Search results
Search for "π–π-interactions" in Full Text gives 110 result(s) in Beilstein Journal of Organic Chemistry.
Chiral anion recognition using calix[4]arene-based ureido receptors in a 1,3-alternate conformation
Beilstein J. Org. Chem. 2020, 16, 2999–3007, doi:10.3762/bjoc.16.249
- completed by the close contacts between carbonyl oxygen atoms (of the urea group) and S atoms (of DMSO), indicating possible chalcogen interactions [47][48], and the C=O···S=O distances were 3.269 and 3.308 Å (Figure 3a). The molecular packing was further strengthened by the π–π interactions of the p
- -acetylphenylalaninate. Design of chiral calix[4]arene-based receptors for anions. X-ray structure of 4a: (a) Top view into the cavity. (b) Side view of the same cavity. X-ray structure of 7a: (a) Hydrogen bonding interactions (black) in a dimeric motif, chalcogen interactions are shown in green. (b) π–π interactions in
Naphthalonitriles featuring efficient emission in solution and in the solid state
Beilstein J. Org. Chem. 2020, 16, 2960–2970, doi:10.3762/bjoc.16.246

- parallel (see Figure 2B), the centroid distance between the rings reaches up to 4.985 Å, suggesting that the intermolecular π–π interactions are absent. In addition to these interactions, there also exist weak supramolecular N–H interactions. Firstly, two Me units form dimeric structures that are connected
Thermodynamic and electrochemical study of tailor-made crown ethers for redox-switchable (pseudo)rotaxanes
Beilstein J. Org. Chem. 2020, 16, 2576–2588, doi:10.3762/bjoc.16.209

- -8 (DBC8, Figure 1), which bind through noncovalent interactions. In detail, these interactions are strong hydrogen bonds between ether oxygen atoms and ammonium protons. In addition, weaker C–H···O hydrogen bonds with the CH2 groups adjacent to the ammonium nitrogen as well as π–π-interactions
- , maximized π–π-interactions with the axle because of the 84° torsional angle between the resorcinol and the NDI unit. Consequently, the complexation of NDIC8 is less enthalpically favored than that of TTFC8. However, the more rigid structure of NDIC8 also leads to a lower degree of conformational fixation in
- the pseudorotaxane of NDIC8, and thus to a more favorable binding entropy compared to the pseudorotaxane of TTFC8. For TTFC8, the increased binding enthalpy can be explained by additional π–π-interactions between the naphthalene and TTF unit of the crown ether and the ammonium axle, resulting in a
Water-soluble host–guest complexes between fullerenes and a sugar-functionalized tribenzotriquinacene assembling to microspheres
Beilstein J. Org. Chem. 2020, 16, 2551–2561, doi:10.3762/bjoc.16.207

- hydrophobic effect and to host–guest π–π interactions. Hydrophobic surface simulations showed that TBTQ-(OG)6 and C60 forms an amphiphilic supramolecular host–guest complex, which further assembles to microspheres with diameters of 0.3–3.5 μm, as determined by scanning electron microscopy. Keywords
- -membered rings of C60 were calculated to be 3.541 and 3.702 Å, respectively. These results suggest significant host–guest π–π interactions between TBTQ-(OG)6 and C60 [57]. In order to intuitively assess the hydrophilicity and hydrophobicity of the complex, the hydrophobic surface diagram was generated and
- the host–guest complexes is primarily due to hydrophobic effects and π–π interactions. The TBTQ-(OG)6 C60 complex was found to further assemble to microspheres with diameters of 0.3–3.5 μm. The inclusion complexation between TBTQ-(OG)6 and fullerenes in aqueous solution may shed light on potential
pH- and concentration-dependent supramolecular self-assembly of a naturally occurring octapeptide
Beilstein J. Org. Chem. 2020, 16, 2017–2025, doi:10.3762/bjoc.16.168

