Search results
Search for "isomerization" in Full Text gives 386 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
A combinatorial approach to improving the performance of azoarene photoswitches
Beilstein J. Org. Chem. 2019, 15, 2753–2764, doi:10.3762/bjoc.15.266

- demonstrate slow thermal Z–E relaxation and the potential to switch with red light, while the o-fluoro compounds reported by Hecht and co-workers [13][14] offer excellent two-way isomerization with visible light and the longest thermal half-life reported for an azobenzene molecule (≈700 days at 25 °C) to date
- (≈1000 days at 25 °C); one of the most stable azo photoswitches reported to date. We further extended the family of arylazopyrazoles with the help of theoretical modelling and discovered 3pzH to be near quantitatively (>98%) switched back and forth between isomers, with a long thermal isomerization half
- drastically increase the isomerization half-life (to months or years) and allow further tuning of the photoaddressability of each isomer. We believe that the structure–property relationships described will guide further development of azoheteroarene photoswitches (particularly arylazopyrazoles), and their use
Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series
Beilstein J. Org. Chem. 2019, 15, 2655–2663, doi:10.3762/bjoc.15.258
- rearrangements had been described previously. Isomerization of o,o’-quaterphenyl (17) with SnCl4/AlCl3 catalysis has been reported to yield a mixture of p,p’- (12), m,p’- (13), and m,m’-quaterphenyl (14) [34]. p,p’-Quaterphenyl (12) and m,m’-quaterphenyl (14) were available commercially. To complete the series
AgNTf2-catalyzed formal [3 + 2] cycloaddition of ynamides with unprotected isoxazol-5-amines: efficient access to functionalized 5-amino-1H-pyrrole-3-carboxamide derivatives
Beilstein J. Org. Chem. 2019, 15, 2623–2630, doi:10.3762/bjoc.15.255
- derivatives can be obtained in up to 99% yield. The reaction mechanism might involve the generation of an unusual α-imino silver carbene intermediate (or a silver-stabilized carbocation) and subsequent cyclization/isomerization to build the significant pyrrole-3-carboxamide motif. The reaction features the
- silver catalyst from E along with the formation of enamide motif affords 3H-pyrrole F. A final aromatization step by isomerization provides the desire cyclic product 10aa. Notably, two possible cyclization routes from D’ (or D) to give 7-membered rings G and H cannot be achieved through the attack of the
- then cyclization/isomerization to complete the formal [3 + 2] cycloaddition process. This reaction highlights the use of an inexpensive catalyst, and simple work-up without column chromatographic purification for most of the products. Furthermore, these products contain the core structure of many
A toolbox of molecular photoswitches to modulate the CXCR3 chemokine receptor with light
Beilstein J. Org. Chem. 2019, 15, 2509–2523, doi:10.3762/bjoc.15.244

- pharmacological alternative, especially with respect to spatial and temporal precision [1][2]. Photochromic ligands usually contain a molecular photoswitch (photoswitchable moiety) that under certain wavelengths of illumination undergoes an isomerization event, thereby changing the properties of the molecule and
- spatial disposition similar to the original biaryl unit and, therefore, a similar biological activity that might change upon isomerization of the azobenzene [8]. The second reason for the success of azobenzene in the photopharmacology field is the robust photoisomerization. It provides typically high
- substituent to the binding site of the receptor [24]. In order to obtain an efficacy photoswitching, we opted for replacing the biaryl moiety for an azobenzene in an azologization approach (Figure 1D) with the expectation that the isomerization of the azobenzene would provide changes in 3D shape that are
In search of visible-light photoresponsive peptide nucleic acids (PNAs) for reversible control of DNA hybridization
Beilstein J. Org. Chem. 2019, 15, 2500–2508, doi:10.3762/bjoc.15.243

