Search results
Search for "metabolites" in Full Text gives 247 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Skeletocutins M–Q: biologically active compounds from the fruiting bodies of the basidiomycete Skeletocutis sp. collected in Africa
Beilstein J. Org. Chem. 2019, 15, 2782–2789, doi:10.3762/bjoc.15.270
- ’ Universite Catholique de Louvain (BCCM/MUCL), Place Croix du Sud 3, B-1348 Louvain-la-Neuve, Belgium Department of Chemistry, Faculty of Science, Egerton University, P.O. Box 536, 20115, Egerton, Kenya 10.3762/bjoc.15.270 Abstract During the course of screening for new metabolites from basidiomycetes, we
- isolated and characterized five previously undescribed secondary metabolites, skeletocutins M–Q (1–5), along with the known metabolite tyromycin A (6) from the fruiting bodies of the polypore Skeletocutis sp. The new compounds did not exhibit any antimicrobial, cytotoxic, or nematicidal activities. However
- , compound 3 moderately inhibited the biofilm formation of Staphylococcus aureus (S. aureus), while compounds 3 and 4 performed moderately in the ʟ-leucine-7-amido-4-methylcoumarin (ʟ-Leu-AMC) inhibition assay. These compounds represent the first secondary metabolites reported to occur in the fruiting bodies
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- : Aspergillus; biosynthesis; drimane; secondary metabolites; sesquiterpenoid; terpenes; Introduction The fungal genus Aspergillus is well recognised as a source of structurally diverse terpenoids comprising monoterpenoids [1], sesquiterpenoids [2][3][4][5], diterpenoids [6], sesterterpenoids [7][8][9
- production of terpenoids as the dominant biosynthetic class of secondary metabolites. Results and Discussion Purification and identification The metabolite profile of A. nanangensis was examined on a limited range of solid and liquid media suitable for fungal metabolite production. The metabolite profile
- Aspergillus type species) and unidentified but metabolically talented fungi (>60,000 spectra from 3,000 species) returned no similar metabolite cohorts, suggesting an unknown species. Individual retention time/UV–vis searches of the dominant 15 secondary metabolites against our in-house pure metabolite
A new approach to silicon rhodamines by Suzuki–Miyaura coupling – scope and limitations
Beilstein J. Org. Chem. 2019, 15, 2569–2576, doi:10.3762/bjoc.15.250

- in super-resolution microscopy [1][2][3][4][5][6][7][8] and as probes for targeting various biomolecules [9][10][11][12] or sensors for metal ions [13][14][15][16][17], pH [15], voltage [18] or metabolites [19][20][21][22]. Since our group is interested in synthesizing new tumor tracers for
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- [1][2][3]. Sesqui- and diterpenes are a diverse class of secondary metabolites derived predominantly from plants, marine invertebrates, fungi and some prokaryotes [4][5][6][7][8]. Properties of these natural products include antitumor, anti-oxidant, anti-inflammatory, antiviral, antimalarial
Synthesis of a dihalogenated pyridinyl silicon rhodamine for mitochondrial imaging by a halogen dance rearrangement
Beilstein J. Org. Chem. 2019, 15, 2333–2343, doi:10.3762/bjoc.15.226

- microscopy [2][3][4][5][6][7][8][9], as direct probes for various biomolecules [10][11][12][13] or as sensors for metal ions [14][15][16][17][18], pH [16], voltage [19] or metabolites [20][21][22][23]. Several attempts were made, partially supported by DFT calculations, to correlate the dyes’ structural
Isolation and biosynthesis of an unsaturated fatty acid with unusual methylation pattern from a coral-associated bacterium Microbulbifer sp.
Beilstein J. Org. Chem. 2019, 15, 2327–2332, doi:10.3762/bjoc.15.225

