Search results
Search for "amino group" in Full Text gives 354 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Pd-catalyzed asymmetric Suzuki–Miyaura coupling reactions for the synthesis of chiral biaryl compounds with a large steric substituent at the 2-position
Beilstein J. Org. Chem. 2020, 16, 966–973, doi:10.3762/bjoc.16.85
- enantioselectivity. Just a slight improvement of the ee value was found along with the introduction of bulkier substituents to the amino group (5b–d). From the reaction results, it can be seen that the Pd···O interaction [13][64] between the carbonyl group and the palladium plays an important role for the reaction
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- thermal decomposition of urea at 550 °C for 2 h afforded the CN polymer, which possesses abundant –NH2 functional groups. The heterogeneous photocatalyst carbon nitride-hemin (CNH) was prepared after an amidation reaction between a carboxyl group of Fe(III) protoporphyrin IX and an amino group of the CN
Synthesis of six-membered silacycles by borane-catalyzed double sila-Friedel–Crafts reaction
Beilstein J. Org. Chem. 2020, 16, 409–414, doi:10.3762/bjoc.16.39
- , Fukuoka 816-8580, Japan 10.3762/bjoc.16.39 Abstract We have developed a catalytic synthetic method to prepare phenoxasilins. A borane-catalyzed double sila-Friedel–Crafts reaction between amino group-containing diaryl ethers and dihydrosilanes can be used to prepare a variety of phenoxasilin derivatives
Combination of multicomponent KA2 and Pauson–Khand reactions: short synthesis of spirocyclic pyrrolocyclopentenones
Beilstein J. Org. Chem. 2020, 16, 200–211, doi:10.3762/bjoc.16.23
- ). The acylation of the amino group was found necessary to allow for the cobalt-catalyzed reaction to proceed under a CO atmosphere. This step was also carried out in one pot after the KA2 reaction by diluting with pyridine and adding the acylating reagent, to achieve the corresponding product in
- slightly lower yield. Attempts to carry out the Pauson–Khand reaction directly on the amino group before the acylation step did not work, nor using a modified approach using ammonium chloride and 1.5 equivalents of Co2(CO)8 under an inert atmosphere, as reported for similar reactions in the presence of
- components in the outcome of the multicomponent coupling reaction. Similarly, the use of piperidone as the ketone component proved to work only when the amino group was protected as Boc, whereas the N-methyl derivative did not proceed to the coupling product (Table 1, entries 10 and 9, respectively). Indeed
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
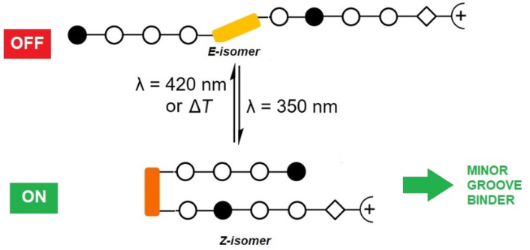
- -methylimidazole units were introduced by employing diamino derivatives 3 (Scheme 2A) [40]. Moreover, it turned out that coupling the subsequent building block to an N-terminal Im building block was problematic because the amino group of the imidazole derivative 2b is a weak nucleophile, and therefore the Fmoc–Py
Facile regiodivergent synthesis of spiro pyrrole-substituted pseudothiohydantoins and thiohydantoins via reaction of [e]-fused 1H-pyrrole-2,3-diones with thiourea
Beilstein J. Org. Chem. 2019, 15, 2864–2871, doi:10.3762/bjoc.15.280
- when the reagents were mixed in the boiling solvent (Table 2, entry 4). Probably, compound 2a was formed not only as a result of the corresponding PTR, but as a result of a concurrent attack of the amino group of thiourea on the C-3a atom of FPD 1a (Scheme 6). Using the optimization data, we obtained a
Design, synthesis and investigation of water-soluble hemi-indigo photoswitches for bioapplications
Beilstein J. Org. Chem. 2019, 15, 2822–2829, doi:10.3762/bjoc.15.275

- in the presence of the co-solvent. The comparison with the reported data on Z-1a [13] and related hemi-indigo derivatives containing a 4-amino group in the phenyl ring [13] allowed to conclude that this substitution pattern is unfavorable for photoswitching in aqueous medium. Thus, a higher content
- of organic co-solvents (20–30% of DMSO, DMF or THF) or/and the presence of triethylamine was required to stimulate the photoswitching of the reported compounds with a 4-amino group in the phenyl ring [13]. Considering the limitations imposed on the nature and content of organic co-solvents used in
Synthesis of aryl-substituted thieno[3,2-b]thiophene derivatives and their use for N,S-heterotetracene construction
Beilstein J. Org. Chem. 2019, 15, 2678–2683, doi:10.3762/bjoc.15.261
- amino group in the corresponding 3-aminothiophene-2-carboxylates with a halogen atom by the Sandmeyer reaction [20][21]. The former transformation is preferable for large-scale syntheses, but we failed to repeat the reported procedures. For example, our attempt to diazotizate methyl 3-amino-5
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein J. Org. Chem. 2019, 15, 2664–2670, doi:10.3762/bjoc.15.259