- tetra-ortho-fluoroazobenzene–PNA conjugates have promising properties (fast reversible isomerization, exceptional thermal stability, high isomer conversions and sensitivity to visible-light irradiation) as reversible modulators to control oligonucleotide hybridization in biological contexts. Furthermore
- after the cleavage from the solid support. Among the initial studied photoswitchable PNAs, the PNA12(oF4Azo) (3) displayed the most promising properties as reversible modulator of oligonucleotide hybridization. Thus, it displayed the fastest reversible isomerization (≈2 s for cis → trans and ≈120 s for
- perform the isomerization after duplex hybridization, which resulted in only a constant 36% cis-isomer rate. The obtained TM values suggested that the localization of the oF4Azo affects both the duplex stability and isomer differences. Thus, the incorporation of oF4Azo near the center of the PNA (7 and 8
Experimental and computational electrochemistry of quinazolinespirohexadienone molecular switches – differential electrochromic vs photochromic behavior
Beilstein J. Org. Chem. 2019, 15, 2473–2485, doi:10.3762/bjoc.15.240
- photochromism results from a spirocyclic ring-opening or other isomerization which results in increased conjugation. Electrochromism is also of increasing materials relevance, e.g., for self-dimming automotive mirror and aircraft window darkening applications [6][7][8]. In electrochromic applications, the color
- as photooxidants, the difference in the reduction potential between LW and SW would need to be increased further, and LW would need to be more reducible to be of use in photooxidation of relevant substrates (e.g., Dewar benzenes, quadricyclanes, or bishomocubanes as quantum amplified isomerization
- above. In the cases of both D0 and T0, the longer bond is the one broken in the isomerization of SW 3a to eLW 5a and pLW 4a, respectively. Similar results are also observed for S0. Though the differences are much more modest in S0, the heteroN–C bond is computed to be both longer and weaker, as in D0
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
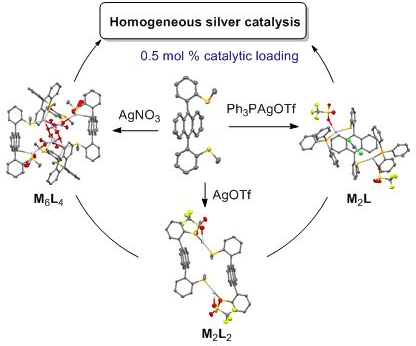
- the 9,10-aryl substituents in respect to the anthracene core, without any indication of a syn-to-anti isomerization. Four silver(I) complexes 1a–d were prepared in excellent yield (77–92%) by mixing the ligand and the following silver salts in a 1:1 ratio in dichloromethane at room temperature: AgOTf
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- , belonging to the hydrazone family. The outstanding properties of this molecule, involving fluorescence toggling, bistability, high isomerization quantum yield and non-negligible two-photon absorption cross section, make it very promising for numerous applications. Here we show that the light induced Z/E
- isomerization occurs on a fast <1 ps timescale in both toluene and acetonitrile, while the excited state lifetime of the Z-form depends on solvent polarity, suggesting a partial charge transfer nature of its low lying excited state. Time-resolved luminescence measurements evidence the presence of a main
- shown in Figure 1. Irradiation of a solution of 1 using 442 nm light induces a Z/E isomerization resulting in a color change, evidenced by the decrease of the absorption at 395 nm and the appearance of a new band with a maximum at 343 nm. The E-form is extremely stable (half-life of 75 years in toluene
Effect of ring size on photoisomerization properties of stiff stilbene macrocycles
Beilstein J. Org. Chem. 2019, 15, 2408–2418, doi:10.3762/bjoc.15.233