- secondary metabolites have received lesser attention [3]. To date, a couple of new compounds were discovered from soft coral-associated bacteria such as pseudoalteromones from Pseudoalteromonas isolated from the cultured octocoral Lobophytum crassum [4][5] and macrolactin V from Bacillus amyloliquefaciens
- of metabolites in a marine-derived Microbulbifer sp. led to the discovery of a unique unsaturated fatty acid, (2Z,4E)-3-methyl-2,4-decadienoic acid (1) in which the carbon originated from the carbonyl carbon of an acetate unit is methylated, providing a quite rare case of C-methylation pattern in
Synthesis of acremines A, B and F and studies on the bisacremines
Beilstein J. Org. Chem. 2019, 15, 2271–2276, doi:10.3762/bjoc.15.219
- relationship with their plant hosts [1], which is mediated by secondary metabolites [2]. In 2005, Torta and co-workers reported the isolation of six meroterpenoid natural products, acremines A–F from A20, a strain of Acreonium byssoides, isolated from grapevine leaves that were artificially inoculated with
Isolation of fungi using the diffusion chamber device FIND technology
Beilstein J. Org. Chem. 2019, 15, 2191–2203, doi:10.3762/bjoc.15.216
- Benjamin Libor Henrik Harms Stefan Kehraus Ekaterina Egereva Max Crusemann Gabriele M. Konig Institute for Pharmaceutical Biology, University of Bonn, Nussallee 6, 53115 Bonn, Germany 10.3762/bjoc.15.216 Abstract Fungi are an important source of bioactive metabolites. The Fungal one-step
- of them being the marine-adapted fungal strain Heydenia cf. alpina. The latter produced two new terpenoids, which are the first secondary metabolites from this genus. Keywords: FIND; fungal one-step isolation device; Heydenia cf. alpina; natural products; terpenes; Introduction Natural products
- antibiotics (e.g., cephalosporins), immunosuppressants (e.g., mycophenolic acid) and immunomodulators (e.g., fingolimod), as well as cholesterol lowering agents (statins). Generally, fungal metabolites were shown to have antiviral, cytotoxic, antineoplastic, cardiovascular, anti-inflammatory, immune
Genome mining in Trichoderma viride J1-030: discovery and identification of novel sesquiterpene synthase and its products
Beilstein J. Org. Chem. 2019, 15, 2052–2058, doi:10.3762/bjoc.15.202

- that has received considerable attention as an effective biocontrol agent against two fungal pathogens, Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes, infecting soybean. This fungus is a competent mycoparasite and strong producer of secondary metabolites [22][23]. However, T. viride
Bipolenins K–N: New sesquiterpenoids from the fungal plant pathogen Bipolaris sorokiniana
Beilstein J. Org. Chem. 2019, 15, 2020–2028, doi:10.3762/bjoc.15.198
- array of secondary metabolites, including sesquiterpenes [1][2][3][4][5][6][7], sesquiterpene-xanthones [8], diterpenes [9], sesterterpenes [10], cochlioquinones and peptides [11]. Moreover, several of these secondary metabolites are known to play important roles in mediating the virulence of these
- horizontal gene transfer [14]. To date, only three studies have explored phytotoxins from B. sorokiniana [2][7][10]. Therefore, in the framework of furthering our understanding of the roles of B. sorokiniana secondary metabolites in crop disease, we investigated the compounds produced by the ToxA-containing
- Cochliobolus sp.) [1][26], were reported in B. sorokiniana for the first time, while, known metabolites 6 and 7 [20], 9 [34], 11 [2], and 12 [16] were previously reported from B. sorokiniana (syn. C. sativus and H. sativum). The terpene synthase responsible for the biosynthesis of the sativene/longifolene
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- analysis revealed 4β-hydroxywithanolide E (1) as the major compound (50 mg) as well as the known metabolites withanolide E (4), withanolide F (5) and perulactone H (6) by comparison to literature data (Figure 1) [9][20]. Two additional compounds attracted our attention based on their unusual 1H NMR spectra
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
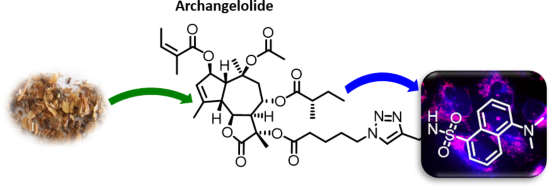
- Sciences of the Czech Republic, Flemingovo náměstí 2, 166 10 Prague 6, Czech Republic Institute of Experimental Medicine, Academy of Sciences of the Czech Republic, v.v.i., 14220 Prague 4, Czech Republic 10.3762/bjoc.15.189 Abstract Sesquiterpene lactones are secondary plant metabolites with sundry
- anti-inflammatory activity of compound 1 using rat macrophages. Results and Discussion L. archangelica metabolite isolation and identification For isolation of the major metabolites of L. archangelica, we used 100 g of fine ground seeds (Supporting Information File 1, Figure S2) and the method of
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- from the involvement of sphingolipid metabolites in an array of important cell processes. ᴅ-threo-PDMP (1R,2R)-81 is a ceramide analogue identified as an inhibitor of glucosylceramide synthase (GCS) at micromolar concentrations [66][67]. It was efficiently synthesized [68][69] employing the alcohol (2R
Fluorine-containing substituents: metabolism of the α,α-difluoroethyl thioether motif
Beilstein J. Org. Chem. 2019, 15, 1441–1447, doi:10.3762/bjoc.15.144
- compounds that are of commercial significance [8][9][10]. Metabolism studies in both of these cases show that the major metabolites are their corresponding sulfoxides (Ar–S(O)CF3) and sulfones (Ar–S(O)2CF3) [8][9]. Indeed, in the case of the insecticide fipronil it is actually the sulfoxide (Ar–S(O)CF3
- , as they are less volatile, to avoid evaporation losses during extended incubations and work-up. Cultures of C. elegans were grown in Saboraud dextrose medium and incubated on an orbital shaker at 28 °C for 72 hours. New metabolites could be conveniently observed by extracting aliquots of the media
- into diethyl ether and dichloromethane (DCM) and then carrying out HPLC analyses. Incubation of thioether 4 with C. elegans led to the identification of three metabolites 6–8 as illustrated in Scheme 1. These were isolated by semi-preparative HPLC. Two of these (6 and 7) displayed an AB system in the
Genomics-inspired discovery of massiliachelin, an agrochelin epimer from Massilia sp. NR 4-1
Beilstein J. Org. Chem. 2019, 15, 1298–1303, doi:10.3762/bjoc.15.128
- the chemistry of the genus Massilia, in general. Because natural product-competent microorganisms typically synthesize multiple compounds [7], strain NR 4-1 appeared as a promising candidate to find further secondary metabolites. Further incentive for the chemical analysis of this bacterium came from
Phylogenomic analyses and distribution of terpene synthases among Streptomyces
Beilstein J. Org. Chem. 2019, 15, 1181–1193, doi:10.3762/bjoc.15.115

