Search results
Search for "cross coupling reaction" in Full Text gives 196 result(s) in Beilstein Journal of Organic Chemistry.
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- intramolecular Heck cross-coupling reaction of the octahydroindoles 9/10 to the pentacycles 11, the process was explored with the simple substrates 8c,d and 8g (Scheme 4). Thus, the Pd(0)-catalyzed cyclization of the latter vinylpyrroles with potassium acetate and using acetonitrile or dimethylacetamide (DMA) as
- the solvent at 100 °C afforded pyrrolo[2,1-a]isoindoles 18a–c in good yields. This annulation process was regioselective, showing a preference of the cross-coupling reaction with the C-5 pyrrolic position and not with the vinyl moiety, which would give the dihydropyrrolo[1,2-b]isoquinoline 19. A
- yields. Once having the optimal reaction conditions for the palladium(0)-catalyzed cross-coupling reaction leading to the cyclization of N-(2-bromobenzyl)pyrroles 8, the endo-octahydropyrrolo[3,4-e]indole-1,3-diones 9g–j and 9m were converted into pentacycles 11a–e through the same methodology (Table 3
Copper-based fluorinated reagents for the synthesis of CF2R-containing molecules (R ≠ F)
Beilstein J. Org. Chem. 2020, 16, 1051–1065, doi:10.3762/bjoc.16.92

- 87% yield) was investigated (Scheme 8) [52]. Finally, the Poisson’s group developed a methodology for the Ullman cross-coupling reaction between the in situ-generated CuCF2PO(OEt)2 and aryl iodides containing a coordinating group (e.g., CO2CH3, COCH3, NO2), at the ortho-position of the halide [52
- (0)-mediated reductive cross-coupling reaction between the iodobenzene and various 2-bromo-1,1,2,2-tetrafluoroethyl derivatives (RCF2CF2Br) was developed presumably involving a RCF2CF2Cu species (Scheme 21) [76]. In 2015, Yagupolskii and co-workers investigated the synthesis of perfluoroalkylcopper
Fluorinated phenylalanines: synthesis and pharmaceutical applications
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- )-iodoalanine 2. The reaction was activated using Pd(0) as a catalyst. A palladium-catalyzed cross-coupling reaction between an organozinc iodide and aryl halides offers a convenient method for the direct preparation of protected fluorinated Phe analogues 3. Thus, cross coupling of the protected iodoalanine 2
Towards triptycene functionalization and triptycene-linked porphyrin arrays
Beilstein J. Org. Chem. 2020, 16, 763–777, doi:10.3762/bjoc.16.70

- material was purified via column chromatography using silica gel. The BODIPY dimer 7 was prepared using 6 [38] and was obtained as a fluorescent orange solution or as pink-orange crystals in a 30% yield. Similarly, the Pd-catalyzed Sonogashira cross-coupling reaction [39] was successful for the reaction
- confirm full conversion. For this reason, it may be that the ethynyl bond was not completely deprotected before the next coupling reaction. After the TBAF deprotection step, the second Sonogashira cross-coupling reaction was carried out with the nickel porphyrin 15 [41] under the same reaction conditions
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
Beilstein J. Org. Chem. 2020, 16, 738–755, doi:10.3762/bjoc.16.68
- synthesis of the (−)-exiguolide enantiomer (25) was reported by Roulland et al. [94]. The method is a mechanistically distinct alternative to the enyne metathesis since it involves a Trost’s Ru-catalyzed enyne cross-coupling reaction associated with a Yamaguchi lactonization, a Grubbs Ru-catalyzed cross
Towards the total synthesis of chondrochloren A: synthesis of the (Z)-enamide fragment
Beilstein J. Org. Chem. 2020, 16, 670–673, doi:10.3762/bjoc.16.64
- best results for the cross-coupling reaction. Conclusion In summary, we established a (Z)-selective Buchwald-type coupling reaction as a key step in the synthesis of an advanced fragment of chondrochloren A (1). The required amide 3 can be synthesized in seven steps with a 16% overall yield [16][17][18
Direct borylation of terrylene and quaterrylene
Beilstein J. Org. Chem. 2020, 16, 621–627, doi:10.3762/bjoc.16.58
- hinderance of Bpin moieties. Finally, to demonstrate the utility of the borylated oligorylenes, the Suzuki–Miyaura cross-coupling reaction of TB4 under the standard conditions was performed (Scheme 3). Coupling of TB4 and 2-bromomesitylene with Pd(PPh3)4, Cs2CO3 and CsF in a mixture of toluene/DMF furnished
- an Ir-catalyzed borylation reaction. Our results provide a highly potential route to various functional oligorylene derivatives. In fact, TB4 could be transformed into the tetraarylterrylene TM4 in good yield by the conventional cross-coupling reaction. TB4 exhibits face-to-face interaction in the
- -dioxaborolan-2-yl. Synthesis of 2,5,12,15-tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quaterrylene (QB4): (a) (Bpin)2 (12 equiv), [Ir(OMe)cod]2 (20 mol %), di-tert-butylbipyridyl (40 mol %), 1,4-dioxane, at 105 °C, 38 h, yield 0.4%. Suzuki–Miyaura cross-coupling reaction of TB4 with 2-bromomesitylene
Controlling alkyne reactivity by means of a copper-catalyzed radical reaction system for the synthesis of functionalized quaternary carbons
Beilstein J. Org. Chem. 2020, 16, 502–508, doi:10.3762/bjoc.16.45
- to produce intermediate C, with concomitant formation of a Cu(I) species. The brominated intermediate C undergoes a cross-coupling reaction with the alkynyl copper species to give the desired product 3. We have detected brominated intermediate C during the reaction. We have also examined the reaction
Synthesis of six-membered silacycles by borane-catalyzed double sila-Friedel–Crafts reaction
Beilstein J. Org. Chem. 2020, 16, 409–414, doi:10.3762/bjoc.16.39
- , the transformation of the amino groups in phenoxasilin 3a into phenyl groups was carried out (Scheme 5). First, the ammonium salt 4 was prepared by treating 3a with MeOTf followed by a palladium-catalyzed cross-coupling reaction with the Grignard reagent (PhMgBr) that afforded the desired diphenylated
Synthesis of 4-(2-fluorophenyl)-7-methoxycoumarin: experimental and computational evidence for intramolecular and intermolecular C–F···H–C bonds
Beilstein J. Org. Chem. 2020, 16, 190–199, doi:10.3762/bjoc.16.22
- cross-coupling reaction [17], Negishi cross-coupling reaction [18] and Wittig reaction [17]. The concept of the incorporation of fluorine into organic molecules has gained much interest since Fried and Sabo reported the improvement of the therapeutic index of cortisol by the incorporation of a fluorine
Allylic cross-coupling using aromatic aldehydes as α-alkoxyalkyl anions
Beilstein J. Org. Chem. 2020, 16, 185–189, doi:10.3762/bjoc.16.21

