Search results
Search for "isoxazoline" in Full Text gives 27 result(s) in Beilstein Journal of Organic Chemistry.
Highly electrophilic, gem- and spiro-activated trichloromethylnitrocyclopropanes: synthesis and structure
Beilstein J. Org. Chem. 2026, 22, 123–130, doi:10.3762/bjoc.22.5
- nitro group upon breaking the C–C bond [16][17]. Substituted nitrocyclopropanes in reactions with various nucleophiles form linear precursors for the synthesis of γ-substituted α-aminobutyric acids [18][19], cyclic nitropyrrolines [20] and isoxazoline N-oxides [18]. The nitrocyclopropane fragment is a
Oxetanes: formation, reactivity and total syntheses of natural products
Beilstein J. Org. Chem. 2025, 21, 1324–1373, doi:10.3762/bjoc.21.101
Recent advances in oxidative radical difunctionalization of N-arylacrylamides enabled by carbon radical reagents
Beilstein J. Org. Chem. 2025, 21, 1207–1271, doi:10.3762/bjoc.21.98
- oxidant and initiator, enabling an efficient intermolecular cascade cyclization process (Scheme 5) [5]. In this strategy, a novel, selective, metal-free synthetic method was introduced for the synthesis of isoxazoline-featured oxindoles through iminoxyl radical-promoted cascade oxyalkylation
- intermediate 12. Subsequent intramolecular cyclization and oxidative aromatization lead to the final isoxazoline-featured oxindole 9. In 2017, Li’s group reported an oxidative divergent bicyclization of 1,n-enynes through α-C(sp3)–H functionalization of alkyl nitriles using a Sc(OTf)3 and Ag2O system (Scheme 7
A study on selective transformation of norbornadiene into fluorinated cyclopentane-fused isoxazolines
Beilstein J. Org. Chem. 2021, 17, 2051–2066, doi:10.3762/bjoc.17.132
- multiple stereogenic centers. The synthesis involved selective cycloadditions, with subsequent ROM of the formed cycloalkene-fused isoxazoline scaffolds and selective CM by chemodifferentiation of the olefin bonds of the resulting alkenylated derivatives. Various experimental conditions were applied for
- the CM transformations with the goal of exploring substrate and steric effects, catalyst influence and chemodifferentiation of the olefin bonds furnishing the corresponding functionalized, fluorine-containing isoxazoline derivatives. Keywords: functionalization; metathesis; nitrile oxide
- they, in particular G-2, provided a superior combined yield of (±)-8a and (±)-8b. Note, that although the yield of the CM is relatively low, a full conversion of the starting isoxazoline could be detected. However, all CM transformations alongside the desired coupled compounds afforded a significant
Oxime radicals: generation, properties and application in organic synthesis
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- rule, a C–O bond is formed in intermolecular reactions, intramolecular cyclization generally occurs with the formation of a five-membered cycle of isoxazoline (C–O bond formation) or nitrone (C–N bond formation). Application of the oxime radicals in organic synthesis: intermolecular reactions Selective
- the radical 65 gives a C-centered radical 66, which is captured by TEMPO to form intermediate 67. Elimination of TEMPOH leads to a β-unsaturated oxime 68, which could undergo cyclization by ionic or radical mechanisms to give isoxazoline 63 [114][115]. A similar cyclization with the formation of
- (products 135a,d–h) and alkyl substituents (products 135b,c) at the oxime fragment (R1). An oxime with a disubstituted double bond (R2 = Me) also reacts with the formation of isoxazoline 135g having a quaternary carbon atom. Under the action of t-BuOOH (TBHP), β,γ-unsaturated oximes 136 undergo a cascade
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
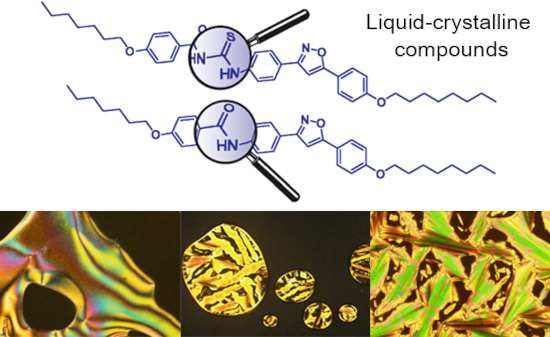
- Ciências e Tecnologia, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal Institute of Chemistry, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil 10.3762/bjoc.16.20 Abstract A series of novel thiourea and amide liquid crystals containing 5-membered isoxazoline and isoxazole rings
- heterocycle. Keywords: amides; isoxazole; isoxazoline; liquid crystal; thiourea; Introduction Thioureas are a structurally diversified group of organic compounds, with technological applications in different areas. The structural diversity of the thiourea moiety is linked to possibly one or both nitrogen
