Search results
Search for "diastereomer" in Full Text gives 273 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Au(III) complexes with tetradentate-cyclam-based ligands
Beilstein J. Org. Chem. 2021, 17, 186–192, doi:10.3762/bjoc.17.18
- [51], which showed that JohnPhos-Au(I) and pyr-menthol-Au(III) complexes provided high amounts of the initially formed cis diastereomer in this model reaction. In contrast, some BOX-Au(III) complexes have the additional ability to rapidly transform the initially formed cis product into the isomerized
Direct synthesis of anomeric tetrazolyl iminosugars from sugar-derived lactams
Beilstein J. Org. Chem. 2021, 17, 115–123, doi:10.3762/bjoc.17.12
- of products As presented in Table 2, only one diastereomer of the desired iminosugar is obtained in almost all cases. This outstanding selectivity has been observed before and is described in our previous works devoted to the functionalization of sugar-derived lactams [23][24][25]. We explain it in
- between protons H2 and H7, suggest that it may be the diastereomer 2-(R), as shown in Figure 3. This result would be in accordance with the previously mentioned Woerpel’s model. The structure of compound 5b was assigned per analogiam, as diagnostic signals in 1H NMR spectrum were also overlapped. We made
All-carbon [3 + 2] cycloaddition in natural product synthesis
Beilstein J. Org. Chem. 2020, 16, 3015–3031, doi:10.3762/bjoc.16.251

- Lee [24] independently in 2003. (Scheme 1C and Scheme 1D) In Krische’s synthesis, a stereospecific intramolecular phosphine-catalyzed [3 + 2] cycloaddition of 2-butynoate with electron-deficient alkene 29 afforded cycloadduct 31 in 88% yield as a single diastereomer [25] (Scheme 1C). Later, Lee`s
- and co-workers disclosed the palladium-catalyzed intermolecular carboxylative TMM [3 + 2] cycloaddition [36] (Scheme 3). Exposure of coumarin 61 to the silyl-substituted TMM precursor 62 in the presence of a catalytic amount of Pd(PPh3)4 afforded adduct 63 in 81% yield as a single diastereomer (Scheme
- the desired alcohol 133 in 75% yield as a single diastereomer. Allylic oxidation of freshly prepared 133 with SeO2 followed by silylation with TBSOTf/Et3N afforded enyne 134. Enyne 134 was subjected to rhodium-catalyzed intramolecular [3 + 2] cycloaddition with a catalytic amount of [Rh(cod)OH]2 to
Ring-closing metathesis of prochiral oxaenediynes to racemic 4-alkenyl-2-alkynyl-3,6-dihydro-2H-pyrans
Beilstein J. Org. Chem. 2020, 16, 2757–2768, doi:10.3762/bjoc.16.226
- (13, 108 mg, 0.624 mmol) were dissolved in CH2Cl2 (4 mL) and the mixture was stirred for 130 h at rt. After evaporation, the mixture was purified by gradient column chromatography (eluent hexane/EtOAc 2.5:1 → 2.4:1) to give the major diastereomer (probably endo-trans-14B, 50 mg, 25%, white solid, Rf
- = 0.17 for hexane/EtOAc 2.5:1) and the minor diastereomer (probably exo-cis-14C, 28 mg, 14%, clear solid, Rf = 0.24 for hexane/EtOAc 2.5:1). A mixture of the two diastereomers (14 mg, 7%, 14B/14C 2:1) and starting N-phenylmaleimide (58 mg, 54%) were also recovered. Major diastereoisomer (probably endo
Recent developments in enantioselective photocatalysis
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197

Lipophilicity trends upon fluorination of isopropyl, cyclopropyl and 3-oxetanyl groups
Beilstein J. Org. Chem. 2020, 16, 2141–2150, doi:10.3762/bjoc.16.182
- , which was explained by the facially polar motif caused by the C–F dipoles present on the same side of the ring. Consequently, the diastereomer B4 possessed a higher lipophilicity, although the lipophilicity of the vicinally difluorinated diastereomers B3 and B5 was identical, and also the same as the
Convenient access to pyrrolidin-3-ylphosphonic acids and tetrahydro-2H-pyran-3-ylphosphonates with multiple contiguous stereocenters from nonracemic adducts of a Ni(II)-catalyzed Michael reaction
Beilstein J. Org. Chem. 2020, 16, 2073–2079, doi:10.3762/bjoc.16.174
- formed (Table 1, entries 6 and 7). After this, the reaction was carried out in an aqueous–organic medium. Fortunately, an individual diastereomer of Henry/acetalyzation product 13a was obtained when K2CO3 in combination with TEBAC was used as a catalyst. A THF/water ratio of 7.5:1 is important in order
Syntheses of spliceostatins and thailanstatins: a review
