Search results
Search for "thiazole" in Full Text gives 103 result(s) in Beilstein Journal of Organic Chemistry.
Enolates ambushed – asymmetric tandem conjugate addition and subsequent enolate trapping with conventional and less traditional electrophiles
Beilstein J. Org. Chem. 2023, 19, 593–634, doi:10.3762/bjoc.19.44
- alkenyl heteroarenes [61]. The aza-enolates were trapped with various Michael acceptors such as unsaturated ketones, esters, and amides (Scheme 25) [62]. The authors noted a strong substrate dependence of this process. The trapping reaction worked best with benzoxazole-derived substrate, while thiazole
One-pot synthesis of 2-arylated and 2-alkylated benzoxazoles and benzimidazoles based on triphenylbismuth dichloride-promoted desulfurization of thioamides
Beilstein J. Org. Chem. 2022, 18, 1479–1487, doi:10.3762/bjoc.18.155
- benzamidine hydrochloride G. The reaction of D with amines may require an excessive amount of D due to competition between aminophenol and the byproduct aniline. A similar mechanism is considered for the construction of benzimidazole and thiazole rings. On the other hand, the released bismuth moiety, Ph3Bi=S
- , and achieved the syntheses of 2-aryl- and 2-alkylbenzazoles, such as oxazoles, imidazoles, and thiazole, in satisfactory yields. The protocol was successfully applied to the synthesis of tafamidis, a clinically used drug for transthyretin amyloid inhibition. The reaction is simple, can be performed in
A one-pot electrochemical synthesis of 2-aminothiazoles from active methylene ketones and thioureas mediated by NH4I
Beilstein J. Org. Chem. 2022, 18, 1249–1255, doi:10.3762/bjoc.18.130
- pharmaceutical activities such as antimicrobial [2][3], antiviral [4], antitumor [5][6], anti-inflammatory [7][8] and so on. Moreover, as a type of important intermediates, thiazole is of prime importance in organic synthesis [9][10] which is used extensively in the preparation of flavors [11], polymers [12
- ], dyes [13], etc. These important features of thiazoles have driven intense interests in their facile synthesis [14][15][16][17]. Among various synthetic routes to the thiazole unit, the Hantzsch condensation of α-halo ketones (dielectrophiles) with various thioureas (dinucleophiles) should be the most
- and would lead to a large quantity of waste. Considering the importance of thiazoles in synthetic and medicinal chemistry, the development of greener and more atom economic processes for the thiazole synthesis has become increasingly necessary with growing awareness of environmental constraints
Post-synthesis from Lewis acid–base interaction: an alternative way to generate light and harvest triplet excitons
Beilstein J. Org. Chem. 2022, 18, 825–836, doi:10.3762/bjoc.18.83

- photophysical changes, have some common structural characteristics. For instance, heterocyclic units containing a nitrogen atom such as pyridine and thiazole, are one of the key structural features either in small molecules or polymers. Thus, the introduction of nitrogen with lone pairs of electrons in
- reaction. In order to clarify the coordination reaction of nitrogen atoms, Bazan’s group designed a conjugated polymer containing pyridine and thiazole groups and small molecule 15 (Figure 8) and compared the 1H NMR spectra and 19F NMR spectra after the addition of 1 equivalent B(C6F5)3 at various
- small molecule 15 containing pyridine and thiazole groups reported by Bazan et al. and pyridine groups-containing diketopyrrolopyrroles (DPP) 16–18 investigated by Huang et al. (a) 1H NMR spectra in the aromatic region and (b) 19F NMR spectra of compound 15 (top) and the mixture with 1 equivalent B(C6F5
BINOL as a chiral element in mechanically interlocked molecules
Beilstein J. Org. Chem. 2022, 18, 508–523, doi:10.3762/bjoc.18.53

