Search results
Search for "amino acid" in Full Text gives 564 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Light-controllable dithienylethene-modified cyclic peptides: photoswitching the in vivo toxicity in zebrafish embryos
Beilstein J. Org. Chem. 2020, 16, 39–49, doi:10.3762/bjoc.16.6

- derivatives of 2 [30]. The library contained 29 compounds grouped into several series: with natural amino acid mutations affecting the amphipathicity (series i), with point mutations that modulate polarity and conformational stability of the β-structural elements (series ii), with backbone N-alkylation and
- (peptides 5, 15–17) was seen to decrease the photoactivation efficiency in vivo. The corresponding LD50 values indicate that an uncharged polar amino acid residue next to the DAE (compounds 6, 7, 8) and the presence of hydroxyleucine residues (12, 13, 14) improve the photoactivation efficiency compared to
- peptides are more toxic than the inactivated ring-closed isomers, with up to two orders of magnitude difference. The most promising modifications of GS appear to be those where a single uncharged polar amino acid has been introduced on the hydrophilic face of the peptide. DAE photoswitch and
Chemical synthesis of tripeptide thioesters for the biotechnological incorporation into the myxobacterial secondary metabolite argyrin via mutasynthesis
Beilstein J. Org. Chem. 2019, 15, 2922–2929, doi:10.3762/bjoc.15.286
- desired thioester probably due to the higher reactivity of the Michael acceptor system. Revision of the synthetic approach into a convergent synthesis with initial formation of the thioester at the amino acid level and subsequent coupling to a dipeptide finally yielded the desired mutasynthon carrying the
Bacterial terpene biosynthesis: challenges and opportunities for pathway engineering
Beilstein J. Org. Chem. 2019, 15, 2889–2906, doi:10.3762/bjoc.15.283

- thiotemplated assembly lines, such as type I polyketide synthases (PKS) and nonribosomal peptide synthetases (NRPS), are modular, with each module contributing a distinct fragment to the final product’s core structure – a short-chain carboxylic acid (PKS) or an amino acid (NRPS). The modularly defined template
Palladium-catalyzed synthesis and nucleotide pyrophosphatase inhibition of benzo[4,5]furo[3,2-b]indoles
Beilstein J. Org. Chem. 2019, 15, 2830–2839, doi:10.3762/bjoc.15.276
- performed to identify binding interactions, as illustrated in Figure 4. The 3D binding interaction study of suramin revealed a number of binding interactions with amino acid residues. Due to its bulky structure, suramin showed linkage inside and over the surface of the enzyme pocket. The important residues
- exhibited π–π T-shaped interactions connecting the indole and furan rings with Tyr340. However, π–alkyl linkage was observed between the benzofuran moiety of compound 5c and amino acid Lys295. The fluorine atom of the 4-fluorophenyl group was involved in the binding with the zinc ion and Ser377. Hydrogen
- , Asn245, Glu275, Tyr289, Leu239, Ser326, Thr205, His329 and Glu322 were involved in the hydrogen binding with suramin. One π–alkyl interaction was found with Pro288 and one metal interaction with zinc. Amino acid Tyr289 was connected with the sulfur atom as shown in Figure 5a. The hydrogen atoms of the
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- 2000, reported for the first time, a crucial modification of a glassy carbon electrode via chemical immobilization of ᴅ-amino acid oxidase (D-AOx) as enzyme and 1-aminopropyl-1'-methyl-4,4'-dipyridinium iodide (ADPy) as electron mediator and thus prepared electrode D-AOx/ADPy/GC. Electrochemical
- to the corresponding amino acid via electrochemical reduction of FAD in AOx by electron mediator ADPy. It should be noted that the authors also reported the limitation of this electrochemical method in terms of substrate selectivity of the enzymes used [44]. Electrochemical asymmetric oxidation using
- aryl sulfides 40 to their corresponding sulfoxides 41 using poly(amino acid)-coated platinum electrodes with moderate yield and good to excellent enantiomeric excess (Scheme 17) [47][48]. Osa and co-workers, in 1994, made an important contribution in the area by developing an enantioselective method
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein J. Org. Chem. 2019, 15, 2664–2670, doi:10.3762/bjoc.15.259
- carbonyl compounds for the synthesis of heterocyclic compounds has been repeatedly demonstrated [2][3]. We recently used a domino reaction of amino acid, ketone and dimethyl fumarate for the one-pot synthesis of a substituted pyrrolidine, which then was converted into a reduction-resistant pyrrolidine
Nanangenines: drimane sesquiterpenoids as the dominant metabolite cohort of a novel Australian fungus, Aspergillus nanangensis
Beilstein J. Org. Chem. 2019, 15, 2631–2643, doi:10.3762/bjoc.15.256

