Search results
Search for "intermolecular" in Full Text gives 693 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- ), as well as and from moderately delocalized one to a highly delocalized one (class III) [1][2][3][4][5]. However, attempts to change the MV characteristics by manipulating the bridge moieties through intermolecular interactions have not been reported for bis(NAr3) derivatives. Redox stimuli are a
- maps of 1b+ (Figure 3, bottom). From these regions, 1b+ can form electrostatic interactions with negatively charged species. Indeed, an optimized structure of a complex of 1b+ and PF6− was obtained and featured an intermolecular H-bonding between the N–H proton and the F atom of hexafluorophosphate
Aggregation-induced emission effect on turn-off fluorescent switching of a photochromic diarylethene
Beilstein J. Org. Chem. 2019, 15, 2204–2212, doi:10.3762/bjoc.15.217

- intermolecular Förster Resonance Energy Transfer (FRET) process between the fluorescent units and the photochromic moieties in their closed form within the aggregated state [29]. The crystal did not show any vapochromism, while a dramatic fluorescent color change from green to pink was observed when chloroform
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- . Tables providing comprehensive directories of fulvene cycloaddition chemistry are provided, including fulvene intramolecular and intermolecular cycloadditions complete with reactant partners and their resulting cyclic adducts, which provide a useful reference source for synthetic chemists working with
- and unique range of fused ring and polycyclic scaffolds. In the subsequent sections, the cycloaddition chemistry of fulvenes will be discussed in terms of their dimerization, and intra- and intermolecular reactions. Whereas the high reactivity and poor stability of triafulvenes have limited studies
- reported as intermolecular cycloaddition reactions, there are some interesting reports regarding the intramolecular cycloaddition of fulvenes, summarised in Table 1. For the intramolecular cycloadditions of pentafulvenes, the fulvene has been reported to react as both diene and dienophile depending on the
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- -donating ethylenediamino moiety toward the electron-accepting ethylenediimino moiety in 3 and delocalization of the positive charges in 6a and 7a (Scheme 3), and are not artifacts arising from the strong intermolecular interactions within the crystals. Losing aromaticity, the benzene ring is becoming less
- of moisture. The photochemistry of both compounds is currently under investigation in our labs. Crystal structures Numerous short intermolecular contacts are revealed upon inspection the crystal lattice of 3, 6a and 7a. There are two independent molecules of 3 in the unit cell with slightly different
- , 6a forms a 2D supramolecular network involving the chloride anion Cl1 as an intermolecular connector between the molecules. Each anion interacts with the two N–H protons of the associated dications (Figure 10). In spite of the asymmetrical structure, the X-ray structure of 7a exhibits the same trend
Synthesis of 1-azaspiro[4.4]nonan-1-oxyls via intramolecular 1,3-dipolar cycloaddition
Beilstein J. Org. Chem. 2019, 15, 2036–2042, doi:10.3762/bjoc.15.200
- one order of magnitude lower for EPR spectra in water (0.005–0.006 mT), obviously because strong intermolecular hydrogen bonds are formed due to solvation. The initial rates of the EPR signal decay were used to obtain the rate constants of the nitroxide reduction by ascorbic acid (see Figure 2 and
Synthesis of benzo[d]imidazo[2,1-b]benzoselenoazoles: Cs2CO3-mediated cyclization of 1-(2-bromoaryl)benzimidazoles with selenium
Beilstein J. Org. Chem. 2019, 15, 2029–2035, doi:10.3762/bjoc.15.199
- adjacent molecules being 3.333 (C8–C24) and 3.380 Å (C18–C13) (Figure 1b). Moreover, there were intermolecular interactions between Se(1) and N(3) atoms, and the Se(1)‒N(3) distance was 3.133 Å, which was 86% of the sum of the van der Waals radii (3.54 Å) of both elements (see Supporting Information File 1