- , mainly due to their structural and electronic features. Isoxazoline and isoxazole cores show strong dipole moments, polarizabilities, anisotropic interactions and geometrical aspects that favor the formation of stable smectic A and nematic mesophases, respectively [19][20]. This work aimed at
Application of chiral 2-isoxazoline for the synthesis of syn-1,3-diol analogs
Beilstein J. Org. Chem. 2019, 15, 1840–1847, doi:10.3762/bjoc.15.179
- Juanjuan Feng Tianyu Li Jiaxin Zhang Peng Jiao Key Laboratory of Radiopharmaceuticals, College of Chemistry, Beijing Normal University, Beijing 100875, P.R. China 10.3762/bjoc.15.179 Abstract The asymmetric cycloaddition of TIPS nitronate catalyzed by “Cu(II)-bisoxazoline” gave the 2-isoxazoline
- product in 95% yield, which was converted into tert-butyl (3S,5R)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate in 14 steps through a β-hydroxy ketone. Keywords: β-hydroxy ketone; cycloaddition; 1,3-diol; isoxazoline; silyl nitronate; Introduction The chiral 1,3-diol structure is widespread in a
- chiral β-hydroxy-δ-lactone moiety or its equivalents, pioneered by Wong [42], is equally competitive. Here, we report the preparations of tert-butyl (3S,5R)-6-hydroxy-3,5-O-isopropylidene-3,5-dihydroxyhexanoate and related syn-1,3-diol analogs from a chiral 2-isoxazoline (Scheme 1b). This work is part of
Introduction of an isoxazoline unit to the β-position of porphyrin via regioselective 1,3-dipolar cycloaddition reaction
Beilstein J. Org. Chem. 2019, 15, 1434–1440, doi:10.3762/bjoc.15.143

- Collaborative Innovation Center of Chemical Science and Engineering, Tianjin 300072, China 10.3762/bjoc.15.143 Abstract Isoxazoline-linked porphyrins have been synthesized by a regioselective 1,3-dipolar cycloaddition reaction between vinylporphyrin 2 and nitrile oxides. The steric interaction directed the
- reaction trajectory, in which only the product with a link between the 5-position of the isoxazoline and the β-position of porphyrin was observed. The isoxazoline-porphyrins 3a,b have been characterized by absorption, emission, 1H NMR and mass spectra. Later, the crystal structure of 3a was obtained and
- ; isoxazoline; macrocycles; nitrile oxide; porphyrin; Introduction In nature, porphyrin-type compounds play a prominent role in life [1]. It is well known that certain vital functions, like O2 transport, photosynthesis etc. depend on the action of porphyrin–metal complexes [2][3][4][5]. Inspired by the natural
Synthesis of unnatural α-amino esters using ethyl nitroacetate and condensation or cycloaddition reactions
Beilstein J. Org. Chem. 2018, 14, 2846–2852, doi:10.3762/bjoc.14.263
- [21] between two equivalents of ethyl nitroacetate (4) and styrene (15), gave the isoxazoline 16 in a 72% yield as a latent α-amino ester [22][23][24]. From this compound, a reductive cleavage of the isoxazoline ring was initiated using palladium over charcoal and a large excess of ammonium formate in
- ethanol to complete the reduction and the amino ester 18 was isolated in a 72% yield. In order to illustrate the synthetic potential of the isoxazoline 16 as an already protected amino acid moiety, we prepared the piperazine-2,5-diones 23a,b in four steps. This was achieved by the hydrolysis of the ester
Ring-opening metathesis of some strained bicyclic systems; stereocontrolled access to diolefinated saturated heterocycles with multiple stereogenic centers
Beilstein J. Org. Chem. 2018, 14, 2698–2707, doi:10.3762/bjoc.14.247
- . Starting from various cyclodienes, the corresponding derived bicyclic lactone, lactam, and isoxazoline derivatives were submitted to ROM under ethenolysis. These functionalized, strained bicyclic systems afforded novel highly-functionalized diolefinated heterocyclic scaffolds in ROM reactions with
- (5 mol %), leading at 20 °C to δ-lactone derivative (±)-15 although with low yield (Scheme 6, Table 4). In continuation, we selected a cyclooctene-fused system, namely isoxazoline (±)-16 which, in turn, was accessed through nitrile–oxide dipolar cycloaddition, by using nitroethane, DMAP and Boc2O