- terpene synthase family, showing similarity to haloacid dehalogenase (HAD)-like hydrolases [21]. Thus, we suspected that a related enzyme may be involved in the biosynthesis of the nanangenines, and used the amino acid sequence of AstC to probe the A. nanangensis genome. We also hypothesised that the acyl
- ionisation-activated cyclisation activity. Two other HAD-like hydrolases, AstI and AstK, with the class I (DDxxD/E) motif, are therefore required to catalyse other dephosphorylation steps. Despite overall low sequence homology, alignment with the amino acid sequences of AstC, AstI, AstK and related homologs
- the AAFTF pipeline (https://github.com/stajichlab/AAFTF). To facilitate the identification of a putative biosynthetic gene cluster, a local BLAST database was created from the resulting assembly [44]. Though Shinohara et al. list the revised annotation of AstC as AORIB40_05408, its amino acid sequence
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- cysteine sulfhydryl side chain to electrophilic Cβ of an O-acetylated Morita–Baylis–Hillman (MBH) adduct residue (Scheme 1). Despite reports describing the use of amino acid residues with nucleophilic side chains to prompt the macrocyclization of peptides and their mimetics, to the best of our knowledge
- repetitive Fmoc-amino acid couplings yielding the linear resin-bound tetrapeptides 5 (Scheme 3) [37][38]. The MBH acids 3 were coupled to the free amine at the N-terminal of 5 to afford the resin-bound linear peptidomimetics 6, which subsequently had the hydroxy group of the MBH residue acylated with acetic
- after preparative HPLC purification and lyophilization. The overall yield for this 11-step synthesis ranged from 7% to 15% for almost all solonamide analogues 9, based on the initial resin’s molarity. The exception relays on those containing ᴅ-Ala and ʟ-Leu amino acid residues sequentially attached to
Sugar-derived oxazolone pseudotetrapeptide as γ-turn inducer and anion-selective transporter
Beilstein J. Org. Chem. 2019, 15, 2419–2427, doi:10.3762/bjoc.15.234

- and Research, Pune, Pune 411008, India 10.3762/bjoc.15.234 Abstract The intramolecular cyclization of a C-3-tetrasubstituted furanoid sugar amino acid-derived linear tetrapeptide afforded an oxazolone pseudo-peptide with the formation of an oxazole ring at the C-terminus. A conformational study of
- transporter for which an anion–anion antiport mechanism was established. Keywords: ion transport; oxazolone; peptidomimetics; pseudo-peptides; sugar amino acid; Introduction Tetrasubstituted α-amino acid (TAA)-derived peptidomimetics offer well-defined turn structures due to the presence of a
- conformation [5]. Amongst these, the use of sugar-derived TAAs in peptidomimetics is less explored. The linear tri-/tetrapeptides and spiro-peptides at the anomeric position of mannofructose are known [6][7][8]. Stick and co-workers have reported the synthesis of tetrasubstituted sugar furanoid amino acid
Current understanding and biotechnological application of the bacterial diterpene synthase CotB2
Beilstein J. Org. Chem. 2019, 15, 2355–2368, doi:10.3762/bjoc.15.228