- a six carbon chain) showed some degree of isomerization when irradiated. DFT calculations of the energy differences between the E- and Z-isomers show the same trend as the experimental results. Interestingly the DFT study highlights that the energy difference between the E- and Z-isomers of even the
- the resulting macrocycle. Keywords: DFT; molecular mechanics; photostability; photo-switch; ring-strain; stiff stilbene; Introduction The stiff stilbene (SS) molecule has drawn a lot of interest due to its photodynamic properties [1]. Stiff stilbenes typically undergo light triggered isomerization
- ). Photoisomerization Photoisomerizing the (Z)-1a–d to the (E)-1a–d isomers requires to stretch the linker. The isomerization was achieved by irradiation of a degassed solution of (Z)-1a–d in chloroform or deuterated chloroform using either a 280 or 300 nm filter (Scheme 3). The conversion was followed by UV–vis or 1H
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- switching to the Z isomer was accompanied by the release of one of the two guests from the cage and the formation of a 1:1 cage/Z-arylazopyrazole inclusion complex. DFT calculations suggest that this process involves a dramatic change in the conformation of the cage. Back-isomerization was induced with
- green light and resulted in the initial 1:2 cage/E-arylazopyrazole complex. This back-isomerization reaction also proceeded in the dark, with a rate significantly higher than in the absence of the cage. Keywords: arylazopyrazoles; coordination cages; inclusion complexes; molecular switches
- affected by their confinement to the surfaces of inorganic nanoparticles. Among other examples, the isomerization kinetics of azobenzene could be tuned by a factor of >5000 by the molecules with which it was co-adsorbed on gold nanoparticles [23], and donor–acceptor Stenhouse adduct (DASA) switches were
Synthesis of acremines A, B and F and studies on the bisacremines
Beilstein J. Org. Chem. 2019, 15, 2271–2276, doi:10.3762/bjoc.15.219
- should then close the tetrahydrofuran ring of the natural product. Upon irradiation of 5 using a Hg lamp, however, the only productive pathway which could be observed was isomerization of the disubstituted double bond (Table 1, entries 17 and 18). Again, we attempted to promote the reaction by tethering
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups
Beilstein J. Org. Chem. 2019, 15, 2213–2270, doi:10.3762/bjoc.15.218
- formation of fluorinated products with an overall retention of the stereochemical configuration suggests a mechanism wherein a palladium-π-allyl intermediate undergoes a rapid π-σ-π isomerization. In 2013, the first example of an allylic C–H fluorination reaction of simple alkenes with Et3N·3HF as a
Harnessing enzyme plasticity for the synthesis of oxygenated sesquiterpenoids
Beilstein J. Org. Chem. 2019, 15, 2184–2190, doi:10.3762/bjoc.15.215
- group could react at C10 to induce a fast 1,11-cyclisation, forming cation 25, which effectively competes with the isomerization of 11 to 8-methoxy-NDP. A subsequent [1,3]-hydride shift leads to 24 (Scheme 3A). Direct deprotonation of 22 at C15 forms the minor reaction product (E)-8-methoxy-β-farnesene
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- physicochemical properties upon irradiation with light, represent one major approach to this. One of the most common light-driven transformations exploited in molecular photoswitches is the E–Z isomerization of double bonds [18]. In this context, the photochemistry of stilbenes and the closely related azobenzenes
- provided deeper insights and clarified the differential behaviour observed in the UV–vis spectra of 2b and 2f after UV irradiation. As anticipated, UV irradiation lead to E→Z isomerization of the C=C double bond in both compounds. The (Z)-isomers were found to be slightly more polar than the respective (E
- reversibly between two states comprising high amounts of (E)-11 and (Z)-11, respectively. The other part of the hypothesis was, that by this photoinduced isomerization a considerable drop of activity would occur due to the conformational change and the resulting changed geometry and polarity. However, this
Characterization of two new degradation products of atorvastatin calcium formed upon treatment with strong acids
Beilstein J. Org. Chem. 2019, 15, 2085–2091, doi:10.3762/bjoc.15.206