- . Ring opening proved to be successful with second generation catalysts, yielding the corresponding diolefinated isoxazoline (±)-17 (Scheme 6). Our studies were continued with the ROM reactions of conformationally restricted γ-lactam (±)-18 (Vince’s lactam) as model compound [24]. The ring opening in
Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry
Beilstein J. Org. Chem. 2018, 14, 2035–2064, doi:10.3762/bjoc.14.179
- in biologically active molecules. This reaction was included due to the ability to introduce chirality into lead structures, something that is valuable in medicinal chemistry. The straightforward synthesis of isoxazoline 29a from the corresponding vinyl aldehyde is a perfect example of the potential
Hypervalent iodine-mediated Ritter-type amidation of terminal alkenes: The synthesis of isoxazoline and pyrazoline cores
Beilstein J. Org. Chem. 2018, 14, 1028–1033, doi:10.3762/bjoc.14.89
- alkene functionalization utilizing a PhI(OAc)2 ((diacetoxyiodo)benzene, PIDA)/Lewis acid combination in order to access isoxazoline and pyrazoline cores. Based on allyl ketone oximes and allyl ketone tosylhydrazones, we have developed an alkene oxyamidation and amido-amidation protocol en route to
- accessing both isoxazoline and pyrazoline cores. Additionally, acetonitrile serves as both the solvent and an amine source in the presence of this PIDA/Lewis acid combination. This operationally straightforward and metal-free protocol provides an easy access to isoxazoline and pyrazoline derivatives
- . Keywords: amido-amidation; hypervalent iodine; isoxazoline; metal-free; oxyamidation; pyrazoline; Introduction Isoxazoline and pyrazoline-containing heterocycles are abundant in natural products and biologically active molecules [1][2][3][4][5]. Thus, these scaffolds are also important from the standpoint
Fluorination of some highly functionalized cycloalkanes: chemoselectivity and substrate dependence
Beilstein J. Org. Chem. 2017, 13, 2364–2371, doi:10.3762/bjoc.13.233
- the corresponding good leaving groups upon treatment with Deoxofluor. When (±)-14 was treated with an excess of Deoxofluor, somewhat surprisingly, the fluorinated isoxazoline derivative (±)-17 was formed with the fluorine attached to the β-position relative to the ester function, whereas the N-atom is
Conjugated nitrosoalkenes as Michael acceptors in carbon–carbon bond forming reactions: a review and perspective
Beilstein J. Org. Chem. 2017, 13, 2214–2234, doi:10.3762/bjoc.13.220
- oximes 95, if the elimination of dimethyl sulfide from 94 proceeds faster than the cyclization. The cyclization/elimination selectivity was found be highly dependent on the nature of substituents in ylide 92. Only when R3 was an acyl or phenacyl group, exclusive formation of isoxazoline products 93 was
- reaction with α-bromo ketoximes 1 (Scheme 36). The reaction requires a copper catalyst, which transforms the diazo compound 96 into a metal carbene complex 97. The latter reacts with a nitrosoalkene intermediate NSA (formed from α-bromo ketoxime 1) producing isoxazoline 93 with recovery of the catalyst
- . Unfortunately, the reaction is complicated by a concurrent [3 + 2]-cycloaddition of diazo compounds 96 to nitrosoalkenes leading to N-nitrosopyrazoles 98 via intermediates 99. A substantial improvement of this isoxazoline ring-forming strategy was recently introduced by Li et al. [85], who achieved the
Combined experimental and theoretical studies of regio- and stereoselectivity in reactions of β-isoxazolyl- and β-imidazolyl enamines with nitrile oxides
Beilstein J. Org. Chem. 2016, 12, 2390–2401, doi:10.3762/bjoc.12.233
- -5-substituted isoxazoles that are otherwise difficult to obtain. Keywords: β-azolyl enamine; [3 + 2]-cycloaddition; isoxazole; isoxazoline; nitrile oxide; Introduction The biological activity and technically useful properties of isoxazoles have made them the focus of both medicinal and materials
- . Investigation of the stepwise mechanism’s pathway allowed locating the only transition state (1a_1−7), which is appropriate to the addition of nitrile oxide 2a onto the double bond of enamine 1a_1, and the respective product – oxime 7. However, the intermediate 7 was found to cyclize to isoxazoline 3a through
