyclocondensation sequences
Beilstein J. Org. Chem. 2019, 15, 2684–2703, doi:10.3762/bjoc.15.262
- 1H-pyridine 8a would have been detected. Therefore, the tentative mechanistic rationale takes into account that the formation of 1H-pyridine 5a rather proceeds via stepwise condensation of alkynone 3 with two equivalents of ethyl cyanoacetate (4) than by reaction with dimer 7 (Scheme 9). First, a
- time on the self-condensation of ethyl cyanoacetate (4) in the presence of the optimized base system.a Michael addition–cyclocondensation synthesis of 1H-pyridine 5 or α-pyrone 6. Photophysical properties of 1H-pyridines 5. Photophysical properties of 1H-pyridines 5a and 5b in the solid state
Synthesis of aryl-substituted thieno[3,2-b]thiophene derivatives and their use for N,S-heterotetracene construction
Beilstein J. Org. Chem. 2019, 15, 2678–2683, doi:10.3762/bjoc.15.261
- . N. Yeltsin, Mira St., 19, Ekaterinburg, 620002, Russia 10.3762/bjoc.15.261 Abstract Fiesselmann thiophene synthesis was applied for the convenient construction of thieno[3,2-b]thiophene derivatives. Thus, new 5- or 6-aryl-3-hydroxythieno[3,2-b]thiophene-2-carboxylates were obtained by condensation
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein J. Org. Chem. 2019, 15, 2664–2670, doi:10.3762/bjoc.15.259
- ). The structure of the compound was finally confirmed by X-ray analysis and elemental analysis data (Figure 1). A possible mechanism for the formation of 1 is presented in Scheme 2. It is well-known that cyclopentanone is prone to self-condensation. In the presence of ammonia these reactions may lead to















































































































































































































































































































































































































































































