Beilstein J. Org. Chem. 2020, 16, 1991–2006, doi:10.3762/bjoc.16.166
- fashion similar to that of the dihydropyranone 58 [16], the 1,4-addition of dimethyl copper lithium to 65 gave the desired cis-50 as a single diastereomer [31], allowing for a convergence with the route in Scheme 7. Alternatively, the Wittig methenylation of 63, followed by a silyl ether cleavage gave 66
Polarity effects in 4-fluoro- and 4-(trifluoromethyl)prolines
Beilstein J. Org. Chem. 2020, 16, 1837–1852, doi:10.3762/bjoc.16.151
- diastereomers exhibited some differences. The cis-diastereomer 1 with the C–F bond pointing in the same direction as the carboxymethyl group, appeared more polar compared to the trans-isomer 2, where the direction was opposite (ΔlogPcis/trans ≈ −0.18). The same effect was observed in 4-hydroxyprolines as well
- one considers that the transition state proceeds through the C4-exo conformation, as shown in Figure 9A. In this scenario, the cis-diastereomer 3 1) readily favors the needed conformational state (vide supra), at the same time, 2) the CF3-group dipole is oriented perpendicular to the carbonyl-group
- dipole, and creates no repulsion. As the result, the transition state is energetically favored, and the rotation becomes faster. Conversely, in the other diastereomer 4, both factors disfavor the transition state: 1) the ring should adopt the disfavored C4-exo conformation with an axial CF3 group, and 2
Rearrangement of o-(pivaloylaminomethyl)benzaldehydes: an experimental and computational study
Beilstein J. Org. Chem. 2020, 16, 1636–1648, doi:10.3762/bjoc.16.136
- experiments, and single-crystal X-ray measurement (Figure 3) as diastereomer racemates. When the reaction of 1e was carried out under the same conditions in THF instead of DCM, HPLC–MS analysis of the crude product mixture showed the presence of dimer-like product 23a (25%), the recovered starting material 1e
- possible in case of a RR–SS diastereomer (23b), with only one of the aromatic hydrogen atoms of the isoindoline moiety [H(7)] located in close proximity above the middle of the other aromatic ring. It can be concluded after all that the NMR data also made it possible to assign the RS–SR diastereomeric
Distinctive reactivity of N-benzylidene-[1,1'-biphenyl]-2-amines under photoredox conditions
Beilstein J. Org. Chem. 2020, 16, 1335–1342, doi:10.3762/bjoc.16.114
- electron density or position of the substituent on the benzylidene moiety. Interestingly, the homocoupling process was highly stereoselective, resulting in the formation of only one diastereomer, probably due to the bulky substitution pattern of the substrates. The structure of the homocoupled 1,2-diamine
Preparation of 2-phospholene oxides by the isomerization of 3-phospholene oxides
Beilstein J. Org. Chem. 2020, 16, 818–832, doi:10.3762/bjoc.16.75
- -diastereomers (cis- and trans-10) were separated by column chromatography, and they were identified by nuclear Overhauser effect (NOE) NMR spectroscopy. In the ROESY spectrum of the cis-diastereomer (cis-10), the phenyl 1H resonances give NOE/ROE crosspeaks with the C(4)H and one of the C(5)H2 1H resonances. By
- contrast, in the spectrum of trans-diastereomer (trans-10), phenyl 1H resonances give NOE/ROE crosspeaks with C(4)-methyl and the other one of the C(5)H2 1H signals (see Supporting Information File 1 for details). Thus, based on the distinctive NOE patterns of phenyl resonances, the two diastereomers (cis
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- addition of benzylic–Cu intermediates to imines proceeds through a flexible linear transition state which is sensitive to the steric environment surrounding the copper catalyst. This observation provided the opportunity to achieve either diastereomer depending on the choice of the ligand L45 or L46. Based
Ultrasonic-assisted unusual four-component synthesis of 7-azolylamino-4,5,6,7-tetrahydroazolo[1,5-a]pyrimidines
Beilstein J. Org. Chem. 2020, 16, 281–289, doi:10.3762/bjoc.16.27
- 2D NMR experiments), and X-ray diffraction analysis. The signals in the 1H and 13C NMR spectra of some of the compounds 4 were duplicated. This could have been caused by two reasons, namely by free rotation of the azolyl fragment in the position 7 or by the presence of a second diastereomer. To