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- -elimination and acid hydrolysis to give the key triptophenolide methyl ether (8) in racemic form (16.5%). In 2008, Sherburn and co-workers developed an approach to the formal synthesis of triptolide (Figure 2, route G, Scheme 5) [71]. Key features of the synthesis include two intermolecular Diels–Alder
- reactions and a newly developed deoxygenative aromatization procedure. The first enantioselective Diels–Alder reaction, which is an intermolecular cycloaddition and lactonization between (Z)-3-iodo-4-methylpenta-2,4-dien-1-ol (29) and methyl acrylate (30) in the presence of Mikami’s (binol)TiCl2 catalyst to
Tautomerism as primary signaling mechanism in metal sensing: the case of amide group
Beilstein J. Org. Chem. 2019, 15, 1898–1906, doi:10.3762/bjoc.15.185

- is strongly solvent-dependent as mentioned above and which can also be seen from Figure 2a. For instance, through intermolecular hydrogen bonding with the carbonyl oxygen atom from the tautomeric backbone, chloroform stabilizes the keto tautomer, absorbing at ≈480 nm, while in acetonitrile the enol
- intermolecular interactions not taken into account by the calculations. The former could be the reason in some of the solvents, which have the capacity to stabilize 6K as proton acceptor (dimethylformamide), proton donor (chloroform) or both (ethanol). The latter could be the reason for the keto tautomer
- stabilization in acetonitrile. The explanation for the sudden stabilization of 6K was found by X-ray measurements of its crystal, obtained in acetonitrile. The crystal structure of 6, shown in Figure 3, clearly indicates that the K form is stabilized through the formation of linear intermolecular associates. It
Halide metathesis in overdrive: mechanochemical synthesis of a heterometallic group 1 allyl complex
Beilstein J. Org. Chem. 2019, 15, 1856–1863, doi:10.3762/bjoc.15.181

- ]∞ in low yield, which was crystallographically characterized as a coordination polymer that displays site disorder of the K+ and Cs+ ions. The entropic benefits of mixed Cs/K metal centers, but more importantly, the generation of multiple intermolecular K…CH3 and Cs…CH3 interactions in [CsKA'2
- ]∞, enable an otherwise unfavorable halide metathesis to proceed with mechanochemical assistance. From this result, we demonstrate that ball milling and unexpected solid-state effects can permit seemingly unfavored reactions to occur. Keywords: caesium; entropy; intermolecular forces; mechanochemistry
- not present in the homometallic allyls. A potentially much more important source of stability in [CsKA'2] is the existence of multiple intermolecular M…CH3 interactions, including Cs…CH3 contacts, obviously energetically significant enough that they support the formation of two-dimensional sheets in
Nanopatterns of arylene–alkynylene squares on graphite: self-sorting and intercalation
Beilstein J. Org. Chem. 2019, 15, 1848–1855, doi:10.3762/bjoc.15.180
- graphite and 1,2,4-trichlorobenzene. Self-sorting leads to the intermolecular interdigitation of alkoxy side chains of identical length. Voids inside and between the squares are occupied by intercalated solvent molecules, which numbers depend on the sizes and shapes of the nanopores. In addition, planar
- formed as a result of intermolecular interactions between directionally bound star-shaped species, such as trimesic acid [10], or 1,3,5-benzenetribenzoic acid [11]. However, they sometimes suffer from a breakdown of an intended packing motif leading to solvent-dependent polymorphism [10]. In addition
- , guest molecules can act as alien species that affect the morphologies of intermolecular nanopores [12]. Another approach for the formation of nanopores relies on the physisorption of shape-persistent macrocycles [13][14]. While there are examples for the deposition of organic molecules into rigid
Norbornadiene-functionalized triazatriangulenium and trioxatriangulenium platforms
Beilstein J. Org. Chem. 2019, 15, 1815–1821, doi:10.3762/bjoc.15.175