- dipole and dipolarophile (Figure 3). Nitroxide Oxidation of 1 with m-CPBA afforded the nitroxide 6 with 48% yield (Scheme 3). It is noteworthy that the oxidation of the amino group is accompanied by the stereospecific hydroxylation at position 4 of the 2,3,4,7-tetrahydroazepine ring. The structure
- distorted half-chair. Presumably, this difference is due to the formation of an intramolecular hydrogen bond O6–H…O5 (H…O 1.95(2) Å, O–H…O 154(2)°) in molecule 6. In the crystal of 1 the amino group participates in intermolecular hydrogen bonding N1–H…O3 (H…O 2.39(2) Å, N–H…O 167(1)°) forming chains of
AgNTf2-catalyzed formal [3 + 2] cycloaddition of ynamides with unprotected isoxazol-5-amines: efficient access to functionalized 5-amino-1H-pyrrole-3-carboxamide derivatives
Beilstein J. Org. Chem. 2019, 15, 2623–2630, doi:10.3762/bjoc.15.255
- can enhance the nucleophilic ability of nitrogen on the isoxazole ring. However, this change could raise at least an issue, involving the direct addition of an amino motif to the ynamide substrate [43][44]. To answer this question, two isoxazoles 7 and 8a with an amino group at the distinct position
Sugar-derived oxazolone pseudotetrapeptide as γ-turn inducer and anion-selective transporter
Beilstein J. Org. Chem. 2019, 15, 2419–2427, doi:10.3762/bjoc.15.234

- peptides [21][22][23]. The free amino group in 2a was acetylated with Ac2O/pyridine in dichloromethane to get –NHAc derivative 2b (Scheme 2). The single crystal formation of oxazolone psudopeptides 1, 2a and 2b were unsuccessful under a variety of solvent conditions. The 1H and 13C NMR spectra of 1, 2a and
- 2b. The influx of Cl‒ ion by these transporters were monitored using EYPC-LUVslucigenin. Additionally, compound 9, which has a free amino group and a free carboxylic acid group, was also subjected to the Cl‒ transport study. The Cl– sensitive dye lucigenin, was entrapped within the lipid vesicles and
- in the presence of valinomycin confirming the transport process occurring through an antiport mechanism via Cl−/NO3− exchange (Figure 8D). Such anion-selective transport can be rationalized based on the binding of anions with the terminal amino group of the transporter through hydrogen bond
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- ‐time photomodulation of sirtuins in vitro. Keywords: azo compounds; epigenetics; photoswitch; sirtuins; stilbenes; Introduction Sirtuins are protein deacylases that cleave off not only acetyl, but also other acyl groups from the ε-amino group of lysines in histones and many other substrate proteins
Synthesis of 1-azaspiro[4.4]nonan-1-oxyls via intramolecular 1,3-dipolar cycloaddition
Beilstein J. Org. Chem. 2019, 15, 2036–2042, doi:10.3762/bjoc.15.200
- in the side chain, the hydroxymethyl group in 9a–c was protected via acylation. Heating of 9a–c with an excess of acetic anhydride in chloroform quantitatively afforded the corresponding esters 10a–c. The products of acylation of the sterically hindered amino group were not detected in the reaction
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
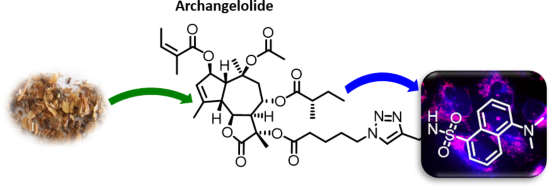
- α-amino group of Ile829 were obvious. The hydrophobic interactions were directed preferentially to Phe256, Val263, Leu260 and Ile829 residues and a hydrophobic part of Lys252. In contrast to simulation 1 with compound 1, there was no indication of compound 2 escaping from the SERCA cavity (Figure 4
Installation of -SO2F groups onto primary amides
Beilstein J. Org. Chem. 2019, 15, 1907–1912, doi:10.3762/bjoc.15.186
- carboxylic amides (1r–u) were well-tolerated and afforded the target products in 56–90% yields. In addition, alkyl carboxylic amides were also smoothly transformed into the corresponding products (2v–z). However, primary amides bearing an amino group or a phenolic hydroxy group were not successfully
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- -2-ones having a 2-chloromethyl substituent 173 was formed. Removal of the 1-phenylethyl moiety and a subsequent replacement of the chlorine atom by the amino group via azide gave imidazolin-2-one dipeptides 174 which were transformed into 12 derivatives 175–177 after hydrolysis of the corresponding
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- more. It was observed that most of the methods utilize 2-aminopyridines as one of the starting materials due to its binucleophilic nature; associated with exocyclic amino group and endocyclic pyridinium nitrogen [19]. Recently, A3 coupling of alkynes, aldehydes, and aminopyridines have been developed
Different reactivity of phosphorylallenes under the action of Brønsted or Lewis acids: a crucial role of involvement of the P=O group in intra- or intermolecular interactions at the formation of cationic intermediates
Beilstein J. Org. Chem. 2019, 15, 1491–1504, doi:10.3762/bjoc.15.151
- species E2 and E4 this carbon is connected to a protonated amino group, and in E1 it is bound to oxygen. The same range of absorbance around 100 ppm for this carbon was observed previously for other oxaphospholium ions [14][16]. Then, we carried out hydrolysis of cations A–H (Scheme 2). Results of