- dehydration of the δ-hydroxy group and some epimers resulting from acid-catalyzed isomerization reactions. In contrast, Vukkum et al. [13] describe, besides lactones 2 and 3, an α,β-unsaturated carboxylic acid 4. Treatment under more drastic conditions (6 M HCl, reflux, 3 h) was reported to result mainly in
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- isomer 43. Treatment of 43 with methoxide ions in methanol at room temperature for 15 min gave the desired C-5 trans-butenolide 8 (40%) along with its C-5 cis-epimer 44 (60%), which is the sole product from the base-catalyzed isomerization of 43. Epoxidation of 43 gave a C-4,5-epoxide intermediate, which
- isomerization of olefin 43, the benzylic oxidation of 8, the use of m-CPBA to introduce the C-9,11 epoxide and the non-stereoselective reduction of the C-14 carbonyl group using sodium borohydride, caused an unacceptable overall yield (1.6%). This pioneering work undoubtedly established the basis for the future
- under the optimal photoredox conditions to provide tetracyclic intermediate 19i (dr = 1:1, Scheme 8, route L), with a cis A-/B-ring connection rather than the desired trans connection. Treatment of 19i with H2SO4, followed by RuCl2(PPh3)3-catalyzed double bond isomerization gave the known intermediate
Norbornadiene-functionalized triazatriangulenium and trioxatriangulenium platforms
Beilstein J. Org. Chem. 2019, 15, 1815–1821, doi:10.3762/bjoc.15.175

- , while the functional groups are protruding upright and freestanding from the central carbon atoms. Azobenzene derivatized TATA’s are known to exhibit extremely fast cis→trans isomerization on metal surfaces, via a peculiar non-adiabatic singlet→triplet→singlet mechanism. We now prepared norbornadienes
- (NBD) and quadricyclanes (QC) attached to TATA and TOTA platforms which can be used to check if these accelerated rates and the spin change mechanism also apply to [2 + 2] cycloreversions (QC→NBD). Keywords: [2 + 2] cycloaddition; [2 + 2] cycloreversion; norbornadiene; photochemical isomerization
- ; quadricyclane; self-assembled monolayers; TATA platform; thermal isomerization; TOTA platform; Introduction Recently, we discovered that the thermochemically forbidden cis–trans isomerization of azobenzenes can be efficiently catalysed by a very peculiar mechanism on bulk gold [1]. In heterogeneous catalysis
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- abstraction from the sp3 carbon atom leading to the formation of six-membered Cu(III) species 42. Furthermore, consecutive isomerization/oxidation/reductive elimination leads to the generation of final compound 37 with regeneration of the Cu(I) catalyst (Scheme 14). The presence of EDGs as compared to EWGs on
- activation of the C–H bond in the alkyne by copper on ZnAl2O4 initiated an intramolecular nucleophilic attack of pyridine nitrogen to the triple bond (135). This was followed by aromatic isomerization to form imidazo[1,2-a]pyridines (Scheme 46). The importance of Cu for this reaction is mentioned in Table 4
Synthesis of ([1,2,4]triazolo[4,3-a]pyridin-3-ylmethyl)phosphonates and their benzo derivatives via 5-exo-dig cyclization
Beilstein J. Org. Chem. 2019, 15, 1563–1568, doi:10.3762/bjoc.15.159
- nucleophilic substitution of chlorine in the chloroethynylphosphonate to form ynamine intermediate A, isomerization of which provides ketenimine B. Further formation of the imine tautomer C enables an intramolecular 5-exo-dig cyclization to furnish the title [1,2,4]triazolo[4,3-a]pyridines (Scheme 6
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- , whereas by the use of visible light the stretched trans,trans-isomer is formed. By means of quantum chemical calculations and CD spectroscopy we could show that the trans→cis isomerization is spatially directed; that means that one of the two different macrocycles performs a definite clockwise rotation to
- the other, caused by irradiation with UV light. For the cis→trans isomerization counterclockwise rotations are found. Furthermore, quantum chemical calculations reveal that the energy of the cis,cis-isomer is only slightly higher than the energy of the cis,trans-isomer. This effect can be explained by
- , the trans→cis isomerization is triggered by UV light whereas the cis→trans back relaxation takes place by visible light or heat [30][42]. Due to the high reversibility, the simple synthesis and the high photostability azobenzene derivatives are the most commonly used switching units. A further
Diazocine-functionalized TATA platforms
Beilstein J. Org. Chem. 2019, 15, 1485–1490, doi:10.3762/bjoc.15.150