- monolayers. A linear, π-conjugated spacer (e.g., an ethynyl unit) is attached to the central carbon atom and the functional molecule is mounted on top. This architecture allows investigating molecules on surfaces under controlled conditions. The size of the platforms determines the intermolecular distances
- temperature (Figure 5a). The molecules form a hexagonally ordered self-assembled monolayer (SAM) with an intermolecular distance of (1.23 ± 0.07) nm. This is in agreement with a (√19 × √19) R23.4° superstructure, which was observed in our previous investigations of adlayers of octyl-TATA derivatives [1][6][19
Complexation of 2,6-helic[6]arene and its derivatives with 1,1′-dimethyl-4,4′-bipyridinium salts and protonated 4,4'-bipyridinium salts: an acid–base controllable complexation
Beilstein J. Org. Chem. 2019, 15, 1795–1804, doi:10.3762/bjoc.15.173

- -polar solvent, acetone hampers or competes the intermolecular non-covalent interactions between the hosts and the guests, and thus resulted in a decrease of the host–guest complexation. ESIMS studies of the formation of host–guest complexes The electrospray ionization (ESI) mass spectra also confirmed
- H5 in the distance of 1.989 Å (R). In addition, C-H···F hydrogen bonds between the two adjacent guests with the distance of 2.420 (S), 2.474 (T) and 2.187 Å (U), respectively, are observed as well. These multiple intermolecular hydrogen-bonding interactions between the host and the guest might be the
- multiple intermolecular non-covalent interactions with distances ranging from 1.651 to 2.575 Å. Compared with hosts H2 and H3, helic[6]arene H1 and its derivatives H4 and H5 all show multiple hydrogen-bonding interactions with the examined guests, which were confirmed by not only X-ray crystal structures
Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–)2A fluorescent dyes
Beilstein J. Org. Chem. 2019, 15, 1712–1721, doi:10.3762/bjoc.15.167

- known that D–π–A fluorescent dyes show bathochromic shifts of the λmax,fl and lower Φf values by changing from the solution state to the solid state. This fact is attributed to the delocalization of excitons or excimers due to the formation of intermolecular π–π interactions [48][49][50][51] between the
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
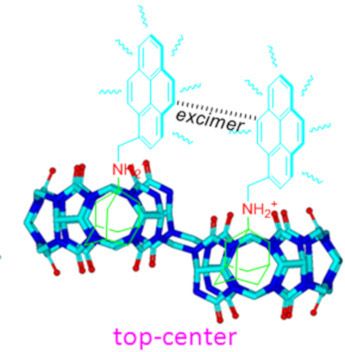
- , the fluorescence intensity of the G2 monomer emissions gradually decreased, while the maximum emission intensity at around 485 nm (typical excimer emissions of pyrene) increased. The excimer emission band of G2 can be attributed to the interaction of two pyrene units resulting in intermolecular π–π
- encapsulation of this moiety. Pyrene group, that are too large for the individual CB[6]–CB[7] sized cavities of ns-CB[10] [23], it is thus believed that the upfield shift of the pyrene protons is attributed the intermolecular π–π stacking between the two pyrenyl moieties as proposed in the plausible inclusion
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- oxidation states (Cu(0), Cu(I), Cu(II), and Cu(III)). Copper has been known for a long time to act as a catalyst for cross-coupling reactions (Ullmann–Goldberg reaction), cyanation of aryl halides (Rosenmund–von Braun reaction), Hurtley reaction and intermolecular oxidative cyclization of haloalkynes [41
- intermolecular oxidative diamination of haloalkynes, mild reaction conditions and efficient conversion of the alkyl-substituted haloalkynes to the halogenated product were great improvements over existing methods. Stimulated by the work of Luz et al. [98] and Phan et al. [99] with Cu(BDC)MOF (BDC: 1,4
- -benzenedicarboxylate), Puthiaraj and co-workers have unprecedently discovered the catalytic activity of this metal-organic framework (MOF) for the synthesis of imidazo[1,2-a]pyridines [100]. The three-component, one-pot reaction between 1, 3 and nitromethane (10, Scheme 5) involved an intermolecular aza-Michael
Different reactivity of phosphorylallenes under the action of Brønsted or Lewis acids: a crucial role of involvement of the P=O group in intra- or intermolecular interactions at the formation of cationic intermediates