- involved in coordination of water molecules in the water network around the catalytic Mg2+ ions. The pyrophosphate sensor motif In a recent review, the presence of a “pyrophosphate sensor”, an arginine 46 amino acid residues upstream of the NSE motif, was discussed as a universal feature of bacterial TPSs
- carbon skeletons of other bioactive natural product classes. In light of this fact, the exchange of only one amino acid changes the product portfolio from cyclooctatin (5) belonging to the fusicoccane diterpene family to compounds of the dolabellane [67] or the cembranoid class [68]. The dolabellane
- performed in the gas phase [92][93]. The latter approach per se disregards the role of the protein scaffold during catalysis. However, there are many examples of mono-, sesqui-, and diterpene synthases, where the contribution of the diphosphate and selected amino acid residues, which decorate the active
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups
Beilstein J. Org. Chem. 2019, 15, 2213–2270, doi:10.3762/bjoc.15.218
- (Scheme 10). Notably, this reaction used an air and moisture-stable chiral palladium complex as the catalyst, which worked well at low catalyst loading (as low as 0.5 mol %). In 2015, Shi et al. [47] introduced a Pd(II)/Pd(IV)-catalyzed fluorination of β-methylene C(sp3)–H bonds of α-amino acid
Azologization and repurposing of a hetero-stilbene-based kinase inhibitor: towards the design of photoswitchable sirtuin inhibitors
Beilstein J. Org. Chem. 2019, 15, 2170–2183, doi:10.3762/bjoc.15.214

- studies proposed a binding mode in which 2a mimics the nicotinamide residue of NAD+, whereas aromatic amino acid residues of the selectivity pocket stabilize the dimethylphenol ring [35]. As photoisomerization in stilbenes and azo dyes is accompanied by a perpendicular twist of the phenyl ring towards the
Genome mining in Trichoderma viride J1-030: discovery and identification of novel sesquiterpene synthase and its products
Beilstein J. Org. Chem. 2019, 15, 2052–2058, doi:10.3762/bjoc.15.202

- three types based on their amino acid sequence. Type I TPSs are metal-dependent enzymes that initiate cyclisation by the elimination of diphosphate groups from precursors and carbocation formation, and type II TPSs initiate the catalytic process by the protonation of an olefinic double bond [7]. The
- of 89.66% and 85.23% with the enzymes from the strain T. virens Gv29-8 [27] and T. reesei QM6a [28], respectively, with only predicted functions. Thereafter, an amino acid sequence alignment with several known terpene synthases showed that Tvi09626 had the typical highly conserved 128DDxxD/E
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197
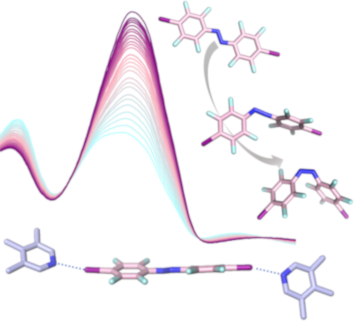
- reaction and as organocatalysts in Diels–Alder reactions [27]. The group of Metrangolo also reported halogen bonding-promoted catalysis in water by exploiting a halogen bonding amino acid, which combines excellent donor properties with good water solubility [28], in addition to their seminal contributions
Isolation and characterisation of irinans, androstane-type withanolides from Physalis peruviana L.
Beilstein J. Org. Chem. 2019, 15, 2003–2012, doi:10.3762/bjoc.15.196

