Search results
Search for "cycloaddition reactions" in Full Text gives 197 result(s) in Beilstein Journal of Organic Chemistry.
Regiodivergent synthesis of functionalized pyrimidines and imidazoles through phenacyl azides in deep eutectic solvents
- Paola Vitale,
- Luciana Cicco,
- Ilaria Cellamare,
- Filippo M. Perna,
- Antonio Salomone and
- Vito Capriati
Beilstein J. Org. Chem. 2020, 16, 1915–1923, doi:10.3762/bjoc.16.158

- (g) heterogeneous “click” cycloaddition reactions [15] using DESs as environmentally responsible and non-innocent reaction media. Telescoped, one-pot transformations of phenacyl halides to symmetrical 2,5-disubstituted pyrazines (A), through phenacyl azides as intermediates, were also found to take

Graphical Abstract

Scheme 1: One-pot synthesis of 2,5-diarylpyrazines (A) (path a) or 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles (B) ...

Scheme 2: Transformation of phenacyl bromide (1a) in ChCl/Gly into phenacyl azide (2a) and 2-benzoyl-(4 or 5)...

Scheme 3: Synthesis of 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles 3. Scope of the reaction. Typical conditions: 1 ...

Scheme 4: Proposed mechanism for the formation of 2-aroyl-(4 or 5)-aryl-(1H)-imidazoles 3/3' from α-phenacyl ...

Scheme 5: Proposed mechanism for the formation of 2-benzoyl-(4 or 5)-phenyl-(1H)-imidazoles 3a/3a' and 2,4-di...

Scheme 6: Scope of the synthesis of 2,4-diaroyl-6-arylpyrimidines 7. Typical conditions: 2 (0.3 mmol), Et3N (...
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
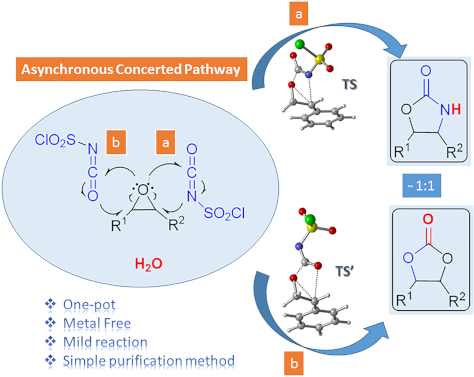
- advantageous: being a one-pot reaction with metal-free reagent, having shorter reaction times, good yields and a very simple purification method. Moreover, using the density functional theory (DFT) method at the M06-2X/6-31+G(d,p) level of theory the mechanism of the cycloaddition reactions has been elucidated

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
Clickable azide-functionalized bromoarylaldehydes – synthesis and photophysical characterization
- Dominik Göbel,
- Marius Friedrich,
- Enno Lork and
- Boris J. Nachtsheim
Beilstein J. Org. Chem. 2020, 16, 1683–1692, doi:10.3762/bjoc.16.139
- oxazoline 24, oxazolidine 27 cyclized already during the reaction, caused by the increased basicity of the ring nitrogen. CuAAC reactions of bromocarbaldehydes We further investigated the reactivity of azide-functionalized bromocarbaldehydes 3, 4, and 5 in copper(I)-catalyzed azide–alkyne cycloaddition
- reactions (CuAAC). For this, we treated the azide-functionalized luminophores with alkynes exhibiting different degrees of steric demand, including 1-decyne (29), phenylacetylene (30), 1-ethynyladamantane (31) and 1,3-di-tert-butyl-5-ethynylbenzene (32, see Scheme 5). All triazoles 33–44, based on the

Graphical Abstract

Scheme 1: a) Schematic depiction of the Jablonski diagram. b) Schematic representation of El-Sayed’s rule.

Figure 1: Top: literature examples of organic compounds showing RTP in the crystalline state (a) and in solut...

Scheme 2: Reaction conditions for para-bromobenzaldehyde 3: a) 1) 2-amino-2-methylpropan-1-ol, 4 Å MS, CH2Cl2...

Scheme 3: Reaction conditions: a) Br2, Fe powder, CHCl3, 0 °C, 4 h, 99%; b) KOH, KI, MeI, DMSO, 25 °C, 18 h, ...

Scheme 4: Reaction conditions: a) 1) NaH, THF, 0 °C, 30 min; 2) MeI, THF, 0 °C to 25 °C, 2 h, 99%; b) 1) MeOT...

Scheme 5: a) CuAAC reactions of azide-functionalized bromocarbaldehydes 3, 4 and 5 with terminal alkynes to t...

Figure 2: a) Normalized UV–vis absorption spectra of 3 (blue line), 34 (olive line), 4 (green line) and 38 (r...

Figure 3: a) Normalized UV–vis absorption spectra of 5 (blue line), 16 (green line), 42 (olive line) and 45 (...
Azidophosphonium salt-directed chemoselective synthesis of (E)/(Z)-cinnamyl-1H-triazoles and regiospecific access to bromomethylcoumarins from Morita–Baylis–Hillman adducts
- Soundararajan Karthikeyan,
- Radha Krishnan Shobana,
- Kamarajapurathu Raju Subimol,
- J. Helen Ratna Monica and
- Ayyanoth Karthik Krishna Kumar
Beilstein J. Org. Chem. 2020, 16, 1579–1587, doi:10.3762/bjoc.16.130
- ]. Among the known synthetic transformations using functionalized MBH adducts, cycloaddition reactions are challenging and attractive for synthetic organic chemists. In this context, acetate-functionalized Morita–Baylis–Hillman adducts have been extensively utilized over other precursors. For example
- ) acetylation, (ii) azidation, and (iii) cycloaddition to produce IV–VIII. In spite of the broad scope and synthetic utility, it is evident that the multistep synthetic methodology is the only existing module for cycloaddition reactions. Our research group is focused on developing one-pot synthetic
- for the synthesis of 3-(bromomethyl)coumarins. Literature-reported cycloaddition reactions of MBH acetates involving azides and alkynes [24][25][26][27][28]. Synthetic methodologies for triazolations of MBH adducts. a) Literature-reported indirect triazolation of MBH adducts [32][33]. b) This work

Graphical Abstract

Scheme 1: Literature-reported cycloaddition reactions of MBH acetates involving azides and alkynes [24-28].

Scheme 2: Synthetic methodologies for triazolations of MBH adducts. a) Literature-reported indirect triazolat...

Scheme 3: Scope of the one-pot cascade reaction of the unprotected Morita–Baylis–Hillman adducts 3a–q.

Figure 1: Proposed mechanism for the synthesis of 1,4-disubstituted triazoles.

Scheme 4: Comparative analysis of the sequential one-pot reaction.

Figure 2: Proposed mechanism for the synthesis of 3-(bromomethyl)coumarins.
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
- Yeersen Patehebieke
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- , oxidations, or isomerizations, disulfides have increasingly proven their power. Herein, we briefly describe the progress in the field of disulfide-involving photocatalysis in recent years for different reaction types. Review Cycloaddition reactions As early as 1988, Feldman and co-workers reported an example
- of a [3 + 2] cycloaddition reactions under UV irradiation with azobis(isobutyronitrile) (AIBN) as the free radical initiator and phenyl disulfide as the catalyst, in which the three-membered rings containing double bonds and substituted olefins were transformed into five-membered-ring structures with
- used similar disulfide-catalyzed [3 + 2] cycloaddition reactions for the synthesis of polycyclic frameworks. The irradiation of vinylcyclopropanes with alkenes or alkynes in the presence of dibutyl disulfide afford the desired bi- or tricyclic products with 54–88% yield (Scheme 2) [6]. In 2014, Maruoka

Graphical Abstract

Scheme 1: [3 + 2] cyclization catalyzed by diaryl disulfide.

Scheme 2: [3 + 2] cycloaddition catalyzed by disulfide.

Scheme 3: Disulfide-bridged peptide-catalyzed enantioselective cycloaddition.

Scheme 4: Disulfide-catalyzed [3 + 2] methylenecyclopentane annulations.

Scheme 5: Disulfide as a HAT cocatalyst in the [4 + 2] cycloaddition reaction.

Scheme 6: Proposed mechanism of the [4 + 2] cycloaddition reaction using disulfide as a HAT cocatalyst.

Scheme 7: Disulfide-catalyzed ring expansion of vinyl spiro epoxides.

Scheme 8: Disulfide-catalyzed aerobic oxidation of diarylacetylene.

Scheme 9: Disulfide-catalyzed aerobic photooxidative cleavage of olefins.

Scheme 10: Disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 11: Proposed mechanism of the disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 12: Disulfide-catalyzed oxidation of allyl alcohols.

Scheme 13: Disulfide-catalyzed diboration of alkynes.

Scheme 14: Dehalogenative radical cyclization catalyzed by disulfide.

Scheme 15: Hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 16: Plausible mechanism of the hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 17: Disulfide-cocatalyzed anti-Markovnikov olefin hydration reactions.

Scheme 18: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 19: Proposed mechanism of the disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 20: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 21: Disulfide-catalyzed conversion of maleate esters to fumarates and 5H-furanones.