- pyrimidine NH group and cross-peaks of the NH group with protons of the aromatic system, thus excluding structure B (Figure 1). Eventually, the structure of tetrahydropyrimidines 4 was proven by X-ray analysis carried out for a single crystal of one diastereomer of compound 4g, which allowed assignment of
Unexpected one-pot formation of the 1H-6a,8a-epiminotricyclopenta[a,c,e][8]annulene system from cyclopentanone, ammonia and dimethyl fumarate. Synthesis of highly strained polycyclic nitroxide and EPR study
Beilstein J. Org. Chem. 2019, 15, 2664–2670, doi:10.3762/bjoc.15.259
- hydrogens in the azepine ring: electrocyclization proceeds via a conrotatory mechanism due to the antisymmetry of the HOMO (Figure 2). The selective formation of a single diastereomer in the 1,3-dipolar cycloaddition reaction is likely a result from secondary interactions of orbitals of the π-systems of the
- : the electrocyclization proceeds via a conrotatory mechanism due to the antisymmetry of the HOMO. Selective formation of a single diastereomer in the 1,3-dipolar cycloaddition reaction. X-ray structure of compound 6 (one of the two enantiomers present in the crystal). A, B) Temperature dependence of
Indium-mediated C-allylation of melibiose
Beilstein J. Org. Chem. 2019, 15, 2458–2464, doi:10.3762/bjoc.15.238
- optimization procedures (Scheme 2). The overall yield of the isolated epimers was 92%. According to Binder et al. [17] and our own experience [18], we expected the syn-product (2-syn) to be the main diastereomer. However, the determination of the configuration is not possible by NMR analysis. Consequently, the
A review of the total syntheses of triptolide
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- 88. Ester exchange of 88 with (+)-8-phenylmenthol gave the key cyclization precursor 16. Oxidative radical cyclization of 16 in the presence of Mn(OAc)3 and Yb(OTf)3·H2O afforded the major tricyclic diastereomer 89 (dr = 38:1). Then the construction of the unsaturated lactone was performed by
- epoxide as a single diastereomer via in situ-generated methyl(trifluoromethyl)dioxirane and the reduction of the C-14 ketone in the presence of Eu(fod)3 to give triptolide (47%) together with its C-14 α-hydroxy epimer epi-triptolide (47%). In 2014, Li’s group reported a divergent synthesis for triptolide
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
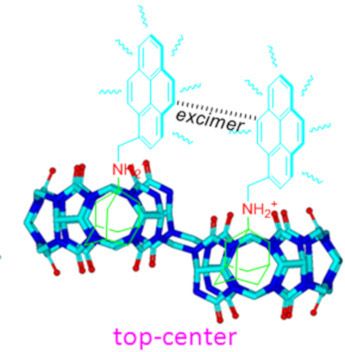
- and Discussion We took advantage of the two novel identical cavities and the simple formation of ternary diastereomer complexes with NS-CB[10]-based host-1 and host-2. The size of each cavity of host-1 is similar in size to Q[7], and the cavity size of host-2 is close to that of Q[6]. We first
Genomics-inspired discovery of massiliachelin, an agrochelin epimer from Massilia sp. NR 4-1
Beilstein J. Org. Chem. 2019, 15, 1298–1303, doi:10.3762/bjoc.15.128
- . A compound which was found to be predominantly produced under iron deficiency was subsequently isolated. Its structural characterization by spectroscopic and bioinformatic analyses revealed a previously not known diastereomer of the cytotoxic alkaloid agrochelin. The structure of this natural
Synthesis of non-racemic 4-nitro-2-sulfonylbutan-1-ones via Ni(II)-catalyzed asymmetric Michael reaction of β-ketosulfones
Beilstein J. Org. Chem. 2019, 15, 1289–1297, doi:10.3762/bjoc.15.127
- . The study of the solvent effect on the reaction are summarized in Table 2. As can be seen from Table 2, the dr value decreases when using more polar solvents. An individual diastereomer 8a is formed when toluene is used as a solvent (Table 2, entry 1). The highest reaction rate and enantioselectivity
- are also achieved in toluene. Considering these factors, toluene was chosen as the best solvent for this reaction. The obtained experimental data show that in some cases the formation of diastereomer (2R,3S)-8a or diastereomer (2S,3S)-9a as the major one is observed depending on the type of catalyst
- have shown, during the first 12 hours, one diastereomer (2R,3S)-8a was formed predominantly (dr 8a:9a of more than 14:1). During this time, the conversion reached 71%. Only after this period, the appearance of the significant amount of second diastereomer (2S,3S)-9a was recorded. After 24 hours, the dr