- [27]. Then, the bifunctional P450c17 acts as a 17α-hydroxylase and 17,20-lyase to give rise to androstanes [27]. Related enzymes have not been reported from plants. We searched transcriptome data of P. peruviana for putative homologues of these enzymes [28]. The best hits only had amino acid sequence
- algorithm. Both enzymes yielded several full-length hits with amino acid sequence identities of 22–28%. Oxidative cleavage of 4β-hydroxywithanolide E (1) to irinan A (2) by NaIO4 57.8 mg of NaIO4 (270.2 µmol, 7.0 equiv) in 400 µL hot H2O was added to 19.4 mg 4ß-hydroxywithanolide E (1, 38.6 µmol, 1.0 equiv
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
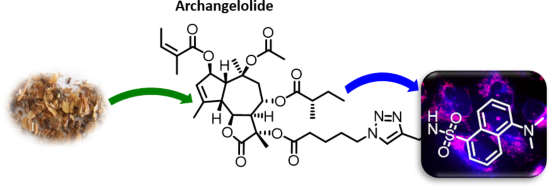
- ). The first round of simulations (1–10 ns) of compound 1 docking into SERCA cavity with compound 1 constrained as described (simulation 1) showed the presence of hydrophobic interactions of ligand side chains with Phe256, Val263, Ile829 and the hydrophobic part of Gln259 amino acid residues. Gln259 was
- -specific dye from [20] (10 min; image C) or pDNA coding mCherry-ER (image G). D, H) merged images. Cartoon representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A, C) archangelolide (1) or B, D) trilobolide (2) after molecular dynamic simulations. Depicted are also amino acid
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- chemistry. In the present study we demonstrate that resorcin[4]arene sulfonic acid (RSA) interacts with chiral amines (amino acid derivatives and aminocavitands) to form inclusion complexes and capsules based on electrostatic interactions. The complexes were characterized by circular dichroism and DOSY NMR
- procedure described in the literature [16]. Complexes of 1 with various amino acid methyl esters (LysOMe·2HCl, ArgOMe·2HCl, HisOMe·2HCl) and with (R or S)-2·4HCl were obtained by dissolution of the components in water (or in water with sodium acetate buffer, pH 7.0) and mixing the solutions in 1:1 (for
- informative and reflect the thermodynamic stability of complexes. They also suggest that this technique may be suitable for estimation of the order of thermodynamic stability of the complexes using only a single-spectrum measurement. Assuming that the size of amino acid derivatives is roughly similar and
Installation of -SO2F groups onto primary amides
Beilstein J. Org. Chem. 2019, 15, 1907–1912, doi:10.3762/bjoc.15.186
- ][15][16][17][18][19]. Among all the developed S(VI)–F species, sulfonyl fluoride (RSO2F) was specifically recognized as unique scaffold for covalent protein inhibitors and biological probes with the affinity-driven activation for forming covalent linkages with the amino acid residues of protein
Design, synthesis and biological evaluation of immunostimulating mannosylated desmuramyl peptides
Beilstein J. Org. Chem. 2019, 15, 1805–1814, doi:10.3762/bjoc.15.174
- -isoGln pharmacophore is essential for the immunostimulatory properties but the introduction of lipophilic substituent into MDP analogues can increase its adjuvant activity [12][18]. Up to now, our research was directed towards isomeric desmuramyl peptides containing lipophilic unnatural amino acid
- racemic Boc-protected (adamant-1-yl)glycine [32] 2 using the carbodiimide EDC/HOBt method [21]. The obtained mixture of BocAdGly-ʟ-Ala-ᴅ-isoGlnOBn diastereoisomers was treated with trifluoroacetic acid in order to remove the Boc protecting group while the diastereoisomer 4 with ᴅ-ʟ-ᴅ amino acid sequence
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- can serve as the starting material to a variety of orthogonally protected derivatives of 2,3-diaminopropanoic acid. The bicyclic amino acid (S)-127 is a major metabolite of isazofos in corn grain and for toxicological studies both enantiomers were required [17]. To this end the aziridine ester (2S,1′R
- presence of acetic anhydride produced a crude acetamido ester (R)-132 which was transformed into the final N-benzyl amide (R)-130 with of 99.9% ee after crystallization. ʟ-(+)-Furanomycin (Scheme 35) is a natural nonproteinogenic amino acid which showed pronounced antibacterial activity. From several
- allyl ether (2R,1'R,1''R)-135 which in the presence of Grubbs 1st generation catalyst produced (2R,1'R,1''R)-136. Transformation of the aziridine portion of 136 into an amino acid fragment of norfuranomycin (2S,2'R)-133 was accomplished starting from the hydrolytic opening of the aziridine ring and was
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