Scheme 22: Disulfide-catalyzed isomerization of difluorotriethylsilylethylene.

Scheme 23: Disulfide-catalyzed isomerization of allyl alcohols to carbonyl compounds.

Scheme 24: Proposed mechanism for the disulfide-catalyzed isomerization of allyl alcohols to carbonyl compound...

Scheme 25: Diphenyl disulfide-catalyzed enantioselective synthesis of ophirin B.

Scheme 26: Disulfide-catalyzed isomerization in the total synthesis of (+)-hitachimycin.

Scheme 27: Disulfide-catalyzed isomerization in the synthesis of (−)-gloeosporone.
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- photocycloaddition of ring-substituted cyclic dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a), affording a series of spirothietanes 245 [72] (Scheme 47). In 1986, Coyle and Rapley reported that the photochemical cycloaddition reactions of N-methylthiophthalimide (237a) and N-methyldithiophthalimide (225
- tetracyclic fused benzoxazolethietane derivative 354 in 20% yield [83] (Scheme 69). In 1991, Sakomto and co-workers started on the synthesis of highly rigid thietane-fused β-lactams. They prepared various derivatives 356 in high yields via the photochemical cycloaddition reactions of N-(α,β-disubstituted

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Ferrocenyl-substituted tetrahydrothiophenes via formal [3 + 2]-cycloaddition reactions of ferrocenyl thioketones with donor–acceptor cyclopropanes
- Grzegorz Mlostoń,
- Mateusz Kowalczyk,
- André U. Augustin,
- Peter G. Jones and
- Daniel B. Werz
Beilstein J. Org. Chem. 2020, 16, 1288–1295, doi:10.3762/bjoc.16.109
- thioketones reacted with donor–acceptor cyclopropanes in dichloromethane at room temperature in the presence of catalytic amounts of Sc(OTf)3 yielding tetrahydrothiophene derivatives, products of formal [3 + 2]-cycloaddition reactions, in moderate to high yields. In all studied cases, dimethyl 2
- thioketones to form nearly equal amounts of both diastereoisomeric tetrahydrothiophenes. A low selectivity was also observed in the reaction of a 2-phthalimide-derived cyclopropane with ferrocenyl phenyl thioketone. Keywords: [3 + 2]-cycloaddition reactions; donor–acceptor cyclopropanes; ferrocenyl
- materials chemistry, and electrochemical studies [19]. In continuation of our studies on organic sulfur compounds and the mechanisms of their reactions, the main goal of the present work was the examination of the formal [3 + 2]-cycloaddition reactions of ferrocenyl-substituted thioketones 8 with D–A

Graphical Abstract

Scheme 1: Synthesis of spirotetrahydrothiophenes 3 via non-concerted [3 + 2]-cycloadditions of thiocarbonyl y...

Scheme 2: Formal [3 + 2]-cycloadditions of thioketones and [4 + 3]-cycloadditions of thiochalcones with donor...

Scheme 3: Formal [3 + 2]-cycloadditions of dimethyl 2-substituted cyclopropane-1,1-dicarboxylates 5a–g with f...

Figure 1: Thermal ellipsoid plots of the molecular structures of cis-9c and trans-9d drawn using 50% probabil...

Scheme 4: Plausible mechanism for the formal [3 + 2]-cycloadditions of ferrocenyl thioketones 8 with D–A cycl...
Synthesis of pyrrolidinedione-fused hexahydropyrrolo[2,1-a]isoquinolines via three-component [3 + 2] cycloaddition followed by one-pot N-allylation and intramolecular Heck reactions
- Xiaoming Ma,
- Suzhi Meng,
- Xiaofeng Zhang,
- Qiang Zhang,
- Shenghu Yan,
- Yue Zhang and
- Wei Zhang
Beilstein J. Org. Chem. 2020, 16, 1225–1233, doi:10.3762/bjoc.16.106
- reactions to make it a green synthetic method with pot, atom and step economy (PASE) [55][56]. Results and Discussion Following the reported procedures for amino ester- and amino acid-based [3 + 2] cycloaddition reactions, pyrrolidine adducts 5 and 6 were synthesized by a three-component reaction of 1 or 2
- with 2-bromobenzaldehydes 3 and maleimides 4 (Scheme 3) [30][37]. The cycloaddition reactions were diastereoselective (>20:1 dr for adducts 5 and >6:1 dr for adducts 6). The major diastereomers of 5 and 6 were isolated for following N-allylation and intramolecular Heck reactions. Adduct 5a generated

Graphical Abstract

Figure 1: Bioactive pyrrolo[2,1-a]isoquinolines and hexahydropyrrolo[2,1-a]isoquinolines.

Scheme 1: [3 + 2] Cycloaddition with amino esters or amino acids.

Scheme 2: Scaffolds derived from the initial [3 + 2] adducts.

Scheme 3: [3 + 2] Cycloaddition with amino esters or amino acids. Conditions: 1:3:4 (1.2:1:1.1), Et3N (1.5 eq...

Scheme 4: Synthesis of pyrrolo[2,1-a]isoquinolines 9. Reaction conditions: 5 (0.5 mmol, 1 equiv), 7 (3 equiv)...

Scheme 5: Synthesis of pyrrolo[2,1-a]isoquinolines 11. Reaction conditions: 6 (0.5 mmol, 1 equiv), 7 (3 equiv...

Scheme 6: Synthesis of pyrrolo[2,1-a]isoquinolines 12. Reaction conditions: 5 or 6 (0.5 mmol, 1 equiv), cinna...

Scheme 7: Plausible mechanism for the synthesis of 9a.
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- (Scheme 26) [61][67][68]. In this section, both reactions are presented and discussed. Singlet oxygen in pericyclic reactions Many important organic transformations can be performed by singlet oxygen including ene, [2 + 2] and [4 + 2] cycloaddition reactions for the formation of hydroperoxides, dioxetanes

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
- Valerian Dragutan,
- Ileana Dragutan,
- Albert Demonceau and
- Lionel Delaude
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- ). Subsequent Diels–Alder cycloaddition reactions with dienophiles and further aromatization reactions paved the way for a convenient access to structurally diverse polycyclic compounds. For instance, installing the C-aryl and spiro-C-aryl glycosides in the same moiety was successfully achieved. An application

Graphical Abstract

Scheme 1: Intramolecular (A) and intermolecular (B) enyne metathesis reactions.

Scheme 2: Ene–yne and yne–ene mechanisms for intramolecular enyne metathesis reactions.

Scheme 3: Metallacarbene mechanism in intermolecular enyne metathesis.

Scheme 4: The Oguri strategy for accessing artemisinin analogs 1a–c through enyne metathesis.

Scheme 5: Access to the tetracyclic core of nanolobatolide (2) via tandem enyne metathesis followed by an Eu(...

Scheme 6: Synthesis of (−)-amphidinolide E (3) using an intermolecular enyne metathesis as the key step.

Scheme 7: Synthesis of amphidinolide K (4) by an enyne metathesis route.

Scheme 8: Trost synthesis of des-epoxy-amphidinolide N (5) [72].

Scheme 9: Enyne metathesis between the propargylic derivative and the allylic alcohol in the synthesis of the...

Scheme 10: Synthetic route to amphidinolide N (6a).

Scheme 11: Synthesis of the stereoisomeric precursors of amphidinolide V (7a and 7b) through alkyne ring-closi...

Scheme 12: Synthesis of the anthramycin precursor 8 from ʟ-methionine by a tandem enyne metathesis–cross metat...

Scheme 13: Synthesis of (−)‐clavukerin A (9) and (−)‐isoclavukerin A (10) by an enyne metathesis route startin...

Scheme 14: Synthesis of (−)-isoguaiene (11) through an enyne metathesis as the key step.

Scheme 15: Synthesis of erogorgiaene (12) by a tandem enyne metathesis/cross metathesis sequence using the sec...

Scheme 16: Synthesis of (−)-galanthamine (13) from isovanilin by an enyne metathesis.

Scheme 17: Application of enyne metathesis for the synthesis of kempene diterpenes 14a–c.

Scheme 18: Synthesis of the alkaloid (+)-lycoflexine (15) through enyne metathesis.

Scheme 19: Synthesis of the AB subunits of manzamine A (16a) and E (16b) by enyne metathesis.

Scheme 20: Jung's synthesis of rhodexin A (17) by enyne metathesis/cross metathesis reactions.

Scheme 21: Total synthesis of (−)-flueggine A (18) and (+)-virosaine B (19) from Weinreb amide by enyne metath...

Scheme 22: Access to virgidivarine (20) and virgiboidine (21) by an enyne metathesis route.

Scheme 23: Enyne metathesis approach to (−)-zenkequinone B (22).

Scheme 24: Access to C-aryl glycoside 23 by an intermolecular enyne metathesis/Diels–Alder cycloaddition.

Scheme 25: Synthesis of spiro-C-aryl glycoside 24 by a tandem intramolecular enyne metathesis/Diels–Alder reac...