Bambusuril analogs based on alternating glycoluril and xylylene units
Beilstein J. Org. Chem. 2019, 15, 1268–1274, doi:10.3762/bjoc.15.124

- in favor of 1a-1 which remained constant at even lower temperature. By application of the Boltzmann distribution to the conformer population, we have calculated that 1a-1 is more stable by 0.13 kcal mol−1 than 1a-2. Conformer 1a-1 is preferred probably due to its higher symmetry. Diastereomer 1b was
- the orientation of one glycoluril unit were obtained. The building blocks within the macrocycles connected via methylene bridges are flexible giving rise to two preferred conformers for each diastereomer. These conformers were identified by molecular modelling and also by low-temperature NMR
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- protocol provided a small library of steroidal 2,5-diketopiperazines 6 obtained as a single diastereomer, thus proving the high diastereoselection of the initial Ugi-4CR with the steroidal carbonyl substrate. In the 2,5-diketopiperazine ring, the configuration of the new asymmetric center C-3’ was S, a
- (3CR) developed by Barluenga et al. [35] to steroids. The original Barluenga’s 3CR comprises the reaction of salicylaldehyde with an alkyl orthoformate and 4-pentyn-1-ol to obtain a 4-alkylchroman spiroketal as a single diastereomer. However, as shown in Scheme 10, the employment of alkynyl-4,5
Alkylation of lithiated dimethyl tartrate acetonide with unactivated alkyl halides and application to an asymmetric synthesis of the 2,8-dioxabicyclo[3.2.1]octane core of squalestatins/zaragozic acids
Beilstein J. Org. Chem. 2019, 15, 1194–1202, doi:10.3762/bjoc.15.116
- (≈80:20 dr) with reactive organohalides (Scheme 4) [17]. The process was valuable, because it allowed direct elaboration of a chiral pool building block that was readily available as either antipode [22][23], with the major alkylated diastereomer 15 being generated in 97:3 er [18]. The study was also
- separable 76:24 mixture of alkylated tartrates 17 and 18, respectively. The relative stereochemistry was assigned by analogy with Seebach’s findings for substituted tartrates: that the diastereomer with the ring methine cis to the ester group (i.e., 17) always displays the higher chemical shift (≈5 ppm vs
- ≈4.5 ppm in CDCl3) [18], and was further supported by 1D NOESY experiments on both alkylated tartrates 17 and 18. The use of DMPU as co-solvent [18][25] reversed the ratio, with the currently undesired diastereomer 18 becoming favoured (37:63, 17:18). However, with HMPA the proportion of 17 improved
Stereo- and regioselective hydroboration of 1-exo-methylene pyranoses: discovery of aryltriazolylmethyl C-galactopyranosides as selective galectin-1 inhibitors
Beilstein J. Org. Chem. 2019, 15, 1046–1060, doi:10.3762/bjoc.15.102
- :19 by HPLC, using the same protocol as is used for purity determinations. The stereochemistry of the β diastereomer, the only one isolated, was assigned using NOESY. [α]D20 31 (c 1, CH2Cl2); 1H NMR (400 MHz, CDCl3) δ 7.41–7.24 (m, 20H), 4.97 (d, J = 9.2 Hz, 1H), 4.94 (d, J = 8.4 Hz, 1H), 4.78 (d, J
- , 78%) as a clear, viscous oil. The diastereoselectivity was determined to be α:β 1:99 by HPLC, using the same protocol as is used for purity determinations. The stereochemistry of the β diastereomer, the only one isolated, was assigned using NOESY. [α]D20 −37 (c 0.1, CH3CN); 1H NMR (400 MHz, CDCl3) δ
Catalytic asymmetric oxo-Diels–Alder reactions with chiral atropisomeric biphenyl diols
Beilstein J. Org. Chem. 2019, 15, 955–962, doi:10.3762/bjoc.15.92
- can be partly separated. Their diastereoselectivities can be further enhanced by slow evaporation of the corresponding diastereomers from acetonitrile and the absolute configuration of one diastereomer was confirmed with X-ray crystal structure (Supporting Information File 1, Figure S2). After





































































































































































































































































































































































































