- remarkable genetic potential to produce a large variety of secondary metabolites with different functions including antibiotics, antifungals, pigments or immunosuppressants [1][2][3]. These are compounds of diverse chemical nature such as polyketides, peptides, aminoglycosides or terpenoids [4][5
- ]. Terpenoids are the largest and the most diverse class of natural compounds known to date and include the initial products of terpene synthases and all derivatives made from them in tailoring steps. This very diverse class of organic compounds is best known as plant metabolites. However, recent studies
Novel (2-amino-4-arylimidazolyl)propanoic acids and pyrrolo[1,2-c]imidazoles via the domino reactions of 2-amino-4-arylimidazoles with carbonyl and methylene active compounds
Beilstein J. Org. Chem. 2019, 15, 1032–1045, doi:10.3762/bjoc.15.101
- derivatives containing pyrrole and 2-aminoimidazole fragments in their structure were found among the metabolites of these marine organisms [1]. This group of compounds is characterized by an exceptional molecular diversity. The main structural types of these substances are shown in Figure 1. The metabolites
- recently from an extract of Pseudoceratina Sp. [6]. The variety of types of pharmacological activity revealed in these marine sponges’ metabolites is not inferior to the chemodiversity of their structure. Many of them are reported to have properties such as α-adrenoreceptors [7] and leukotriene B4 receptor
- metabolites of marine sponges with interesting biological properties has received considerable attention from both chemists and pharmacologists. In the middle of 2000s, the authors of the studies [17][18][19] proposed a facile one-pot two-step procedure for the synthesis of diversely substituted 2
New terpenoids from the fermentation broth of the edible mushroom Cyclocybe aegerita
Beilstein J. Org. Chem. 2019, 15, 1000–1007, doi:10.3762/bjoc.15.98
- ) is one of the most praised cultivated edible mushrooms and is being cultivated at large scale for food production. Furthermore, the fungus serves as a model organism to study fruiting body formation and the production of secondary metabolites during the life cycle of Basidiomycota. By studying the
- clusters. Keywords: bioinformatics; gene cluster analysis; natural products; secondary metabolites; structure elucidation; terpenes; Introduction The basidiomycete Agrocybe aegerita (synonym: A. cylindracea) was traditionally accommodated in the genus Agrocybe (family Bolbitiaceae) until a recent
- liquid culture and could eventually serve as hosts for heterologous production of secondary metabolites derived from other Basidiomycota that are more difficult or even impossible to culture. With these goals in mind, we have initiated extensive studies of the secondary metabolism of the aforementioned
Diastereo- and enantioselective preparation of cyclopropanol derivatives
Beilstein J. Org. Chem. 2019, 15, 752–760, doi:10.3762/bjoc.15.71
- transformations [1]. The cyclopropane subunit is also present in many biologically important compounds such as pheromones, fatty acid metabolites, unusual amino acids and possess interesting herbicidal, insecticidal, antibiotic, antibacterial, antifungal, antiviral and antitumor activities [2]. For these reasons
New sesquiterpenoids from the South China Sea soft corals Clavularia viridis and Lemnalia flava
Beilstein J. Org. Chem. 2019, 15, 695–702, doi:10.3762/bjoc.15.64