Scheme 26: Pathways to (−)-exiguolide (25) by Trost’s Ru-catalyzed enyne cross-coupling and cross-metathesis [94].
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
- Pezhman Shiri and
- Jasem Aboonajmi
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52
- -, oxygen-, and sulfur-containing ligands were investigated soon after the disclosure of the auxiliary effect of ligands on copper-catalyzed alkyne–azide cycloaddition reactions [16][17]. Heterogeneous catalysts are safer than their homogeneous counterparts. They also offer some advantages, such as the ease
- to be highly active in [3 + 2] cycloaddition reactions of halides, nonactivated terminal alkynes, and sodium azide (Scheme 21). Copper(I) ions were attached to the surface of carbon graphene as outlined in Scheme 21. Therein, GO was generated by Hummer’s method, and ascorbic acid was used to produce

Graphical Abstract

Scheme 1: Chemical structure of the catalysts 1a and 1b and their catalytic application in CuAAC reactions.

Scheme 2: Synthetic route to the catalyst 11 and its catalytic application in CuAAC reactions.

Scheme 3: Synthetic route of dendrons, illustrated using G2-AMP 23.

Scheme 4: The catalytic application of CuYAu–Gx-AAA–SBA-15 in a CuAAC reaction.

Scheme 5: Synthetic route to the catalyst 36.

Scheme 6: Application of the catalyst 36 in CuAAC reactions.

Scheme 7: The synthetic route to the catalyst 45 and catalytic application of 45 in “click” reactions.

Scheme 8: Synthetic route to the catalyst 48 and catalytic application of 48 in “click” reactions.

Scheme 9: Synthetic route to the catalyst 58 and catalytic application of 58 in “click” reactions.

Scheme 10: Synthetic route to the catalyst 64 and catalytic application of 64 in “click” reactions.

Scheme 11: Chemical structure of the catalyst 68 and catalytic application of 68 in “click” reactions.

Scheme 12: Chemical structure of the catalyst 69 and catalytic application of 69 in “click” reactions.

Scheme 13: Synthetic route to, and chemical structure of the catalyst 74.

Scheme 14: Application of the cayalyst 74 in “click” reactions.

Scheme 15: Synthetic route to, and chemical structure of the catalyst 78 and catalytic application of 78 in “c...

Scheme 16: Synthetic route to the catalyst 85.

Scheme 17: Application of the catalyst 85 in “click” reactions.

Scheme 18: Synthetic route to the catalyst 87 and catalytic application of 87 in “click” reactions.

Scheme 19: Chemical structure of the catalyst 88 and catalytic application of 88 in “click” reactions.

Scheme 20: Synthetic route to the catalyst 90 and catalytic application of 90 in “click” reactions.

Scheme 21: Synthetic route to the catalyst 96 and catalytic application of 96 in “click” reactions.

Scheme 22: Synthetic route to the catalyst 100 and catalytic application of 100 in “click” reactions.

Scheme 23: Synthetic route to the catalyst 102 and catalytic application of 23 in “click” reactions.

Scheme 24: Synthetic route to the catalysts 108–111.

Scheme 25: Catalytic application of 108–111 in “click” reactions.

Scheme 26: Synthetic route to the catalyst 121 and catalytic application of 121 in “click” reactions.

Scheme 27: Synthetic route to 125 and application of 125 in “click” reactions.

Scheme 28: Synthetic route to the catalyst 131 and catalytic application of 131 in “click” reactions.

Scheme 29: Synthetic route to the catalyst 136.

Scheme 30: Application of the catalyst 136 in “click” reactions.

Scheme 31: Synthetic route to the catalyst 141 and catalytic application of 141 in “click” reactions.

Scheme 32: Synthetic route to the catalyst 144 and catalytic application of 144 in “click” reactions.

Scheme 33: Synthetic route to the catalyst 149 and catalytic application of 149 in “click” reactions.

Scheme 34: Synthetic route to the catalyst 153 and catalytic application of 153 in “click” reactions.

Scheme 35: Synthetic route to the catalyst 155 and catalytic application of 155 in “click” reactions.

Scheme 36: Synthetic route to the catalyst 157 and catalytic application of 157 in “click” reactions.

Scheme 37: Synthetic route to the catalyst 162.

Scheme 38: Application of the catalyst 162 in “click” reactions.

Scheme 39: Synthetic route to the catalyst 167 and catalytic application of 167 in “click” reactions.

Scheme 40: Synthetic route to the catalyst 169 and catalytic application of 169 in “click” reactions.

Scheme 41: Synthetic route to the catalyst 172.

Scheme 42: Application of the catalyst 172 in “click” reactions.
Influence of the cis/trans configuration on the supramolecular aggregation of aryltriazoles
- Sara Tejera,
- Giada Caniglia,
- Rosa L. Dorta,
- Andrea Favero,
- Javier González-Platas and
- Jesús T. Vázquez
Beilstein J. Org. Chem. 2019, 15, 2881–2888, doi:10.3762/bjoc.15.282
- supramolecular studies and report herein the ability of ditriazoles to form gels, their physical properties, as well as the dependence of these properties on the cis/trans relative configuration. Results and Discussion A large set of mono- and ditriazoles was synthesized using cycloaddition reactions based on

Graphical Abstract

Scheme 1: Structures of 4-substituted 1-glucopyranosyltriazoles 1a–g and 2a–g [15].

Scheme 2: Synthesis of 1,2-cis-/trans-bistriazoles 7a–7g and 8a–8g [15].

Scheme 3: Compounds 9 (trans) and 10 (cis) [15].

Scheme 4: Synthesis of (1R,2R)- and (1R,2S)-1,2-bis-(4-(4-bromophenyl)-1H-triazol-1-yl)cyclohexane (12 and 14...

Figure 1: Tube inversion test: gels formed by compounds 7f, 8f, 10, 12, and 14.

Figure 2: SEM images of the xerogels of compounds 7f (DMSO, top left), 8f (DMSO/H2O, 3:1, v/v, top right), 10...

Figure 3: ORTEP representation of the molecular structure of compound 12 (trans configuration) obtained from ...

Figure 4: Crystal packing of compound 12 (trans configuration) in DMSO.

Figure 5: Crystal packing of 10 (cis configuration) in DMSO/H2O (1:1, v/v). Colored lines: π–π stacking inter...

Figure 6: CD spectra of compound 10 (cis) in DMSO/H2O (1:2, v/v) in solution (in black) and as gel (in blue).
An overview of the cycloaddition chemistry of fulvenes and emerging applications
- Ellen Swan,
- Kirsten Platts and
- Anton Blencowe
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- The unusual electronic properties and unique reactivity of fulvenes have interested researchers for over a century. The propensity to form dipolar structures at relatively low temperatures and to participate as various components in cycloaddition reactions, often highly selectively, makes them ideal
- interest as a result of their unique reactivity resulting from their exocyclic double bond [9][29][30][31][32], and more recently, as intermediates in the synthesis of more complex polycyclic scaffolds via cycloaddition reactions. While this highlight article will focus primarily on the cycloaddition
- chemistry of fulvenes and its applications, a brief introduction to the properties and reactivity of fulvenes, important to understanding their participation in cycloaddition reactions, is initially provided. For a more general background on the chemistry of pentafulvenes, in particular their fundamental

Graphical Abstract

Figure 1: General structure of fulvenes, named according to the number of carbon atoms in their ring. Whilst ...

Figure 2: Generic structures of commonly referenced heteropentafulvenes, named according to the heteroatom su...

Scheme 1: Resonance structures of (a) pentafulvene and (b) heptafulvene showing neutral (1 and 2), dipolar (1a...

Scheme 2: Resonance structures of (a) pentafulvenes and (b) heptafulvenes showing the influence of EDG and EW...

Scheme 3: Reaction of 6,6-dimethylpentafulvene with singlet state oxygen to form an enol lactone via the mult...

Scheme 4: Photosensitized oxygenation of 8-cyanoheptafulvene with singlet state oxygen to afford 1,4-epidioxi...

Figure 3: A representation of HOMO–LUMO orbitals of pentafulvene and the influence of EWG and EDG substituent...

Scheme 5: Reactions of (a) 6,6-dimethylpentafulvene participating as 2π and 4π components in cycloadditions w...

Scheme 6: Proposed mechanism for the [6 + 4] cycloaddition of tropone with dimethylfulvene via an ambimodal [...

Scheme 7: Triafulvene dimerization through the proposed 'head-to-tail' mechanism. The dipolar transition stat...

Scheme 8: Dimerization of pentafulvenes via a Diels–Alder cycloaddition pathway whereby one fulvene acts as a...

Scheme 9: Dimerization of pentafulvenes via frustrated Lewis pair chemistry as reported by Mömming et al. [116].

Scheme 10: Simplified reaction scheme for the formation of kempane from an extended-chain pentafulvene [127].

Scheme 11: The enantioselective (>99% ee), asymmetric, catalytic, intramolecular [6 + 2] cycloaddition of fulv...