- -capping. The resulting compounds were treated with chloroacetic anhydride and then with thiazole. After anion exchange the chiral thiazolium salts (R)-35a/b, which differ in the chain length of the axle, were obtained in 9%/42% overall yield (see Figure 8a). For comparison, a rotaxane containing a BINOL
- -based axle and an achiral macrocycle was also synthesized. This design was chosen to investigate the difference between a covalently and a mechanically linked chiral unit with regard to the chiral induction in asymmetric catalysis. By acylative end-capping, followed by introduction of the thiazole unit
Regioselective synthesis of methyl 5-(N-Boc-cycloaminyl)-1,2-oxazole-4-carboxylates as new amino acid-like building blocks
Beilstein J. Org. Chem. 2022, 18, 102–109, doi:10.3762/bjoc.18.11
- combining thiazole, selenazole, pyrazole, indazole, and indole moieties with both carboxyl functional groups and cycloaminyl units [27][28][29][30][31]. In recent decades, various methods of constructing 1,2-oxazole ring systems have been developed [1][2][3][4][5][6][7][32]. The two primary pathways to 1,2
- the range of 14.4–14.7 Hz, while the 3JHN values were in the range of 1–3 Hz [42][43]. The coupling constants 13C,15N of a series of 15N-labeled pyrazoles gave 1JCN values of 8–11 Hz, while 2JCN were less than 2 Hz [44]. The 15N-labeled 1,2-thiazole moiety was readily determined by measuring the
Silica gel and microwave-promoted synthesis of dihydropyrrolizines and tetrahydroindolizines from enaminones
Beilstein J. Org. Chem. 2021, 17, 2543–2552, doi:10.3762/bjoc.17.170
- , was reported as recently as 2018 [55]. More interestingly, conventional heating in acetic acid of an enaminone bearing a phenacylsulfanyl substituent adjacent to nitrogen on the C=C bond produced ethyl pyrrolo[2,1-b]thiazole-5-carboxylates in moderate yield, while the corresponding microwave-assisted
Chemical approaches to discover the full potential of peptide nucleic acids in biomedical applications
Beilstein J. Org. Chem. 2021, 17, 1641–1688, doi:10.3762/bjoc.17.116
- modified natural nucleobase that did not quench the fluorescence upon hybridization [148]. Köhler and Seitz introduced thiazole orange (TO, Figure 10), an intercalator dye originally designed for DNA [149], as a forced intercalation (FIT) probe in PNA. Because of rotation around the methine bond connecting
- thiazole and quinoline, TO fluorescence is almost completely quenched in ssPNA, but increases significantly upon hybridization to the complementary DNA [150]. The intercalation of TO in PNA–DNA duplex restricts rotation around the methine bond enforcing planarity of the two TO’s aromatic system, which
- base pairs in dsRNA [153][154]. Replacement of thiazole in TO with another quinoline gives bis-quinoline (BisQ, Figure 10), a red-shifted PNA nucleobase analogous to TO [155]. Although binding of BisQ with all four natural DNA nucleobases has not been explored in detail, BisQ-modified FIT PNAs showed
Photoinduced post-modification of graphitic carbon nitride-embedded hydrogels: synthesis of 'hydrophobic hydrogels' and pore substructuring
Beilstein J. Org. Chem. 2021, 17, 1323–1334, doi:10.3762/bjoc.17.92

- group stretching relying on buried g-CN structure in hydrogel network. The vTA photografting can be revealed via distinctive signals such as the emergent peak at 3081 cm−1, corresponding to C–H aromatic stretchings arising from the thiazole ring, the neck at 1687 cm−1 signifies the C=N vibration band
- , and at last the intensified peak at 1419 cm−1 indicates a C–N stretching of the thiazole ring. Proceeding the examination by UV–vis spectroscopy, overlaid absorption spectra of the samples revealed the photophysical differences as expected, since HG does not consist of photoactive g-CN particles in
Microwave-assisted multicomponent reactions in heterocyclic chemistry and mechanistic aspects
Beilstein J. Org. Chem. 2021, 17, 819–865, doi:10.3762/bjoc.17.71