- enamine 1a, whereas transition states based on 1a_1 geometry of the enamine lead to isoxazolines 3a and 8 with trans orientation of the Az and NMe2 groups (Scheme 4). Thus, one could conclude, that the configuration around the double bond of the enamine controls the stereoconfiguration of the isoxazoline
Cationic Pd(II)-catalyzed C–H activation/cross-coupling reactions at room temperature: synthetic and mechanistic studies
Beilstein J. Org. Chem. 2016, 12, 1040–1064, doi:10.3762/bjoc.12.99
- the product from their 5-membered isoxazoline-containing palladacycle [160][168][225]. Although BQ is sometimes used as a ligand for palladium to accelerate reductive elimination [103][226][227][228][229][230], its presence was not necessary in our stoichiometric reaction of a cationic 6-membered ring
Copper-mediated synthesis of N-alkenyl-α,β-unsaturated nitrones and their conversion to tri- and tetrasubstituted pyridines
Beilstein J. Org. Chem. 2015, 11, 2097–2104, doi:10.3762/bjoc.11.226
- of N-alkenyl-α,β-unsaturated nitrones 8 to pyridines 9, two mechanistic experiments were evaluated (Scheme 4). The conversion of nitrone 8ae to pyridine 9ae was monitored by 1H and 13C NMR spectroscopy. Surprisingly, after heating 8ae for 4 h at 140 °C in DMSO-d6, a 1:1:1 mixture of isoxazoline 15ae
N–O Cleavage reactions of heterobicycloalkene-fused 2-isoxazolines
Beilstein J. Org. Chem. 2014, 10, 2200–2205, doi:10.3762/bjoc.10.227
- yields (66–95%) and without the need for chromatographic purification. Keywords: azabicycloalkene; β-hydroxyketone; 2-isoxazoline; oxabicycloalkene; Raney nickel; Introduction 2-Isoxazolines 1 are practical precursors to countless structural motifs found in nature. Various carbonyl compounds 2, β
- isoxazoline-cleavage methodology to the preparation of biologically active natural products, including novel antibacterial, antifungal and anticancer treatments [8][9][10][11]. Some common methods of reductive N–O bond cleavage in isoxazolines include hydrogenolysis using Raney nickel, reduction by LiAlH4
- as β-hydroxycarbonyl derivatives 12 and γ-aminoalcohols 13, as well as carbonyl compounds including phthalan 14 and isoindoline derivatives 15 (R2 = Ar; Scheme 2). Results and Discussion For our investigation of heterobicycloalkene-fused 2-isoxazoline cleavage, we initially planned to follow our
A one-pot synthesis of 3-trifluoromethyl-2-isoxazolines from trifluoromethyl aldoxime
Beilstein J. Org. Chem. 2013, 9, 2387–2394, doi:10.3762/bjoc.9.275
- yield the corresponding trifluoromethylated γ-amino alcohols. Keywords: aldoxime; amino alcohol; fluorine; isoxazole; isoxazoline; organo-fluorine; Introduction 2-Isoxazolines are five-membered heterocyclic compounds that have been widely applied in medicinal and organic chemistry. This nucleus is
- optimized reagents and conditions, the scope of the reaction was explored with regard to the substrates (Table 2). As observed in the earliest works [11], the cycloaddition of 4 with terminal olefins led to the corresponding 3-trifluoromethyl-5-substituted-2-isoxazoline 1 with complete regioselectivity. No
- trace of the regioisomer 3-trifluoromethyl-4-substituted-2-isoxazoline could be detected even when yields of cycloadducts were low. From functionalized olefins such as NH–Cbz and NH–Boc allylamines the desired product could be isolated in excellent yields (90% and 91%, respectively, Table 2, entries 2
Diastereoselective synthesis of nitroso acetals from (S,E)-γ-aminated nitroalkenes via multicomponent [4 + 2]/[3 + 2] cycloadditions promoted by LiCl or LiClO4
Beilstein J. Org. Chem. 2013, 9, 838–845, doi:10.3762/bjoc.9.96
- respective carbonylated isoxazolines and, therefore, causing a decrease in yield. These last compounds could be identified through 1H and 13C NMR spectroscopy. The signals at 9.5 ppm, and 201 and 159 ppm revealed the presence of an aldehyde function and a sp2 carbon bond of an isoxazoline ring (spectra not
Aryl nitrile oxide cycloaddition reactions in the presence of pinacol boronic acid ester
Beilstein J. Org. Chem. 2012, 8, 606–612, doi:10.3762/bjoc.8.67