Scheme 12: Intramolecular [8 + 6] cycloaddition of the heptafulvene-pentafulvene derivative [22,27].

Scheme 13: Reaction scheme for (a) [2 + 2] cycloaddition of 1,2-diphenylmethylenecyclopropene and 1-diethylami...

Scheme 14: Diels–Alder cycloaddition of pentafulvenes derivatives participating as dienes with (i) maleimide d...

Scheme 15: Generic schemes showing pentafulvenes participating as dienophiles in Diels–Alder cycloadditions wi...

Scheme 16: Reaction of 8,8-dicyanoheptafulvene and styrene derivatives to afford [8 + 2] and [4 + 2] cycloaddu...

Scheme 17: Reaction of 6-aminofulvene and maleic anhydride, showing observed [6 + 2] cycloaddition; the [4 + 2...

Scheme 18: Schemes for Diels–Alder cycloadditions in dynamic combinatorial chemistry reported by Boul et al. R...

Scheme 19: Polymerisation and dynamer formation via Diels–Alder cycloaddition between fulvene groups in polyet...

Scheme 20: Preparation of hydrogels via Diels–Alder cycloaddition with fulvene-conjugated dextran and dichloro...

Scheme 21: Ring-opening metathesis polymerisation of norbornene derivatives derived from fulvenes and maleimid...
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
- Gagandeep Kour Reen,
- Ashok Kumar and
- Pratibha Sharma
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- , inexpensive, water-tolerant Lewis acid catalyst in the formation of both carbon–carbon and carbon–heteroatom bonds, and thereby the formation of various biologically promising organic compounds [68]. Important advances in scandium-catalyzed chemistry include [4 + 2] and [2 + 2] cycloaddition reactions, Baeyer

Graphical Abstract

Figure 1: Various drugs having IP nucleus.

Figure 2: Participation percentage of various TMs for the syntheses of IPs.

Scheme 1: CuI–NaHSO4·SiO2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 2: Experimental examination of reaction conditions.

Scheme 3: One-pot tandem reaction for the synthesis of 2-haloimidazopyridines.

Scheme 4: Mechanistic scheme for the synthesis of 2-haloimidazopyridine.

Scheme 5: Copper-MOF-catalyzed three-component reaction (3-CR) for imidazo[1,2-a]pyridines.

Scheme 6: Mechanism for copper-MOF-driven synthesis.

Scheme 7: Heterogeneous synthesis via titania-supported CuCl2.

Scheme 8: Mechanism involving oxidative C–H functionalization.

Scheme 9: Heterogeneous synthesis of IPs.

Scheme 10: One-pot regiospecific synthesis of imidazo[1,2-a]pyridines.

Scheme 11: Vinyl azide as an unprecedented substrate for imidazo[1,2-a]pyridines.

Scheme 12: Radical pathway.

Scheme 13: Cu(I)-catalyzed transannulation approach for imidazo[1,5-a]pyridines.

Scheme 14: Plausible radical pathway for the synthesis of imidazo[1,5-a]pyridines.

Scheme 15: A solvent-free domino reaction for imidazo[1,2-a]pyridines.

Scheme 16: Cu-NPs-mediated synthesis of imidazo[1,2-a]pyridines.

Scheme 17: CuI-catalyzed synthesis of isoxazolylimidazo[1,2-a]pyridines.

Scheme 18: Functionalization of 4-bromo derivative via Sonogashira coupling reaction.

Scheme 19: A plausible reaction pathway.

Scheme 20: Cu(I)-catalyzed intramolecular oxidative C–H amidation reaction.

Scheme 21: One-pot synthetic reaction for imidazo[1,2-a]pyridine.

Scheme 22: Plausible reaction mechanism.

Scheme 23: Cu(OAc)2-promoted synthesis of imidazo[1,2-a]pyridines.

Scheme 24: Mechanism for aminomethylation/cycloisomerization of propiolates with imines.

Scheme 25: Three-component synthesis of imidazo[1,2-a]pyridines.

Figure 3: Scope of pyridin-2(1H)-ones and acetophenones.

Scheme 26: CuO NPS-promoted A3 coupling reaction.

Scheme 27: Cu(II)-catalyzed C–N bond formation reaction.

Scheme 28: Mechanism involving Chan–Lam/Ullmann coupling.

Scheme 29: Synthesis of formyl-substituted imidazo[1,2-a]pyridines.

Scheme 30: A tandem sp3 C–H amination reaction.

Scheme 31: Probable mechanistic approach.

Scheme 32: Dual catalytic system for imidazo[1,2-a]pyridines.

Scheme 33: Tentative mechanism.

Scheme 34: CuO/CuAl2O4/ᴅ-glucose-promoted 3-CCR.

Scheme 35: A tandem CuOx/OMS-2-based synthetic strategy.

Figure 4: Biomimetic catalytic oxidation in the presence of electron-transfer mediators (ETMs).

Scheme 36: Control experiment.

Scheme 37: Copper-catalyzed C(sp3)–H aminatin reaction.

Scheme 38: Reaction of secondary amines.

Scheme 39: Probable mechanistic pathway.

Scheme 40: Coupling reaction of α-azidoketones.

Scheme 41: Probable pathway.

Scheme 42: Probable mechanism with free energy calculations.

Scheme 43: MCR for cyanated IP synthesis.

Scheme 44: Substrate scope for the reaction.

Scheme 45: Reaction mechanism.

Scheme 46: Probable mechanistic pathway for Cu/ZnAl2O4-catalyzed reaction.

Scheme 47: Copper-catalyzed double oxidative C–H amination reaction.

Scheme 48: Application towards different coupling reactions.

Scheme 49: Reaction mechanism.

Scheme 50: Condensation–cyclization approach for the synthesis of 1,3-diarylated imidazo[1,5-a]pyridines.

Scheme 51: Optimized reaction conditions.

Scheme 52: One-pot 2-CR.

Scheme 53: One-pot 3-CR without the isolation of chalcone.

Scheme 54: Copper–Pybox-catalyzed cyclization reaction.

Scheme 55: Mechanistic pathway catalyzed by Cu–Pybox complex.

Scheme 56: Cu(II)-promoted C(sp3)-H amination reaction.

Scheme 57: Wider substrate applicability for the reaction.

Scheme 58: Plausible reaction mechanism.

Scheme 59: CuI assisted C–N cross-coupling reaction.

Scheme 60: Probable reaction mechanism involving sp3 C–H amination.

Scheme 61: One-pot MCR-catalyzed by CoFe2O4/CNT-Cu.

Scheme 62: Mechanistic pathway.

Scheme 63: Synthetic scheme for 3-nitroimidazo[1,2-a]pyridines.

Scheme 64: Plausible mechanism for CuBr-catalyzed reaction.

Scheme 65: Regioselective synthesis of halo-substituted imidazo[1,2-a]pyridines.

Scheme 66: Synthesis of 2-phenylimidazo[1,2-a]pyridines.

Scheme 67: Synthesis of diarylated compounds.

Scheme 68: CuBr2-mediated one-pot two-component oxidative coupling reaction.

Scheme 69: Decarboxylative cyclization route to synthesize 1,3-diarylimidazo[1,5-a]pyridines.

Scheme 70: Mechanistic pathway.

Scheme 71: C–H functionalization reaction of enamines to produce diversified heterocycles.

Scheme 72: A plausible mechanism.

Scheme 73: CuI-promoted aerobic oxidative cyclization reaction of ketoxime acetates and pyridines.

Scheme 74: CuI-catalyzed pathway for the formation of imidazo[1,2-a]pyridine.

Scheme 75: Mechanistic pathway.

Scheme 76: Mechanistic rationale for the synthesis of products.

Scheme 77: Copper-catalyzed synthesis of vinyloxy-IP.

Scheme 78: Regioselective product formation with propiolates.

Scheme 79: Proposed mechanism for vinyloxy-IP formation.

Scheme 80: Regioselective synthesis of 3-hetero-substituted imidazo[1,2-a]pyridines with different reaction su...

Scheme 81: Mechanistic pathway.

Scheme 82: CuI-mediated synthesis of 3-formylimidazo[1,2-a]pyridines.

Scheme 83: Radical pathway for 3-formylated IP synthesis.

Scheme 84: Pd-catalyzed urea-cyclization reaction for IPs.

Scheme 85: Pd-catalyzed one-pot-tandem amination and intramolecular amidation reaction.

Figure 5: Scope of aniline nucleophiles.

Scheme 86: Pd–Cu-catalyzed Sonogashira coupling reaction.

Scheme 87: One-pot amide coupling reaction for the synthesis of imidazo[4,5-b]pyridines.

Scheme 88: Urea cyclization reaction for the synthesis of two series of pyridines.

Scheme 89: Amidation reaction for the synthesis of imidazo[4,5-b]pyridines.

Figure 6: Amide scope.

Scheme 90: Pd NPs-catalyzed 3-component reaction for the synthesis of 2,3-diarylated IPs.

Scheme 91: Plausible mechanistic pathway for Pd NPs-catalyzed MCR.

Scheme 92: Synthesis of chromenoannulated imidazo[1,2-a]pyridines.