Synthesis of (Z)-3-[amino(phenyl)methylidene]-1,3-dihydro-2H-indol-2-ones using an Eschenmoser coupling reaction
Beilstein J. Org. Chem. 2021, 17, 527–539, doi:10.3762/bjoc.17.47
- ) or leads to a complex and inseparable mixture of products (when starting from 2a,b). The addition of a base (e.g., triethylamine, N-methylmorpholine, ammonia) which was originally found to be beneficial [33] for the rearrangement of the kinetically formed thiazole to the desired product now caused a
- desired Eschenmoser coupling reaction (route b). Therefore, the nucleophilicity of the conjugated base of the nitrogen (benzenecarbimidothioate or thioimidate) is exerted towards the oxindole carbonyl to give the thiazole. Moreover, if both benzene rings contain electron-withdrawing groups, enhancing
- either the leaving ability of the oxindole nitrogen (R1: Cl, NO2) or the acidity of the >C=NH2+ group (R2: Cl, CF3) increasing the proportion of the nucleophile >C=NH, then the formation of the thiazole (route a) is further accelerated (Scheme 2). Although the addition of a base generates the required C3
Regioselective synthesis of heterocyclic N-sulfonyl amidines from heteroaromatic thioamides and sulfonyl azides
Beilstein J. Org. Chem. 2020, 16, 2937–2947, doi:10.3762/bjoc.16.243
- , Department of Chemistry, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium 10.3762/bjoc.16.243 Abstract N-Sulfonyl amidines bearing 1,2,3-triazole, isoxazole, thiazole and pyridine substituents were successfully prepared for the first time by reactions of primary, secondary and tertiary heterocyclic
Synthesis and investigation of quadruplex-DNA-binding, 9-O-substituted berberine derivatives
Beilstein J. Org. Chem. 2020, 16, 2795–2806, doi:10.3762/bjoc.16.230
- a lower affinity [38]. Along the same line, the influence of the length and substituents of the side chains at the G4-DNA ligands have been assessed for quinolinium [43], indoloquinoline [44][45], phenanthroline [46], phenothiazine [47], and thiazole orange [48] derivatives. In these studies, the
Access to highly substituted oxazoles by the reaction of α-azidochalcone with potassium thiocyanate
Beilstein J. Org. Chem. 2020, 16, 2108–2118, doi:10.3762/bjoc.16.178
- ; potassium persulfate; thiazole; vinyl azide; Introduction Vinyl azide is one of the most versatile and potent building blocks explored in the synthesis of several heterocycles [1][2][3][4][5]. It can undergo photolysis or thermolysis to afford highly strained three-membered 2H-azirine, which can act as the
- -azirine, an efficient source of nitrogen, was employed as starting material for the synthesis of oxazole with various coupling partners such as aldehyde, trifluoroacetic acid, etc. [8][45][46][47][48][49][50] (Scheme 1). Thiazole is a common structural motif that is found in a wide variety of naturally
- existing alkaloids and a number of pharmaceutically active compounds [51][52][53]. 2-Aminothiazole has a thiourea-like character with a tendency to modulate promiscuously multiple biological targets. Thiazole derivatives also exhibit a broad spectrum of biological activities including antiviral, antiprion
Synthesis of 3(2)-phosphonylated thiazolo[3,2-a]oxopyrimidines
Beilstein J. Org. Chem. 2020, 16, 1947–1954, doi:10.3762/bjoc.16.161
- Thiazolopyrimidines, whose molecules includes both thiazole and pyrimidine rings, have a structural analogy with the antipsychotic drugs ritanserin and setoperone (Figure 1) [1][2][3]. To date, a wide spectrum of biological activity of thiazolopyrimidines has been determined: anticancer [4][5], antimicrobial [6][7
- the latter [22][23][24][25][26][27][28]. Herein, we report the synthesis of a new series of phosphonylated thiazolopyrimidines. In our studies, chloroethynylphosphonate was used as the phosphonylating agent, which allowed the formation of a thiazole ring with simultaneous phosphonylation of the latter
- the 1H NMR spectra, the proton of the thiazole ring of both isomers is represented by a doublet signal in the low field region at δH 7.91 (3JHP = 3.2 Hz) and 8.41 ppm (3JHP = 7.5 Hz), whereas the corresponding proton of the uracil ring resonated as a singlet at δH 6.61 and 6.71 ppm, respectively. Note
Copper-based fluorinated reagents for the synthesis of CF2R-containing molecules (R ≠ F)
Beilstein J. Org. Chem. 2020, 16, 1051–1065, doi:10.3762/bjoc.16.92

- oxazoles (17 examples, up to 87% yield) were difluoromethylated but a variety of other heteroarenes turned out to be suitable such as pyridine, imidazole, benzo[d]thiazole, benzo[b]thiophene, benzo[d]oxazole, thiazole and thiophene derivatives (Scheme 6). Copper-based CF2FG-containing reagents Besides the
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
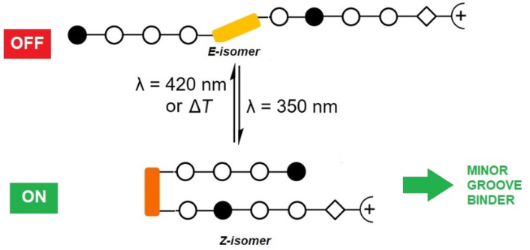
- ligand to examine the propensity of these derivatives to bind to DNA. The known intercalator thiazole orange (TO) was chosen as indicator because it exhibits an intense fluorescence band at 526 nm when bound to DNA, whereas it is only weakly fluorescent in solution. The displacement of TO from its DNA
Thermal stability of N-heterocycle-stabilized iodanes – a systematic investigation
Beilstein J. Org. Chem. 2019, 15, 2311–2318, doi:10.3762/bjoc.15.223

- Supporting Information File 1) were observed at remarkable high Tpeak values (193.9–210.1 °C). Following these results, it has been intriguing to analyze the influence of the heteroatom in the heterocyclic moiety on the thermal decomposition process. The change of one nitrogen atom to sulfur as in thiazole
- model reaction, especially N-substituted triazoles 4 and 5. In contrast, the reactivity of triazole 3 is comparable to that of benzoxazoles 13 and 14 as well as pyrazole 7 and indazole 8. In our view thiazole 12 is the best compromise in this regard since it is thermally even more stable than 7 and 8
Click chemistry towards thermally reversible photochromic 4,5-bisthiazolyl-1,2,3-triazoles
Beilstein J. Org. Chem. 2019, 15, 2161–2169, doi:10.3762/bjoc.15.213