- sesquiterpenoids and diterpenoids with various intriguing carbon skeletons, such as nardosinanes, neolemnanes, and ylanganes [10]. Many of these secondary metabolites have attracted a lot of attention for further synthetic and pharmacological studies due to their potent bioactivities ranging from neuroprotective
- , cytotoxic, to anti-inflammatory properties [10]. In the framework of our ongoing research for the bioactive metabolites from South China Sea soft corals [11][12], we made the collection of the title samples Clavularia viridis and Lemnalia flava off the Xisha Islands, Hainan Province, China. The chemical
- , eight sesquiterpenoids (1–8), belonging to four different structural types, were isolated from two South China Sea soft corals (C. viridis and L. flava) for the first time. The discovery of these metabolites extended the structural diversity and complexity of sesquiterpenoids derived from soft corals C
Synthesis of the aglycon of scorzodihydrostilbenes B and D
Beilstein J. Org. Chem. 2019, 15, 610–616, doi:10.3762/bjoc.15.56
- multiple phenolic functionality. These natural products that form as secondary metabolites on a branch of flavonoid biosynthesis found wide interest for their various biological effects like anti-oxidative and biofouling-preventing activity [1]. From crude extracts of the Mongolian medicinal plant
Back to the future: Why we need enzymology to build a synthetic metabolism of the future
Beilstein J. Org. Chem. 2019, 15, 551–557, doi:10.3762/bjoc.15.49

- avoided some problems upfront [58]. Yet, it needs to be mentioned that even if complex synthetic metabolic networks can be realized in vitro, this does not mean that these metabolic networks can be easily transplanted into living cells. The introduction of new reactions and metabolites into a host cell is
- expected to create interactions with the native metabolic and regulatory network of the host. Again, promiscuity poses a major challenge. Even though the metabolites and reactions might be completely non-native to the cell, these intermediates might be still drained due to unwanted side reactions or create
- there are likely to be hundreds if not thousands of unknown reactions and metabolites, often described as catalytic or metabolic “dark matter” [53][60]. Thus, a more detailed understanding of the promiscuity of native enzymes and the interaction of small molecules with the native regulatory network of
Synthesis and biological activity of methylated derivatives of the Pseudomonas metabolites HHQ, HQNO and PQS
Beilstein J. Org. Chem. 2019, 15, 187–193, doi:10.3762/bjoc.15.18
- metabolites [16]. For example, Mycobacterium abscessus, which like P. aeruginosa and S. aureus can occur in the lung of cystic fibrosis patients [17][18][19], is able to methylate HQNO to give 2-heptyl-1-methoxy-4(1H)-quinolone (HMOQ). HMOQ is a significantly less efficient inhibitor of the respiratory chain
Ring-closing-metathesis-based synthesis of annellated coumarins from 8-allylcoumarins
Beilstein J. Org. Chem. 2018, 14, 2991–2998, doi:10.3762/bjoc.14.278
- to antineurodegenerative activities [2][3]. The majority of natural coumarins are secondary metabolites isolated from plants [5][6][7]. A commonly used taxonomy for these natural products (which has been extended to the non-natural analogues) is based on the coumarin structure (Figure 1) [4][8]. It
Volatiles from the hypoxylaceous fungi Hypoxylon griseobrunneum and Hypoxylon macrocarpum
Beilstein J. Org. Chem. 2018, 14, 2974–2990, doi:10.3762/bjoc.14.277
- mycotoxins [2], a class of highly bioactive secondary metabolites that belong to the strongest known inhibitors of protein biosynthesis in eukaryotes [3]. Similarly, the sesquiterpene aristolochene (2) is the parent hydrocarbon of PR toxin [4][5] and has been used as a marker to differentiate between toxin
- in secondary metabolites [20], but not much is known about volatiles from these fungi [21]. In continuation of this work, here we present the volatiles emitted by Hypoxylon griseobrunneum MUCL 53754 and Hypoxylon macrocarpum STMA 130423. These strains were selected, because both species released a
- ). Small amounts of matsutake alcohol (3) were also found. This volatile is frequently accompanied by other C8 metabolites [1], which is reflected for H. griseobrunneum by the detection of octan-3-one (8). Trace amounts of a series of alkylated pyrazines including methylpyrazine (9), 2,5-dimethylpyrazine











































































































































































































