Scheme 93: Mechanism for the synthesis of chromeno-annulated IPs.

Scheme 94: Zinc oxide NRs-catalyzed synthesis of imidazo[1,2-a]azines/diazines.

Scheme 95: Zinc oxide-catalyzed isocyanide based GBB reaction.

Scheme 96: Reaction pathway for ZnO-catalyzed GBB reaction.

Scheme 97: Mechanistic pathway.

Scheme 98: ZnO NRs-catalyzed MCR for the synthesis of imidazo[1,2-a]azines.

Scheme 99: Ugi type GBB three-component reaction.

Scheme 100: Magnetic NPs-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 101: Regioselective synthesis of 2-alkoxyimidazo[1,2-a]pyridines catalyzed by Fe-SBA-15.

Scheme 102: Plausible mechanistic pathway for the synthesis of 2-alkoxyimidazopyridine.

Scheme 103: Iron-catalyzed synthetic approach.

Scheme 104: Iron-catalyzed aminooxygenation reaction.

Scheme 105: Mechanistic pathway.

Scheme 106: Rh(III)-catalyzed double C–H activation of 2-substituted imidazoles and alkynes.

Scheme 107: Plausible reaction mechanism.

Scheme 108: Rh(III)-catalyzed non-aromatic C(sp2)–H bond activation–functionalization for the synthesis of imid...

Scheme 109: Reactivity and selectivity of different substrates.

Scheme 110: Rh-catalyzed direct C–H alkynylation by Li et al.

Scheme 111: Suggested radical mechanism.

Scheme 112: Scandium(III)triflate-catalyzed one-pot reaction and its mechanism for the synthesis of benzimidazo...

Scheme 113: RuCl3-assisted Ugi-type Groebke–Blackburn condensation reaction.

Scheme 114: C-3 aroylation via Ru-catalyzed two-component reaction.

Scheme 115: Regioselective synthetic mechanism.

Scheme 116: La(III)-catalyzed one-pot GBB reaction.

Scheme 117: Mechanistic approach for the synthesis of imidazo[1,2-a]pyridines.

Scheme 118: Synthesis of imidazo[1,2-a]pyridine using LaMnO3 NPs under neat conditions.

Scheme 119: Mechanistic approach.

Scheme 120: One-pot 3-CR for regioselective synthesis of 2-alkoxy-3-arylimidazo[1,2-a]pyridines.

Scheme 121: Formation of two possible products under optimization of the catalysts.

Scheme 122: Mechanistic strategy for NiFe2O4-catalyzed reaction.

Scheme 123: Two-component reaction for synthesizing imidazodipyridiniums.

Scheme 124: Mechanistic scheme for the synthesis of imidazodipyridiniums.

Scheme 125: CuI-catalyzed arylation of imidazo[1,2-a]pyridines.

Scheme 126: Mechanism for arylation reaction.

Scheme 127: Cupric acetate-catalyzed double carbonylation approach.

Scheme 128: Radical mechanism for double carbonylation of IP.

Scheme 129: C–S bond formation reaction catalyzed by cupric acetate.

Scheme 130: Cupric acetate-catalyzed C-3 formylation approach.

Scheme 131: Control experiments for signifying the role of DMSO and oxygen.

Scheme 132: Mechanism pathway.

Scheme 133: Copper bromide-catalyzed CDC reaction.

Scheme 134: Extension of the substrate scope.

Scheme 135: Plausible radical pathway.

Scheme 136: Transannulation reaction for the synthesis of imidazo[1,5-a]pyridines.

Scheme 137: Plausible reaction pathway for denitrogenative transannulation.

Scheme 138: Cupric acetate-catalyzed C-3 carbonylation reaction.

Scheme 139: Plausible mechanism for regioselective C-3 carbonylation.

Scheme 140: Alkynylation reaction at C-2 of 3H-imidazo[4,5-b]pyridines.

Scheme 141: Two-way mechanism for C-2 alkynylation of 3H-imidazo[4,5-b]pyridines.

Scheme 142: Palladium-catalyzed SCCR approach.

Scheme 143: Palladium-catalyzed Suzuki coupling reaction.

Scheme 144: Reaction mechanism.

Scheme 145: A phosphine free palladium-catalyzed synthesis of C-3 arylated imidazopyridines.

Scheme 146: Palladium-mediated Buchwald–Hartwig cross-coupling reaction.

Figure 7: Structure of the ligands optimized.

Scheme 147: Palladium acetate-catalyzed direct arylation of imidazo[1,2-a]pyridines.

Scheme 148: Palladium acetate-catalyzed mechanistic pathway.

Scheme 149: Palladium acetate-catalyzed regioselective arylation reported by Liu and Zhan.

Scheme 150: Mechanism for selective C-3 arylation of IP.

Scheme 151: Pd(II)-catalyzed alkenylation reaction with styrenes.

Scheme 152: Pd(II)-catalyzed alkenylation reaction with acrylates.

Scheme 153: A two way mechanism.

Scheme 154: Double C–H activation reaction catalyzed by Pd(OAc)2.

Scheme 155: Probable mechanism.

Scheme 156: Palladium-catalyzed decarboxylative coupling.

Scheme 157: Mechanistic cycle for decarboxylative arylation reaction.

Scheme 158: Ligand-free approach for arylation of imidazo[1,2-a]pyridine-3-carboxylic acids.

Scheme 159: Mechanism for ligandless arylation reaction.

Scheme 160: NHC-Pd(II) complex assisted arylation reaction.

Scheme 161: C-3 arylation of imidazo[1,2-a]pyridines with aryl bromides catalyzed by Pd(OAc)2.

Scheme 162: Pd(II)-catalyzed C-3 arylations with aryl tosylates and mesylates.

Scheme 163: CDC reaction for the synthesis of imidazo[1,2-a]pyridines.

Scheme 164: Plausible reaction mechanism for Pd(OAc)2-catalyzed synthesis of imidazo[1,2-a]pyridines.

Scheme 165: Pd-catalyzed C–H amination reaction.

Scheme 166: Mechanism for C–H amination reaction.

Scheme 167: One-pot synthesis for 3,6-di- or 2,3,6-tri(hetero)arylimidazo[1,2-a]pyridines.

Scheme 168: C–H/C–H cross-coupling reaction of IPs and azoles catalyzed by Pd(II).

Scheme 169: Mechanistic cycle.

Scheme 170: Rh-catalyzed C–H arylation reaction.

Scheme 171: Mechanistic pathway for C–H arylation of imidazo[1,2-a]pyridine.

Scheme 172: Rh(III)-catalyzed double C–H activation of 2-phenylimidazo[1,2-a]pyridines and alkynes.

Scheme 173: Rh(III)-catalyzed mechanistic pathway.

Scheme 174: Rh(III)-mediated oxidative coupling reaction.

Scheme 175: Reactions showing functionalization of the product obtained by the group of Kotla.

Scheme 176: Mechanism for Rh(III)-catalyzed oxidative coupling reaction.

Scheme 177: Rh(III)-catalyzed C–H activation reaction.

Scheme 178: Mechanistic cycle.

Scheme 179: Annulation reactions of 2-arylimidazo[1,2-a]pyridines and alkynes.

Scheme 180: Two-way reaction mechanism for annulations reaction.

Scheme 181: [RuCl2(p-cymene)]2-catalyzed C–C bond formation reaction.

Scheme 182: Reported reaction mechanism.

Scheme 183: Fe(III) catalyzed C-3 formylation approach.

Scheme 184: SET mechanism-catalyzed by Fe(III).

Scheme 185: Ni(dpp)Cl2-catalyzed KTC coupling.

Scheme 186: Pd-catalyzed SM coupling.

Scheme 187: Vanadium-catalyzed coupling of IP and NMO.

Scheme 188: Mechanistic cycle.

Scheme 189: Selective C3/C5–H bond functionalizations by mono and bimetallic systems.

Scheme 190: rGO-Ni@Pd-catalyzed C–H bond arylation of imidazo[1,2-a]pyridine.

Scheme 191: Mechanistic pathway for heterogeneously catalyzed arylation reaction.

Scheme 192: Zinc triflate-catalyzed coupling reaction of substituted propargyl alcohols.
Synthesis of pyrrolo[1,2-a]quinolines by formal 1,3-dipolar cycloaddition reactions of quinolinium salts
- Anthony Choi,
- Rebecca M. Morley and
- Iain Coldham
Beilstein J. Org. Chem. 2019, 15, 1480–1484, doi:10.3762/bjoc.15.149
- could be converted to other derivatives by Suzuki–Miyaura coupling, reduction or oxidation reactions. Keywords: azomethine ylide; cycloaddition; heterocycle; pyrrolidine; stereoselective; Introduction Cycloaddition reactions of azomethine ylides are an important class of pericyclic reactions that give
- with a quinoline. Pyrroloquinolines have been found to show antibacterial and antifungal activity, to be ligands for the NK1 receptor, and to be effective against the Hif hypoxia pathway in cancer cell lines [36][37][38]. Almost all of the examples of dipolar cycloaddition reactions involving

Graphical Abstract

Scheme 1: Reaction of ketone 1 with electron-deficient alkenes 2.