Beilstein J. Org. Chem. 2019, 15, 1491–1504, doi:10.3762/bjoc.15.151
- phosphonic acids and other phosphorus-containing compounds. Contrary to Brønsted acids, 3-methylbuta-1,2-dien-1-ylphosphonic dichloride [Cl2(O=)P–HC=C=CMe2] reacted with the Lewis acid AlCl3 in an intermolecular way forming noncyclic intermediates, which were investigated by NMR spectroscopy and DFT
- intermolecular hydroarylation of allenes bearing electron-withdrawing substituents. Plausible reaction mechanisms have been proposed on the basis of the investigated reactions, and NMR analysis and DFT studies of the intermediate cationic species. Keywords: aluminum chloride; cation; intermediate
- ][16]. It should be especially emphasized that intermolecular reactions of phosphorylallenes with arenes have not been yet achieved. In general, intermolecular hydroarylation of allenes has been developed for reactions catalyzed by complexes of various metals [17], such as Pd [18][19][20], Pt [21], Au
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- confirmed the basic features of the NMR-derived structure. Furthermore, a pair of enantiomers of 3a presented in the crystal, which formed a dimeric complex through intermolecular coordination between the Zn2+ center and the carbonyl group of the second molecule. Keywords: dipolar cycloaddition
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- often emit strongly in their dilute solution as isolated molecules, but once aggregation occurred, fluorescence is quenched owing to intermolecular packing. These ACQ dyes generally possess large conjugated coplanar molecular structures which lead to strong intermolecular interactions. Fl is one of the
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- of the heterotriacenes should be expected planar, a slight curvature of the π-system was found for DST 3, whose α-carbon atoms are bent relative to the central thiophene plane by about 2.5 degrees (Figure 2b). This effect might be due to strong intermolecular π–π interactions in pairs of molecules
- (Figure S8, Table S3, and Table S6 in Supporting Information File 1). In the case of DTT 1 only three non-bonding contacts between sulfur atoms in the b-axis direction were found which imply a 1-dimensional intermolecular electronic coupling in the molecular columns separated from each other by distances
- signals at expected strong π–π intermolecular distances of 3.5–3.3 Å (2Θ = 25–26°), at offset π–π intermolecular distances of 4.1 Å (2Θ = 21.5°), and at herringbone intermolecular interaction distances of 8.2 Å (2Θ = 10.8°) were found and correlated with the Miller indices obtained in the X-ray single
One-pot activation–alkynylation–cyclization synthesis of 1,5-diacyl-5-hydroxypyrazolines in a consecutive three-component fashion
Beilstein J. Org. Chem. 2019, 15, 1360–1370, doi:10.3762/bjoc.15.136
- to spectroscopic assignment the structure of 6a has now been corroborated by an X-ray structure analysis showing infinite chains of molecules 6a formed by intermolecular hydrogen bonding between the pyrazole N1 and the carbonyl O1 (Figure 3) [53]. The first assumption was that the tentative
- ) and antibacterial activity (center and right). ORTEP plot of 5-benzoyl-3-phenyl-1H-pyrazole (6a) (thermal ellipsoids at 30% probability); the direction of intermolecular N−H···O hydrogen bonding is indicated by dashed lines. Ellipsoid plot of 1-Boc-5-benzoyl-5-hydroxypyrazoline 5a. ORTEP plot and
Formation of an unexpected 3,3-diphenyl-3H-indazole through a facile intramolecular [2 + 3] cycloaddition of the diazo intermediate
Beilstein J. Org. Chem. 2019, 15, 1347–1354, doi:10.3762/bjoc.15.134
- -3H-indazole core of 8 carries both benzhydryl and germinal diphenyl groups. These aromatic substituents make significantly different intermolecular contacts in the crystal. The phenyl rings of the former (C1A to C6A, and C1B to C6B) make complementary C–H···π interactions with the related molecule