Synthesis of non-racemic 4-nitro-2-sulfonylbutan-1-ones via Ni(II)-catalyzed asymmetric Michael reaction of β-ketosulfones
Beilstein J. Org. Chem. 2019, 15, 1289–1297, doi:10.3762/bjoc.15.127
- coordinated to Ni in more Lewis acidic equatorial position, whereas the nitroalkene is positioned in apical by avoiding the steric repulsion of benzyl groups (TS2-I and 2-II vs TS1-I and 1-II, Scheme 2). Additional hydrogen bonding between the hydrogen atom of the amino group and the oxygen atom of the
Electrophilic oligodeoxynucleotide synthesis using dM-Dmoc for amino protection
Beilstein J. Org. Chem. 2019, 15, 1116–1128, doi:10.3762/bjoc.15.108
- 14 to give the target monomer 3a (Scheme 2). The dA phosphoramidite monomer 3b was synthesized similarly starting from 15 [48]. The amino group of 15 was carbamylated with 5 in the presence of two equivalents LDA to give 16. The silyl groups were removed, and compound 17 was tritylated to give 18
- isolated and the exo-amino group was carbamylated directly with 5 in the presence of two equivalents LDA giving 21 in 55% yield. The silyl protecting groups were removed to give 22, which was tritylated to give 23 and phosphitylated to give the target monomer 3c (Scheme 2). As will be discussed later, we
Novel (2-amino-4-arylimidazolyl)propanoic acids and pyrrolo[1,2-c]imidazoles via the domino reactions of 2-amino-4-arylimidazoles with carbonyl and methylene active compounds
Beilstein J. Org. Chem. 2019, 15, 1032–1045, doi:10.3762/bjoc.15.101
- selectively led to the corresponding Knoevenagel–Michael adducts containing a free amino group in the imidazole fragment. The adducts derived from Meldrum’s acid have been smoothly converted into 1,7-diaryl-3-amino-6,7-dihydro-5H-pyrrolo[1,2-c]imidazol-5-ones and 3-(2-amino-4-aryl-1H-imidazol-5-yl)-3
- intensity of the C5–NH2 group of the imidazo[1,2-c]pyrrole cycle at 7.74–7.84 ppm. A characteristic feature is the appearance of another broad singlet at the 6.37–6.46 ppm, inherent to the amino group of the C2 atom of the imidazole ring, whose chemical shift is affected by the character of the substituents
- the formation of both unexplored heterocyclic systems containing a free amino group open for chemical modifications and the corresponding hetarylpropanoic acids providing useful templates for the synthesis of some marine alkaloids or their analogues. In domino reactions of the 2-amino-4-arylimidazoles
Hoveyda–Grubbs catalysts with an N→Ru coordinate bond in a six-membered ring. Synthesis of stable, industrially scalable, highly efficient ruthenium metathesis catalysts and 2-vinylbenzylamine ligands as their precursors
Beilstein J. Org. Chem. 2019, 15, 769–779, doi:10.3762/bjoc.15.73
- styrenes 5 with a cyclic tertiary amino group from 1,2,3,4-tetrahydroisoquinoline (Scheme 2). In this case, the initial isoquinoline was reduced in the presence of formic acid and then converted into the desired products 5 by a one-pot solvent-free reaction under the action of the corresponding dihalide in
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- group removal followed by methyl ester hydrolysis and Fmoc protection of the Nα-amino group. Fmoc-Tyr(3-I,Me)-OH was coupled using COMU, Oxyma and DIPEA in DMF overnight. An aliquot of resin 10 was cleaved providing the expected linear peptide in 98% purity. Microwave-assisted intramolecular Suzuki
The LANCA three-component reaction to highly substituted β-ketoenamides – versatile intermediates for the synthesis of functionalized pyridine, pyrimidine, oxazole and quinoxaline derivatives
Beilstein J. Org. Chem. 2019, 15, 655–678, doi:10.3762/bjoc.15.61
- added carboxylic acid at the nitrogen gives an allenyl iminium/aminobutadienyl cation intermediate B that accepts the present carboxylate at the electrophilic carbon to provide an acyloxy-substituted aminobutadiene derivative C. The acyl group is subsequently transferred to the close amino group giving
Selectivity in multiple multicomponent reactions: types and synthetic applications
Beilstein J. Org. Chem. 2019, 15, 521–534, doi:10.3762/bjoc.15.46
- of aminomethyltetrazoles arising from two consecutive isocyanide-MCRs shows excellent selectivity and broad scope, and although it combines two different transformations, the amine component (ammonia in the starting mixture and a primary amino group in the first adduct) reacts at different rates in
































































































































































































































































































































































































































































































































































