Scheme 2: Reactions of ester 4 and amide 5 with electron-deficient alkenes 6.

Figure 1: Single crystal X-ray structure for 7c.

Scheme 3: Reactions of ester 4 and amide 5 with N-methylmaleimide.

Figure 2: Single crystal X-ray structure for 9.

Scheme 4: Reduction and oxidation of adducts 9 and 10.

Scheme 5: Formation of amides 15a and 15b and Suzuki–Miyaura coupling to yield 16.
Synthesis of non-racemic 4-nitro-2-sulfonylbutan-1-ones via Ni(II)-catalyzed asymmetric Michael reaction of β-ketosulfones
- Alexander N. Reznikov,
- Anastasiya E. Sibiryakova,
- Marat R. Baimuratov,
- Eugene V. Golovin,
- Victor B. Rybakov and
- Yuri N. Klimochkin
Beilstein J. Org. Chem. 2019, 15, 1289–1297, doi:10.3762/bjoc.15.127
- cytotoxicity [9]. However, it is of great importance to obtain all stereoisomers for the study of biological activity. Therefore, the development of methods for the asymmetric synthesis of polyfunctional sulfones is valuable. The most notable of them are Ag- and Cu-catalyzed 1,3-dipolar cycloaddition reactions

Graphical Abstract

Figure 1: Рharmacologically active sulfones.

Figure 2: Structures of the ligands L1–L8.

Figure 3: Evolution of the conversion of 5 and diastereomeric composition of the products of reaction of 5a w...

Figure 4: Time profile of epimerization and retro-Michael reaction of (2R,3S)-8a in chloroform-d solution.

Figure 5: ORTEP diagram of (2R,3S)-8d.

Scheme 1: The proposed mechanism of asymmetric addition of β-ketosulfones to nitroalkenes.

Scheme 2: Transition state models for asymmetric addition of β-ketosulfones to nitroalkenes.
Unnatural α-amino ethyl esters from diethyl malonate or ethyl β-bromo-α-hydroxyiminocarboxylate
- Eloi P. Coutant,
- Vincent Hervin,
- Glwadys Gagnot,
- Candice Ford,
- Racha Baatallah and
- Yves L. Janin
Beilstein J. Org. Chem. 2018, 14, 2853–2860, doi:10.3762/bjoc.14.264
- + 3] cycloaddition reactions with nitroethane or 1-nitropropane gave the isoxazole-bearing compounds 44a,b in 23 and 33% yield, respectively. Then, oximation of these compounds gave the α-hydroxyimino esters 45a,b in 50 and 58% and upon their reduction, the expected α-amino esters 46a,b. As depicted

Graphical Abstract

Scheme 1: Malonate-based retrosynthesis of α-amino esters.

Scheme 2: Some side products and synthesis of α-amino ester 10.

Scheme 3: Syntheses of α-amino esters 22, 24, 26, 28 and 33.

Scheme 4: Syntheses of α-amino esters 38, 41 and 46a,b.

Scheme 5: Syntheses of α-amino esters 53 and 58.
Synthesis of unnatural α-amino esters using ethyl nitroacetate and condensation or cycloaddition reactions
- Glwadys Gagnot,
- Vincent Hervin,
- Eloi P. Coutant,
- Sarah Desmons,
- Racha Baatallah,
- Victor Monnot and
- Yves L. Janin
Beilstein J. Org. Chem. 2018, 14, 2846–2852, doi:10.3762/bjoc.14.263

Graphical Abstract

Scheme 1: α-Amino esters from ethyl nitroacetate (4).

Scheme 2: Preparations of α-amino esters 10, 12 and 14.

Scheme 3: Syntheses of α-amino ester 18 and piperazinediones 23a,b.

Scheme 4: Syntheses of α-hydroximino ester 29 and α-amino ester 36.

Scheme 5: Synthesis of α-amino ester 43.
Transition metal-free oxidative and deoxygenative C–H/C–Li cross-couplings of 2H-imidazole 1-oxides with carboranyl lithium as an efficient synthetic approach to azaheterocyclic carboranes
- Lidia A. Smyshliaeva,
- Mikhail V. Varaksin,
- Pavel A. Slepukhin,
- Oleg N. Chupakhin and
- Valery N. Charushin
Beilstein J. Org. Chem. 2018, 14, 2618–2626, doi:10.3762/bjoc.14.240
- of promising heterocyclic carboranes: (i) condensation of decaborane (B10H14) with substituted acetylenes [27][28][29][30], (ii) carboryne-based cycloaddition reactions [31][32][33], and (iii) C–X/C–M cross coupling of halogenated azaheterocycles (X = Br, Cl, F) with carborane organometallic

Graphical Abstract

Scheme 1: C–H/C–Li cross-coupling reactions of 2H-imidazole 1-oxides 1a–d and carboranyl lithium 2. The react...

Figure 1: The 1H NMR spectra of 1-(5-(4-bromophenyl)-2-ethyl-2-methyl-2H-imidazol-4-yl)-1,2-dicarba-closo-dod...

Figure 2: Fragment of the 2D 1H–13C{1H} HSQC (a) and HMBC (b) spectra of imidazolyl carborane 5d in CDCl3 at ...

Figure 3: Molecular structure of 5d. Selected bond distances (Å) and angles (deg) for molecule 1: C(3)–C(14),...
Synthesis of cis-hydrindan-2,4-diones bearing an all-carbon quaternary center by a Danheiser annulation
- Gisela V. Saborit,
- Carlos Cativiela,
- Ana I. Jiménez,
- Josep Bonjoch and
- Ben Bradshaw
Beilstein J. Org. Chem. 2018, 14, 2597–2601, doi:10.3762/bjoc.14.237
- Lycopodium alkaloids carinatine A [11][14][15], 8-deoxyserratinine [10], fawcettidine [10], fawcettimine [7][10], lycojaponicumin C [10], lycopladine A [11][13][14][15], lycoposerramine R [13][14], and sieboldine [19][20] (Figure 2). Results and Discussion Despite the potential of [3 + 2] cycloaddition
- reactions [27] to achieve cis-hydrindan-2,4-diones, their application to rapidly assemble the five-membered ring of the targeted 6,5-bicylic system has not been reported until now. Two examples using cycloaddition processes in this field should be mentioned: Diels–Alder [28] and Pauson–Khand [29] reactions

Graphical Abstract

Figure 1: Previous synthetic approaches to 3a-substituted cis-hydrindan-2,4-diones.

Scheme 1: Decahydroquinoline 1 as a versatile building block for Lycopodium alkaloid synthesis.

Figure 2: Examples of Lycopodium alkaloids synthesized from 3a-substituted hydrindan-2,4-diones.

Scheme 2: A de novo approach to 3a-substituted cis-hydrindan-2,4-diones.

Scheme 3: Synthesis of enone 4 and the Danheiser annulation. The depicted compounds are all racemic.

Scheme 4: Transformation of the vinylsilane moiety to ketone 8.

Figure 3: Stereoview of cis-hydrindane 8.
Microfluidic light-driven synthesis of tetracyclic molecular architectures
- Javier Mateos,
- Nicholas Meneghini,
- Marcella Bonchio,
- Nadia Marino,
- Tommaso Carofiglio,
- Xavier Companyó and
- Luca Dell’Amico
Beilstein J. Org. Chem. 2018, 14, 2418–2424, doi:10.3762/bjoc.14.219
- synthetic method for the direct assembly of highly functionalized tetracyclic pharmacophoric cores. Coumarins and chromones undergo diastereoselective [4 + 2] cycloaddition reactions with light-generated photoenol intermediates. The reactions occur by aid of a microfluidic photoreactor (MFP) in high yield

Graphical Abstract

Figure 1: a) Light-driven reaction between 2-MBP A and maleimide B for the synthesis of C through a [4 + 2] c...

Figure 2: Generality and limits of the light-driven [4 + 2] cyclization reaction between 2-MBP 1a–g and couma...

Figure 3: Generality and limits of the light-driven [4 + 2] cyclization reaction between 2-MBP 1a–f and chrom...

Scheme 1: MFP parallel setup for higher scale production of 4a (top) and different molecular scaffolds 6a–9a ...
Catalyst-free synthesis of 4-acyl-NH-1,2,3-triazoles by water-mediated cycloaddition reactions of enaminones and tosyl azide
- Lu Yang,
- Yuwei Wu,
- Yiming Yang,
- Chengping Wen and
- Jie-Ping Wan
Beilstein J. Org. Chem. 2018, 14, 2348–2353, doi:10.3762/bjoc.14.210
- synthesis of 4-acyl-NH-1,2,3-triazoles has been accomplished with high efficiency through the cycloaddition reactions between N,N-dimethylenaminones and tosyl azide. This method is featured with extraordinary sustainability by employing water as the sole medium, free of any catalyst or additive
- intermediate 5 can undergo aminolysis and/or hydrolysis to provide the target products 3. The participation of water throughout the reaction also explains the high efficiency of the method using water as reaction medium. Conclusion In summary, by means of the cycloaddition reactions between tertiary enaminones

Graphical Abstract

Figure 1: Scope of the water-mediated synthesis of 4-acyl-NH-1,2,3-triazoles. General conditions: enaminone 1...