- secondary structures, which lead to nanostructured materials [16][17]. Noncovalent interactions, such as hydrogen bonding, van der Waals interactions, hydrophobic interactions, electrostatic interactions, and π–π interactions [18][19][20][21][22] are common driving forces in peptide self-assembly. These
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- 8b/7c and 8c/7c (Figure 4a,b and Supporting Information File 1, Appendix 6). All the observable π···π interactions were parallel-displaced (offset) π-stacking, and the distance values were within the range of the known values, whether determined from X-ray structures or calculated values (3.2–4.0 Å
Synthesis, antiinflammatory activity, and molecular docking studies of bisphosphonic esters as potential MMP-8 and MMP-9 inhibitors
Beilstein J. Org. Chem. 2020, 16, 1277–1287, doi:10.3762/bjoc.16.108
- of the phosphonate moieties, while 5 and 6 had interactions through the C=O oxygen atom of the ester group. Because the interactions of the benzyl group of 5 and 6, respectively, with the Phe110 residue present at the catalytic site through π–π interactions are possible, the orientation of the
- molecules also presented this type of interactions between an ethyl fragment of the ligand with Leu160, and less with His207 and Val194. Weak hydrophobic interactions were also observed from Tyr219 and the aromatic ring of 6 as well as π–π interactions to His197. The molecules were not adequately occupying
- ligand to the zinc ion, with the same applying to 5. This could explain why no predicted hydrogen bonding was present in 6 and just very weak ones in 5. For MMP-9, the hydrophobic zones were formed by Ile159, Leu160, and Ala161, as was the case for Gly158 and Val194. As stated before, π–π interactions
Synthesis and anticancer activity of bis(2-arylimidazo[1,2-a]pyridin-3-yl) selenides and diselenides: the copper-catalyzed tandem C–H selenation of 2-arylimidazo[1,2-a]pyridine with selenium
Beilstein J. Org. Chem. 2020, 16, 1075–1083, doi:10.3762/bjoc.16.94

- phenyl rings that face each other were almost parallel (mean-interplanar angles: Ph(A)–ImdazoPy(B): 4.59° and Ph(B)–ImdazoPy(A): 4.64°, Figure S2, Supporting Information File 1), and 2a exhibited intramolecular π–π interactions over the whole molecule. The monoselenide 3a exhibited a C1A–Se–C2A angle of
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- adjacent layers have strong π–π interactions that could be beneficial for the charge mobility. On the other hand, the three-dimensionally organized (3D-COF) allows open sites. Therefore, for the first time, the photocatalytic activity of the same porphyrin was evaluated in distinct dimensional frameworks
Design and synthesis of diazine-based panobinostat analogues for HDAC8 inhibition
Beilstein J. Org. Chem. 2020, 16, 628–637, doi:10.3762/bjoc.16.59