- ], and continuous-flow processes [3][4]. An important consideration when using these reactions for multistep syntheses is whether they are chemically compatible, without having to resort to protection/deprotection systems. For example, to generate a library of 3-bi(hetero)aryl isoxazoline analogues 3 a
- shifts for the cycloadduct isoxazoline ring protons. The resonances of protons on C4 of the isoxazoline ring appear 1–2 ppm upfield from those of C5 protons on the isoxazoline rings [42], and hence 5-substituted isoxazolines are easily distinguished from 4-substituted isoxazolines. All of the
Synthesis of highly functionalized β-aminocyclopentanecarboxylate stereoisomers by reductive ring opening reaction of isoxazolines
Beilstein J. Org. Chem. 2012, 8, 100–106, doi:10.3762/bjoc.8.10
- opening of the isoxazoline reductive ring to the corresponding highly functionalized 2-aminocyclopentanecarboxylates occurred stereoselectively with good yields. Keywords: amino acids; cycloaddition; functionalization; isoxazolines; reduction; Introduction Isoxazoline-fused amino acids are important
- Peramivir analogues has recently been investigated as potential antiviral agents [46][47]. Results and Discussion We recently reported a regio- and stereoselective procedure for the formation of a series of isoxazoline-fused cispentacin and transpentacin regio- and stereoisomers (2–6) from bicyclic β-lactam
- 1 [48][49] (Scheme 1). The syntheses consisted of a dipolar cycloaddition of nitrile oxide (generated with Boc2O, Et3N and DMAP) to the olefinic bond of cis-ethyl 2-aminocyclopent-3-enecarboxylate derived from 1, during which the isoxazoline-fused amino ester regio- and stereoisomers (2 and 4) were
Gold-catalyzed propargylic substitutions: Scope and synthetic developments
Beilstein J. Org. Chem. 2011, 7, 866–877, doi:10.3762/bjoc.7.99
- reactions of propargylic alcohol 1 with PhSO2NHOH in the presence of NaAuCl4 neither the propargylic substitution product 22 nor the expected isoxazoline 23 could be observed in the crude product. Instead a 1:1 mixture of unreacted alcohol 1 and compound 24 formally resulting from the addition of a second
- isoxazolines cannot be directly obtained from propargylic alcohols 1 in the presence of gold(III) catalysts. From these preliminary experiments, it appears that the addition of hydroxylamine to the isoxazoline double bond is much faster than propargylic substitution. Consequently, the only possibility to
- 15 proved to be efficient and compounds 23a–i were obtained in 38–86% yield (Scheme 15). Prompted by the apparently simplicity of the addition of hydroxylamine to the isoxazoline double bond (Scheme 12), we next tried to promote the gold-catalyzed addition of various nucleophiles. As illustrated in
Photocycloaddition of aromatic and aliphatic aldehydes to isoxazoles: Cycloaddition reactivity and stability studies
Beilstein J. Org. Chem. 2011, 7, 127–134, doi:10.3762/bjoc.7.18
- evaporated. The crude product was obtained as a yellow oil and used immediately without further purification. 3-Methyl-5-trimethylsilyloxy-2-isoxazoline (2) [19]. A mixture of acrylonitrile (10.61 g, 0.2 mol, 13.2 mL), triethylamine (10.12 g, 0.1 mol, 13.9 mL) and 1 (28.73 g, 0.2 mol) in toluene (90 mL) was
Bioorthogonal metabolic glycoengineering of human larynx carcinoma (HEp-2) cells targeting sialic acid
Beilstein J. Org. Chem. 2010, 6, No. 24, doi:10.3762/bjoc.6.24

- ), 62.23 (C-3), 61.21 (C(CH3)3, 53.71 (C-1’’), 39.07 (C-4), 35.92 (C-2’), 25.91 (C-4’), 25.91 (C(CH3)3), 25.85 (C-3’), 14.45 (OCH2CH3); MS (ESI): m/z [M+Na]+ calculated for C21H36N2O8[Na]+ 467.2, found 467.2. N-(Hex-5’-ynoyl)neuraminic acid (3) Isoxazoline (2.50 g, 5.63 mmol) and NaOMe (0.74 mL of 5.4 M




















































































































































































































































































































































































































































































































































