Scheme 1: The gram scale synthesis of 3a: (a) before reaction; (b) completed reaction; (c) the purified produ...

Scheme 2: The proposed reaction mechanism.
First thia-Diels–Alder reactions of thiochalcones with 1,4-quinones
- Grzegorz Mlostoń,
- Katarzyna Urbaniak,
- Paweł Urbaniak,
- Anna Marko,
- Anthony Linden and
- Heinz Heimgartner
Beilstein J. Org. Chem. 2018, 14, 1834–1839, doi:10.3762/bjoc.14.156
- cycloaddition reactions not only as heterodienes [17][18][19], but also as heterodipolarophiles [15]. In two recent publications we reported new thia-Diels–Alder reactions of aryl, hetaryl and ferrocenyl-substituted thiochalcones with acetylenic dienophiles, which lead to the corresponding 4H-thiopyrans in a
- cycloadducts could be expected, but the 1H NMR analysis of the crude products showed that only one product was present in each case and, therefore, the studied [4 + 2] cycloaddition reactions occurred with complete regioselectivity. Based on the assumption that the nucleophilic S-atom of the thiochalcone

Graphical Abstract

Scheme 1: Reactions of aryl/hetarylthiochalcones 1a–d with 1,4-naphthoquinone (2b).

Scheme 2: Reactions of thiochalcones 1a–d with 1,4-anthraquinones 2c and 2d.

Figure 1: ORTEP plot [29] of the molecular structure of 4k showing the major conformation of the disordered thiop...

Figure 2: Products of the reactions of thiochalcones 1a and 1b with 1,4-benzoquinone (2a) and of 1a with mena...
A survey of chiral hypervalent iodine reagents in asymmetric synthesis
- Soumen Ghosh,
- Suman Pradhan and
- Indranil Chatterjee
Beilstein J. Org. Chem. 2018, 14, 1244–1262, doi:10.3762/bjoc.14.107

- iodobiarenes to synthesize a new class of I(III) and I(V) reagents 17. These were applied for the hydroxylative dearomatization of phenolic derivatives 42 followed by the successive use of the hydroxylated products as dienes in [4 + 2] cycloaddition reactions [42]. This new reagent promoted oxygen transfer in

Graphical Abstract

Scheme 1: An overview of different chiral iodine reagents or precursors thereof.

Scheme 2: Asymmetric oxidation of sulfides by chiral hypervalent iodine reagents.

Scheme 3: Oxidative dearomatization of naphthol derivatives by Kita et al.

Scheme 4: [4 + 2] Diels–Alder dimerization reported by Birman et al.

Scheme 5: m-CPBA guided catalytic oxidative naphthol dearomatization.

Scheme 6: Oxidative dearomatization of phenolic derivatives by Ishihara et al.

Scheme 7: Oxidative spirocyclization applying precatalyst 11 developed by Ciufolini et al.

Scheme 8: Asymmetric hydroxylative dearomatization.

Scheme 9: Enantioselective oxylactonization reported by Fujita et al.

Scheme 10: Dioxytosylation of styrene (47) by Wirth et al.

Scheme 11: Oxyarylation and aminoarylation of alkenes.

Scheme 12: Asymmetric diamination of alkenes.

Scheme 13: Stereoselective oxyamination of alkenes reported by Wirth et al.

Scheme 14: Enantioselective and regioselective aminofluorination by Nevado et al.

Scheme 15: Fluorinated difunctionalization reported by Jacobsen et al.

Scheme 16: Aryl rearrangement reported by Wirth et al.

Scheme 17: α-Arylation of β-ketoesters.

Scheme 18: Asymmetric α-oxytosylation of carbonyls.

Scheme 19: Asymmetric α-oxygenation and α-amination of carbonyls reported by Wirth et al.

Scheme 20: Asymmetric α-functionalization of ketophenols using chiral quaternary ammonium (hypo)iodite salt re...

Scheme 21: Oxidative Intramolecular coupling by Gong et al.

Scheme 22: α-Sulfonyl and α-phosphoryl oxylation of ketones reported by Masson et al.

Scheme 23: α-Fluorination of β-keto esters.

Scheme 24: Alkynylation of β-ketoesters and amides catalyzed by phase-transfer catalyst.

Scheme 25: Alkynylation of β-ketoesters and dearomative alkynylation of phenols.
One hundred years of benzotropone chemistry
- Arif Dastan,
- Haydar Kilic and
- Nurullah Saracoglu
Beilstein J. Org. Chem. 2018, 14, 1120–1180, doi:10.3762/bjoc.14.98
- complete electron delocalization, whereas the carbonium ion 200 (and 201) formed from 190 indicated considerably less electron delocalization (Scheme 34). 3.2.3. Cycloaddition reactions: 2,3-Benzotropone (12) possesses a high functional tolerance towards both the diene and dienophile and undergoes the

Graphical Abstract

Scheme 1: Tropone (1), tropolone (2) and their resonance structures.

Figure 1: Natural products containing a tropone nucleus.

Figure 2: Possible isomers 11–13 of benzotropone.

Scheme 2: Synthesis of benzotropones 11 and 12.

Scheme 3: Oxidation products of benzotropylium fluoroborate (16).

Scheme 4: Oxidation of 7-bromo-5H-benzo[7]annulene (22).

Scheme 5: Synthesis of 4,5-benzotropone (11) using o-phthalaldehyde (27).

Scheme 6: Synthesis of 4,5-benzotropone (11) starting from oxobenzonorbornadiene 31.

Scheme 7: Acid-catalyzed cleavage of oxo-bridge of 34.

Scheme 8: Synthesis of 4,5-benzotropone (11) from o-xylylene dibromide (38).

Scheme 9: Synthesis of 4,5-benzotropone (11) via the carbene adduct 41.

Scheme 10: Heck coupling strategy for the synthesis of 11.

Scheme 11: Synthesis of benzofulvalenes via carbonyl group of 4,5-benzotropone (11).

Figure 3: Some cycloheptatrienylium cations.

Scheme 12: Synthesis of condensation product 63 and its subsequent oxidative cyclization products.

Figure 4: A novel series of benzo[7]annulenes prepared from 4,5-benzotropone (11).

Scheme 13: Preparation of substituted benzo[7]annulene 72 using the Mukaiyama-Michael reaction.

Figure 5: Possible benzo[7]annulenylidenes 73–75.

Scheme 14: Thermal and photochemical decomposition of 7-diazo-7H-benzo[7]annulene (76) and the trapping of int...

Scheme 15: Synthesis of benzoheptafulvalene 86.

Scheme 16: Synthesis of 7-(diphenylmethylene)-7H-benzo[7]annulene (89).

Scheme 17: Reaction of 4,5-benzotropone (11) with dimethyl diazomethane.

Scheme 18: Synthesis of dihydrobenzomethoxyazocine 103.

Scheme 19: Synthesis and reducibility of benzo-homo-2-methoxyazocines.

Scheme 20: Synthesis of 4,5-benzohomotropones 104 and 115 from 4,5-benzotropones 11 and 113.

Scheme 21: A catalytic deuterogenation of 4,5-benzotropone (11) and synthesis of 5-monosubstituted benzo[7]ann...

Scheme 22: Synthesis of methyl benzo[7]annulenes 131 and 132.

Scheme 23: Ambident reactivity of halobenzo[7]annulenylium cations 133a/b.

Scheme 24: Preparation of benzo[7]annulenylidene–iron complexes 147.

Scheme 25: Synthesis of 1-ethynylbenzotropone (150) and the etheric compound 152 from 4,5-benzotropone (11) wi...

Scheme 26: Thermal decomposition of 4,5-benzotropone (11).

Scheme 27: Reaction of 4,5-benzotropone (11) with 1,2-ethanediol and 1,2-ethanedithiol.

Scheme 28: Conversions of 1-benzosuberone (162) to 2,3-benzotropone (12).

Scheme 29: Synthesis strategies for 2,3-bezotropone (12) using 1-benzosuberones.

Scheme 30: Oxidation-based synthesis of 2,3-benzotropone (12) via 1-benzosuberone (162).

Scheme 31: Synthesis of 2,3-benzotropone (12) from α-tetralone (171) via ring-expansion.

Scheme 32: Preparation of 2,3-benzotropone (12) by using of benzotropolone 174.

Figure 6: Benzoheptafulvenes as condensation products of 2,3-benzotropone (12).

Scheme 33: Conversion of 2,3-benzotropone (12) to tosylhydrazone salt 182 and gem-dichloride 187.

Figure 7: Benzohomoazocines 191–193 and benzoazocines 194–197.

Scheme 34: From 2,3-benzotropone (12) to carbonium ions 198–201.