- of this class of inhibitors in HDACs [31][32][33][34]. Again, our work here extends the investigation focusing on HDAC8 due to the need for novel neuroblastoma therapeutics. Each compound was also shown to have two parallel-displaced π–π interactions, one with Phe-152 and the other with Phe-208. The
- interaction with the Zn2+ ion. TOI3-rev, like that of TOI1, TOI2, and TOI4 produces two parallel-displaced π–π interactions with Phe-152 and Phe-208 (Figure 2). The pyrimidine ring of TOI3-rev also lays planar in the gorge similar to the pyrazine and pyrimidine rings of TOI1 and TOI2. The indole ring of TOI3
Six-fold C–H borylation of hexa-peri-hexabenzocoronene
Beilstein J. Org. Chem. 2020, 16, 391–397, doi:10.3762/bjoc.16.37
- organic solvents greatly decreases due to the π–π interactions. Hexa-peri-hexabenzocoronene (HBC) is a notable compound investigated in the fields from synthetic chemistry to astrophysics among PAHs [4][8][9][10], and also known as a poorly soluble PAH [11]. Against the context of difficulty in
Synthesis and herbicidal activities of aryloxyacetic acid derivatives as HPPD inhibitors
Beilstein J. Org. Chem. 2020, 16, 233–247, doi:10.3762/bjoc.16.25
- [10][19][32][33][34]. The results show that two main interactions exist between I12 and the AtHPPD active site (Figure 4), as was observed for mesotrione; the 1,3-dicarbonyl unit is chelated to the iron ion, and the aromatic ring moiety formed π–π interactions with Phe403 and Phe360. Electron
Preparation of anthracene-based tetraperimidine hexafluorophosphate and selective recognition of chromium(III) ions
Beilstein J. Org. Chem. 2019, 15, 2847–2855, doi:10.3762/bjoc.15.278
- anthracene, and the dihedral angle between two perimidine units of each arm was determined to be 18.1(4)°. Two of the four perimidine groups were parallel to the anthracene plane, with intramolecular π–π interactions [43] being present in this setup (with a face-to-face distance of 3.566(1) Å between
- intermolecular π–π interactions between perimidine moieties (with a face-to-face distance of 3.558(4) Å and a center-to-center distance of 3.566(1) Å), as shown in Supporting Information File 1, Figure S1a. Besides, a 2D supramolecular layer was formed by the 1D supramolecular chains through two types of C–H···F
Emission solvatochromic, solid-state and aggregation-induced emissive α-pyrones and emission-tuneable 1H-pyridines by Michael addition–cyclocondensation sequences
Beilstein J. Org. Chem. 2019, 15, 2684–2703, doi:10.3762/bjoc.15.262
- -phenyl ring in C-H···N [36][37][38][39] and C-H···π [40][41][42][43][44][45][46][47][48][49] interactions (Figure 2, for details, see Supporting Information File 1). It is noteworthy to mention that there are no significant π···π interactions in the solid-state structure of 5a (for details, see
Synthesis of benzo[d]imidazo[2,1-b]benzoselenoazoles: Cs2CO3-mediated cyclization of 1-(2-bromoaryl)benzimidazoles with selenium
Beilstein J. Org. Chem. 2019, 15, 2029–2035, doi:10.3762/bjoc.15.199
- independent molecules, and the benzimidazole and the fused benzoselenophene rings are virtually coplanar (mean deviation 0.0169 and 0.0359 Å, respectively) to each other. The molecules show head-to-tail (antiparallel) stacking with π···π interactions, with distances between the nearest neighbor atoms on
Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–)2A fluorescent dyes
Beilstein J. Org. Chem. 2019, 15, 1712–1721, doi:10.3762/bjoc.15.167

- known that D–π–A fluorescent dyes show bathochromic shifts of the λmax,fl and lower Φf values by changing from the solution state to the solid state. This fact is attributed to the delocalization of excitons or excimers due to the formation of intermolecular π–π interactions [48][49][50][51] between the
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
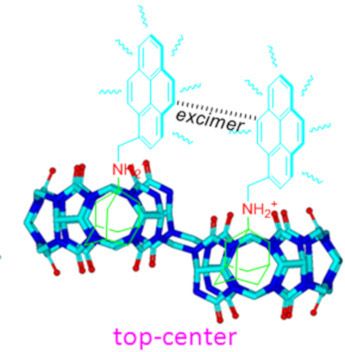
- guests in its cavity through host-stabilized charge-transfer or π–π interactions [14][15]. This novel property of Q[8] has been utilized as molecular container for biological substrates [16][17], as well as in the construction of various supramolecular assemblies with specific structures and properties
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

Reversible end-to-end assembly of selectively functionalized gold nanorods by light-responsive arylazopyrazole–cyclodextrin interaction
Beilstein J. Org. Chem. 2019, 15, 1407–1415, doi:10.3762/bjoc.15.140