- (thiazole) [39], and 10c (imidazole) [40] in non-polar solvents, 1c has a much longer absorption maximum in toluene (Scheme 4, Table 2). It should also be noted that the absorption maximum wavelength is longer when the central ethene moiety is part of the aromatic ring than when it is an isolated ethene in
- AcOEt than in toluene, and the thermal back reaction rate in AcOEt was increased. Conclusion We have synthesized three novel thermally reversible 4,4'-(1-benzyl-1H-1,2,3-triazole-4,5-diyl)bis(5-methyl-2-(4-substituted-phenyl)thiazole)s 1o–3o by Ru(I)-catalysed Huisgen cyclization, which is a type of
Identification of optimal fluorescent probes for G-quadruplex nucleic acids through systematic exploration of mono- and distyryl dye libraries
Beilstein J. Org. Chem. 2019, 15, 1872–1889, doi:10.3762/bjoc.15.183

- , the widely used fluorescent probes, such as thioflavin T (ThT, Φ = 0.25, in the presence of 22AG/K+ conditions) and thiazole orange (TO, Φ = 0.19 in the presence of 22AG/K+ conditions) [92], and approach the brightest G4-DNA probes developed so far, such as trialryimidazole IZCM-7 (Φ = 0.52, in the
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

Synthesis of ([1,2,4]triazolo[4,3-a]pyridin-3-ylmethyl)phosphonates and their benzo derivatives via 5-exo-dig cyclization
Beilstein J. Org. Chem. 2019, 15, 1563–1568, doi:10.3762/bjoc.15.159
- ]thiadiazole [2], and benzo[4,5]imidazo[2,1-b]thiazole [3], as well as due to the simultaneous presence of a biologically active phosphorus function in the molecules. Recently, we have shown that the reaction of chloroethynylphosphonates with 2-aminopyridines proceeds through a 5-endo-dig cyclization to form
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- , but with the tetrahydropyrane ring having α orientation. A mixture of epimers at position 4' was also obtained when using the C-5 epimer of substrate 33. Asif et al. [36] developed another 3CR for the synthesis of steroidal thiazole derivatives. As shown in Scheme 11, cholestanic ketone 7 was reacted
- with a thiosemicarbazide and 2-bromo-1-phenylethan-1-one under microwave irradiation to form the steroid–thiazole hybrid 35 in very good yield. As previously mentioned, due to the poor reactivity of steroidal ketones and their imine derivatives, most MCRs with ketosteroids described in the literature
- with an alkynyl seco-cholestane [34]. Multicomponent synthesis of steroid–thiazole hybrids from a steroidal ketone [36]. Synthesis of cholanic pseudo-peptide derivatives by novel MCRs based on the reactivity of ynamide [37][38]. Synthesis of steroid-fused pyrimidines and pyrimidones using the Biginelli
Microwave-assisted synthesis of N,N-bis(phosphinoylmethyl)amines and N,N,N-tris(phosphinoylmethyl)amines bearing different substituents on the phosphorus atoms
Beilstein J. Org. Chem. 2019, 15, 469–473, doi:10.3762/bjoc.15.40
- elaborated by us for the synthesis of several (aminomethyl)phosphine oxides [10][11]. As regards α-aminophosphine oxides with different P-substituents, only two different types were reported. Olszewski and co-workers synthesized chiral thiazole-substituted aminophosphine oxides 2 through the Pudovik reaction
- of alkylphenylphosphine oxides and the corresponding aldimine derivatives of thiazole 1 (Scheme 1) [12]. Cherkasov and his group applied the Kabachnik–Fields reaction to synthesize a P-chiral aminophosphine oxide with a 2-pyridyl substituent 3 (Scheme 2) [13]. Bis(aminophosphine oxide) derivatives
- isolated in high yields and fully characterized. Synthesis of chiral thiazole-substituted aminophosphine oxides. Synthesis of a P-chiral aminophosphine oxide containing a 2-pyridyl moiety. Condensation of (octylaminomethyl)dihexylphosphine oxide with paraformaldehyde and di(p-tolyl)phosphine oxide
Annulation of 1H-pyrrole-2,3-diones by thioacetamide: an approach to 5-azaisatins
Beilstein J. Org. Chem. 2019, 15, 364–370, doi:10.3762/bjoc.15.32
- corresponding 1H-pyrrolo[3,2-c]pyridine-2,3-dione 4l. The most possible explanation of this fact is the steric hindrance introduced by the N-phenyl substituent, which obstructs the thiazole ring opening. Conclusion In summary, we have developed a novel approach to 1H-pyrrolo[3,2-c]pyridine-2,3-diones (5























































































































































































































































































































































































































































































































































































































