- Abstract Recently, it has been shown that the thermochemical cis→trans isomerization of azobenzenes is accelerated by a factor of more than 1000 by electronic coupling to a gold surface via a conjugated system with 11 bonds and a distance of 14 Å. The corresponding molecular architecture consists of a
- to the trans-form, which slowly returns back to the stable cis-isomer. To investigate the thermal trans→cis isomerization as a function of the conjugation to the metal surface, we connected the acetylene spacer in meta (weak conjugation) and in para (strong conjugation) position. Both isomers form
- ordered monolayers on Au(111) surfaces. Keywords: cis–trans isomerization; diazocine; molecular switch; photochrome; self-assembled monolayers; TATA platform; Introduction Catalysts increase chemical reaction rates by lowering the activation energies and thus create more favorable reaction pathways [1
Reversible end-to-end assembly of selectively functionalized gold nanorods by light-responsive arylazopyrazole–cyclodextrin interaction
Beilstein J. Org. Chem. 2019, 15, 1407–1415, doi:10.3762/bjoc.15.140

- -functionalized with the CD, no assembly could be observed after addition of the divalent azobenzene linker. Moreover, the assemblies could only once be disassembled by the combination of UV irradiation and physical forces by sonication. The light-induced back-isomerization of azobenzenes did not form similar end
- photophysical properties like nearly quantitative isomerization, straight forward synthesis in excellent yields and very long Z-isomer half-life times up to 1000 days due to less steric repulsion [37]. In previous reports we could show that the AAP guest inclusion properties to β-CD are comparable to
Precious metal-free molecular machines for solar thermal energy storage
Beilstein J. Org. Chem. 2019, 15, 1096–1106, doi:10.3762/bjoc.15.106

- constants of metal complexes were determined and are in good agreement with the literature data for reference dyes. The temporal evolution of trans-to-cis isomerization was observed in a real-time regime. The dyes demonstrated a low intrinsic fluorescence of their Ba2+ complexes and high yield of E/Z
- depends strongly on the Ba2+ concentration. The cis isomers formed upon irradiation are thermally unstable and revert to the trans isomers in the dark [23]. The rates of trans-to-cis isomerization were determined for several Ba2+ concentrations in the range of 2 × 10−3 M up to 1 M at a fixed dye
- ), as for the free dye 4b, increased with the irradiation time, suggesting that isomerization from trans to a cis form of the free dye or its Ba2+ complex proceeded until a photostationary state was reached. As can be seen from Figure 4 the trans-to-cis isomerization takes place to a higher extent in
Multicomponent reactions (MCRs): a useful access to the synthesis of benzo-fused γ-lactams
Beilstein J. Org. Chem. 2019, 15, 1065–1085, doi:10.3762/bjoc.15.104
- Heck cyclization reaction between the substituted alkyne and aryl bromide in 133 takes place to form a cyclic palladium intermediate 134 with E-configuration, resulting from a syn-addition mechanism of this step. The addition of a silver salt reduces the probability of isomerization of the double bond
Easy, efficient and versatile one-pot synthesis of Janus-type-substituted fullerenols
Beilstein J. Org. Chem. 2019, 15, 901–905, doi:10.3762/bjoc.15.87

- from the isomerization and fragmentation of the oxygen species in addition to the fragmentation of the substituents. The signals can be assigned with a general formula [M − xH2O − yH − zO − v(HNR)]a−/+. The molecular ion peak of the janus-fullerenol with 1 as substituent can be identified at m/z 1798.6



























































































































































































































































































































































































































































































































































