- aggregates can be realized more or less efficiently through various approaches based on supramolecular interactions like metal–metal and π–π interactions [11], DNA mediated [12] or by host–guest chemistry [13]. Most of these approaches require selective functionalization of the ends of the AuNR and take
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- of the heterotriacenes should be expected planar, a slight curvature of the π-system was found for DST 3, whose α-carbon atoms are bent relative to the central thiophene plane by about 2.5 degrees (Figure 2b). This effect might be due to strong intermolecular π–π interactions in pairs of molecules
- stacked and offset molecules leading to π–π interactions with distances as close as 3.42 Å for DST 3 (Figure 3a and b), 3.24 to 3.49 Å for DTS 2 (Figure S6a–c in Supporting Information File 1), and 3.28 to 3.58 Å for DSS 4 (Figure S8a–c in Supporting Information File 1). Interestingly, the symmetry of the
- DST 3: (a) partial overlap of stacked and displaced molecules leading to π–π interactions with distances down to 3.42 Å (side view); (b) and top view (64% molecular overlap). (c) Intermolecular interactions between heteroatoms and hydrogen-heteroatoms (labelled cyan and blue, respectively). DFT
Mechanochemical synthesis of hyper-crosslinked polymers: influences on their pore structure and adsorption behaviour for organic vapors
Beilstein J. Org. Chem. 2019, 15, 1154–1161, doi:10.3762/bjoc.15.112
- explained by the strong interactions of the benzene molecules with the aromatic surface of the polymer via π–π interactions. The rather strong adsorption at the inner surface is overlapped by pore filling due to a condensation-like effect. Thereby, swelling results in a reversible structural change
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- , the cyclization of linear peptides through aryl–aryl bond formation confers a relative conformational constraint on the peptide scaffold. Moreover, the resulting biaryl motif is able to participate in π–π interactions with aromatic and hydrophobic residues, and also in π-cation interactions with
Adhesion, forces and the stability of interfaces
Beilstein J. Org. Chem. 2019, 15, 106–129, doi:10.3762/bjoc.15.12

- even known. Instead, attraction is attributed to π–π interactions or CH–π interactions of unclear physical origin. That deformation of molecules induces static multipoles is also not well known; the bending of non-polar planar molecules that have a quadrupole as their lowest static multipole (e.g
Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers
Beilstein J. Org. Chem. 2018, 14, 2627–2645, doi:10.3762/bjoc.14.241

- hydrophobic interactions were observed in the tail region, in particular with leucins 189 and 208 as well as Y258. Mutations at these specific residues indicated that the π–π interactions of Y258 are crucial for M64's full inhibition with respect to pyocyanin production, which was only weakly inhibited in an
Synthesis of 3-aminocoumarin-N-benzylpyridinium conjugates with nanomolar inhibitory activity against acetylcholinesterase
Beilstein J. Org. Chem. 2018, 14, 2545–2552, doi:10.3762/bjoc.14.231
- the amide group and dimethoxy substituents on the chromene ring to the amino acid residues Tyr72, Tyr124, Trp286, and Tyr341 in the PAS; and (5) the hydrogen atoms of phenyl ring to Glu202 and a methoxy group to Ser293. Moreover, C–H–π and π–π interactions were found for compound 9h. The C–H–π
- interactions were found between hydrogen atoms of the methoxy group to the PAS amino acid residue Tyr72 and the pyridinium ring to the CAS amino acid residue Phe338, whereas π–π interactions were found among the chromene ring, pyridinium, and phenyl ring to Trp286, Phe338, and Trp86, respectively. When the
- chromene ring moved away from Tyr72 in the PAS; thus, compounds 9e and 9i lost H-bond interactions to Tyr72. Moreover, the C–H–π interactions of compounds 9e and 9i to Tyr72 became weaker. All other H-bonds, C–H–π and π–π interactions of 9e and 9i to amino acid residues in the binding site were similar to
















































































































































































































































































































































































































































































