- . Possible compounds with the molecular formula C33H26N2O (structure 7 contains 27 hydrogen atoms). ORTEP view of the molecule 8 showing the atom labelling (ellipsoids are drawn at 50% probability level). Significant intermolecular interactions made by the benzhydryl group (a, upper) and the gem-diphenyl
Synthesis of dipolar molecular rotors as linkers for metal-organic frameworks
Beilstein J. Org. Chem. 2019, 15, 1331–1338, doi:10.3762/bjoc.15.132
- molecular rotors have been synthesized by several groups. Molecular rotors with permanent dipole moments can be oriented by an external electric field as shown by Michl [26][27] and Price [28][29], or undergo spontaneous ordering by intermolecular dipole–dipole interactions. The ultimate goal is the
- ordered state with aligned dipoles depends on the arrangement of the rotors and the strength of their interactions. MOFs [31][32] und particular SURMOFs [33][34] are ideally suited to achieve an ordered 3D arrangement and to maximize intermolecular interactions, because the dipolar rotors are used as
- an intermolecular distance of 10 Å), which is a typical distance in MOF lattices. The structures are fully optimized at the PBE-D3/defSVP level of theory within the point groups C2v, C2h and D2h. Relative energies (Erel) are given in kcal mol−1 and in meV (in brackets). The arrows indicate the
Bambusuril analogs based on alternating glycoluril and xylylene units
Beilstein J. Org. Chem. 2019, 15, 1268–1274, doi:10.3762/bjoc.15.124

- urea groups can participate in intermolecular hydrogen bonding resulting in the formation of tubular [5][6][7] or gel-like [8] structures. Ureas lacking of N–H hydrogen atoms such as ethyleneureas, glycolurils, and biotin are also important building blocks of macrocyclic receptors such as cucurbiturils
- good agreement with the structure of the 1b-1 conformer calculated by DFT. The parallel glycoluril units are placed on one side of the plane defined by the xylylene moieties. The macrocycles form a layered structure stabilized by intermolecular hydrogen bonding interaction between the carbonyl oxygens
Self-assembly behaviors of perylene- and naphthalene-crown macrocycle conjugates in aqueous medium
Beilstein J. Org. Chem. 2019, 15, 1203–1209, doi:10.3762/bjoc.15.117

- through hydrogen bonding and metal ion coordination in nonpolar solvents [66][67][68]. Compared with NDI, PDI has more aromatic rings to generate stronger intermolecular π−π stacking, leading to molecular aggregates more easily, and these aggregates or supramolecular assemblies can give rise to desirable
Switchable selectivity in Pd-catalyzed [3 + 2] annulations of γ-oxy-2-cycloalkenones with 3-oxoglutarates: C–C/C–C vs C–C/O–C bond formation
Beilstein J. Org. Chem. 2019, 15, 1107–1115, doi:10.3762/bjoc.15.107
- -2-cycloalkenones, simply differing on the reaction temperature, are disclosed. These domino transformations allow C–C/O–C or C–C/C–C [3 + 2] annulations at will, via an intermolecular Pd-catalyzed C-allylation/intramolecular O- or C-1,4-addition sequence, respectively. In particular, exploiting the
- , resonance-stabilized acetamides and cyclic α,β-unsaturated-γ-oxicarbonyl derivatives are used as bis-nucleophile and bis-electrophile partners, respectively. This process involves an intermolecular Pd(0)-catalyzed C-allylation (Tsuji–Trost reaction)/intramolecular nitrogen 1,4-addition sequence (Scheme 1
- stoichiometric intermolecular 1,4-addition step (i.e., the potential initial addition of the nucleophile to the C-β of the bis-electrophile) has to be slower than the intermolecular addition of the nucleophile to the catalytically generated η3-allylpalladium complex, or it has to be at least a reversible process











































































































































































































































































































































































































