- , perhaps because the α,β-unsaturated double bond affected the iodination. Mohan et al. successfully developed an efficient copper-catalyzed aerobic oxidative amination of C(sp3)–H bonds to synthesize imidazo[1,5-a]pyridine derivatives [122]. The reaction was also applicable to amino acid derivatives, as
Synthesis and conformational preferences of short analogues of antifreeze glycopeptides (AFGP)
Beilstein J. Org. Chem. 2019, 15, 1581–1591, doi:10.3762/bjoc.15.162

- conformation of the molecule as well an on the stereochemistry of amino acid residues. The desired monoglycosylated analogues with acetylated amino termini and the carboxy termini in form of N-methylamide have been synthesized. Conformational nuclear magnetic resonance (NMR) studies of the designed analogues
- have shown a strong influence of the stereochemistry of amino acid residues on the peptide chain stability, which could be connected to the antifreeze activity of these compounds. A better understanding of the mechanism of action of antifreeze glycopeptides would allow applying these materials, e.g
- the saccharide moiety is conformationally well-defined by the peptide backbone and the whole structure adopts a polyproline II (PP II) helix [17]. Antifreeze activity strongly relies on the stereochemistry of the amino acid residues. Earlier studies have shown that AFGP analogues containing either an
Molecular basis for the plasticity of aromatic prenyltransferases in hapalindole biosynthesis
Beilstein J. Org. Chem. 2019, 15, 1545–1551, doi:10.3762/bjoc.15.157
- prenyl donor [12]. This plasticity is due to the hydrophobic nature of the prenyl acceptor binding site and the fluctuations of the amino acids forming the cation shield, similarly to AmbP3. Comparison of the AmbP1 and AmbP3 amino acid sequences with other ABBA PTases The AmbP1 and AmbP3 amino acid
- amino acid sequence alignment of AmbP1, AmbP3, and other ABBA PTases, visualized by ESPript3 [34]. Secondary structure elements: α, α-helices; β, β-strands; η, 310-helices; TT, strict β-turns. The red frames indicate amino acids that anchor pyrophosphate, the blue frames indicate the residues that
An azobenzene container showing a definite folding – synthesis and structural investigation
Beilstein J. Org. Chem. 2019, 15, 1534–1544, doi:10.3762/bjoc.15.156
- for such rigid systems are macrocycles which stem from Lissoclinum cyclopeptide alkaloids (Figure 1) [2][3]. Here, the required recognition units can be introduced via the amino acid side chains or via the side chains of the azole rings. The orientation and the distance between the recognition units
- are determined by the type and size of the macrocyclic platform, e.g., if all of the amino acid side chains are of the same configuration, they are presented on one face of the macrocycle in a convergent manner. The artificial Lissoclinum cyclopeptide platforms feature C2, C3 and C4 symmetry [3]. So
- the amino acid side chains, are described in the literature [49][50]. One example is the platform 4, which consists of two imidazole building blocks connected by two azobenzene units (Figure 2) [50]. Irradiation of the platform 4 with UV light results in a trans→cis isomerization accompanied by a
Anomeric sugar boronic acid analogues as potential agents for boron neutron capture therapy
Beilstein J. Org. Chem. 2019, 15, 1355–1359, doi:10.3762/bjoc.15.135
- . Galactose and fructose also allow tumor growth in the absence of glucose. Boronic acid derivatives have gained interest in the last years in different fields such as the development of enzyme inhibitors, drug delivery polymers, saccharide sensors and as boron carriers for BNCT, e.g., amino acid derivatives



























































































































































































































































































































































































































































































































































































































