Scheme 35: Cycloaddition reactions of 2,3-benzotropone (12).

Scheme 36: Reaction of 2,3-benzotropone (12) with various reagents and compounds.

Figure 8: 3,4-Benzotropone (13) and its resonance structure.

Scheme 37: Synthesis of 6,7-benzobicyclo[3.2.0]hepta-3,6-dien-2-one (230).

Figure 9: Photolysis and thermolysis products of 230.

Figure 10: Benzotropolones and their tautomeric structures.

Scheme 38: Synthesis strategies of 4,5-benzotropolone (238).

Scheme 39: Synthesis protocol for 2-hydroxy-4,5-benzotropone (238) using oxazole-benzo[7]annulene 247.

Figure 11: Some quinoxaline and pyrazine derivatives 254–256 prepared from 4,5-benzotropolone (238).

Scheme 40: Nitration product of 4,5-benzotropolone (238) and its isomerization to 1-nitro-naphthoic acid (259)....

Scheme 41: Synthesis protocol for 6-hydroxy-2,3-benzotropone (239) from benzosuberone (162).

Scheme 42: Various reactions via 6-hydroxy-2,3-benzotropone (239).

Scheme 43: Photoreaction of 6-hydroxy-2,3-benzotropone (239).

Scheme 44: Synthesis of 7-hydroxy-2,3-benzotropone (241) from benzosuberone (162).

Scheme 45: Synthesis strategy for 7-hydroxy-2,3-benzotropone (241) from ketone 276.

Scheme 46: Synthesis of 7-hydroxy-2,3-benzotropone (241) from β-naphthoquinone (280).

Scheme 47: Synthesis of 7-hydroxy-2,3-benzotropone (241) from bicyclic endoperoxide 213.

Scheme 48: Synthesis of 7-hydroxy-2,3-benzotropone (241) by ring-closing metathesis.

Figure 12: Various monosubstitution products 289–291 of 7-hydroxy-2,3-benzotropone (241).

Scheme 49: Reaction of 7-hydroxy-2,3-benzotropone (241) with various reagents.

Scheme 50: Synthesis of 4-hydroxy-2,3-benzotropones 174 and 304 from diketones 300/301.

Scheme 51: Catalytic hydrogenation of diketones 300 and 174.

Scheme 52: Synthesis of halo-benzotropones from alkoxy-naphthalenes 306, 307 and 310.

Figure 13: Unexpected byproducts 313–315 during synthesis of chlorobenzotropone 309.

Figure 14: Some halobenzotropones and their cycloadducts.

Scheme 53: Multisep synthesis of 2-chlorobenzotropone 309.

Scheme 54: A multistep synthesis of 2-bromo-benzotropone 26.

Scheme 55: A multistep synthesis of bromo-2,3-benzotropones 311 and 316.

Scheme 56: Oxidation reactions of 8-bromo-5H-benzo[7]annulene (329) with some oxidants.

Scheme 57: Synthesis of 2-bromo-4,5-benzotropone (26).

Scheme 58: Synthesis of 6-chloro-2,3-benzotropone (335) using LiCl and proposed intermediate 336.

Scheme 59: Reaction of 7-bromo-2,3-benzotropone (316) with methylamine.

Scheme 60: Reactions of bromo-2,3-benzotropones 26 and 311 with dimethylamine.

Scheme 61: Reactions of bromobenzotropones 311 and 26 with NaOMe.

Scheme 62: Reactions of bromobenzotropones 26 and 312 with t-BuOK in the presence of DPIBF.

Scheme 63: Cobalt-catalyzed reductive cross-couplings of 7-bromo-2,3-benzotropone (316) with cyclic α-bromo en...

Figure 15: Cycloadduct 357 and its di-π-methane rearrangement product 358.

Scheme 64: Catalytic hydrogenation of 2-chloro-4,5-benzotropone (311).

Scheme 65: Synthesis of dibromo-benzotropones from benzotropones.

Scheme 66: Bromination/dehydrobromination of benzosuberone (162).

Scheme 67: Some transformations of isomeric dibromo-benzotropones 261A/B.

Scheme 68: Transformations of benzotropolone 239B to halobenzotropolones 369–371.

Figure 16: Bromobenzotropolones 372–376 and 290 prepared via bromination/dehydrobromination strategy.

Scheme 69: Synthesis of some halobenzotropolones 289, 377 and 378.

Figure 17: Bromo-chloro-derivatives 379–381 prepared via chlorination.

Scheme 70: Synthesis of 7-iodo-3,4-benzotropolone (382).

Scheme 71: Hydrogenation of bromobenzotropolones 369 and 370.

Scheme 72: Debromination reactions of mono- and dibromides 290 and 375.

Figure 18: Nitratation and oxidation products of some halobenzotropolenes.

Scheme 73: Azo-coupling reactions of some halobenzotropolones 294, 375 and 378.

Figure 19: Four possible isomers of dibenzotropones 396–399.

Figure 20: Resonance structures of tribenzotropone (400).

Scheme 74: Two synthetic pathways for tribenzotropone (400).

Scheme 75: Synthesis of tribenzotropone (400) from dibenzotropone 399.

Scheme 76: Synthesis of tribenzotropone (400) from 9,10-phenanthraquinone (406).

Scheme 77: Synthesis of tribenzotropone (400) from trifluoromethyl-substituted arene 411.

Figure 21: Dibenzosuberone (414).

Figure 22: Reduction products 415 and 416 of tribenzotropone (400).

Figure 23: Structures of tribenzotropone dimethyl ketal 417 and 4-phenylfluorenone (412) and proposed intermed...

Figure 24: Structures of benzylidene- and methylene-9H-tribenzo[a,c,e][7]annulenes 419 and 420 and chiral phos...

Figure 25: Structures of tetracyclic alcohol 422, p-quinone methide 423 and cation 424.

Figure 26: Structures of host molecules 425–427.

Scheme 78: Synthesis of non-helical overcrowded derivatives syn/anti-431.

Figure 27: Hexabenzooctalene 432.

Figure 28: Structures of possible eight isomers 433–440 of naphthotropone.

Scheme 79: Synthesis of naphthotropone 437 starting from 1-phenylcycloheptene (441).

Scheme 80: Synthesis of 10-hydroxy-11H-cyclohepta[a]naphthalen-11-one (448) from diester 445.

Scheme 81: Synthesis of naphthotropone 433.

Scheme 82: Synthesis of naphthotropones 433 and 434 via cycloaddition reaction.

Scheme 83: Synthesis of naphthotropone 434 starting from 452.

Figure 29: Structures of tricarbonyl(tropone)irons 458, and possible cycloadducts 459.

Scheme 84: Synthesis of naphthotropone 436.

Scheme 85: Synthesis of precursor 465 for naphthotropone 435.

Scheme 86: Generation of naphthotropone 435 from 465.

Figure 30: Structures of tropylium cations 469 and 470.

Figure 31: Structures of tropylium ions 471+.BF4−, 472+.BF4−, and 473+.BF4−.

Scheme 87: Synthesis of tropylium ions 471+.BF4− and 479+.ClO4−.

Scheme 88: Synthesis of 1- and 2-methylanthracene (481 and 482) via carbene–carbene rearrangement.

Figure 32: Trapping products 488–490.

Scheme 89: Generation and chemistry of a naphthoannelated cycloheptatrienylidene-cycloheptatetraene intermedia...

Scheme 90: Proposed intermediates and reaction pathways for adduct 498.

Scheme 91: Exited-state intramolecular proton transfer of 505.

Figure 33: Benzoditropones 506 and 507.

Scheme 92: Synthesis of benzoditropone 506e.

Scheme 93: Synthetic approaches for dibenzotropone 507 via tropone (1).

Scheme 94: Formation mechanisms of benzoditropone 507 and 516 via 515.

Scheme 95: Synthesis of benzoditropones 525 and 526 from pyromellitic dianhydride (527).

Figure 34: Possible three benzocyclobutatropones 534–536.

Scheme 96: Synthesis of benzocyclobutatropones 534 and 539.

Scheme 97: Synthesis attempts for benzocyclobutatropone 545.

Scheme 98: Generation and trapping of symmetric benzocyclobutatropone 536.

Scheme 99: Synthesis of chloro-benzocyclobutatropone 552 and proposed mechanism of fluorenone derivatives.

Scheme 100: Synthesis of tropolone analogue 559.

Scheme 101: Synthesis of tropolones 561 and 562.

Figure 35: o/p-Tropoquinone rings (563 and 564) and benzotropoquinones (565–567).

Scheme 102: Synthesis of benzotropoquinone 566.

Scheme 103: Synthesis of benzotropoquinone 567 via a Diels–Alder reaction.

Figure 36: Products 575–577 through 1,2,3-benzotropoquinone hydrate 569.

Scheme 104: Structures 578–582 prepared from tropoquinone 567.

Figure 37: Two possible structures 583 and 584 for dibenzotropoquinone, and precursor compound 585 for 583.

Scheme 105: Synthesis of saddle-shaped ketone 592 using dibenzotropoquinone 584.