- homoallylic alcohol derivatives [12][13][14]. Results and Discussion Specifically, the three-component allylic cross-coupling reaction of benzaldehyde (1a, 0.4 mmol), tert-butyl cinnamyl carbonate (2a, 0.2 mmol) and (dimethylphenylsilyl)boronic acid pinacol ester [PhMe2SiB(pin)] (0.4 mmol) occurred in the
- )CuCl (53%) in terms of the chemical yield. Notably, the allylic cross-coupling reaction did not occur at all without Pd(OCOCF3)2–DPPF or (SIPr)CuCl, and thus the palladium and copper catalysts cooperatively acted in the allylic cross-coupling. Scheme 3 shows the substrate range of aromatic aldehydes 1
An improved, scalable synthesis of Notum inhibitor LP-922056 using 1-chloro-1,2-benziodoxol-3-one as a superior electrophilic chlorinating agent
Beilstein J. Org. Chem. 2019, 15, 2790–2797, doi:10.3762/bjoc.15.271
- biology of Notum and build target validation to underpin new drug discovery programs. Results: An improved, scalable synthesis of 1 is reported. Key modifications include: (1) the introduction of the C7-cyclopropyl group was most effectively achieved with a Suzuki–Miyaura cross-coupling reaction with MIDA
- group was most effectively achieved with a Suzuki–Miyaura cross-coupling reaction with MIDA-boronate 11 (5 → 6); and (2) C6 chlorination was performed with 1-chloro-1,2-benziodoxol-3-one (12) (6 → 7) as a mild selective electrophilic chlorination agent. 4-Chlorothieno[3,2-d]pyrimidine (3) was either
- modifications include: (1) the introduction of the C7-cyclopropyl group was most effectively achieved with a Suzuki–Miyaura cross-coupling reaction with MIDA-boronate 11 (5 → 6); and (2) C6 chlorination was performed with 1-chloro-1,2-benziodoxol-3-one (12) (6 → 7) as a mild selective electrophilic chlorination
A chiral self-sorting photoresponsive coordination cage based on overcrowded alkenes
Beilstein J. Org. Chem. 2019, 15, 2767–2773, doi:10.3762/bjoc.15.268

- formed revealing that a chiral self-sorting process takes place. In addition, two of the cage isomers can bind a tosylate anion in solution by formation of a host–guest complex. Results and Discussion Ligands Z-1 and E-1 (Scheme 1) were synthesized by a Suzuki cross-coupling reaction of 3
A new approach to silicon rhodamines by Suzuki–Miyaura coupling – scope and limitations
Beilstein J. Org. Chem. 2019, 15, 2569–2576, doi:10.3762/bjoc.15.250

- catalyst, different substituted boroxines were assessed to explore the scope of the Pd-catalyzed cross-coupling reaction. Conclusions: A number of silicon rhodamines were synthesized under the optimized conditions in up to 91% yield without the necessity of HPLC purification. Moreover, silicon rhodamines
- atom of 9b or sterical reasons (the chlorine in 2’-position might lead to repulsion during the cross-coupling reaction). The reaction of the triflate with cyano-substituted phenylboroxines 10b and 11b led to silicon rhodamine dyes 14 and 15 in poor yields of 23 and 19%, respectively. The reaction
- carbonate resulted in no reaction at all (Table 1, entry 5). Whereby usage of potassium phenyltrifluoroborate (19) resulted in a yield comparable to boroxine 18b (Table 1, entry 6), usage of pinacol ester 20 showed no reaction in the cross-coupling reaction (Table 1, entry 7). Although described
Self-assembled coordination thioether silver(I) macrocyclic complexes for homogeneous catalysis
Beilstein J. Org. Chem. 2019, 15, 2465–2472, doi:10.3762/bjoc.15.239
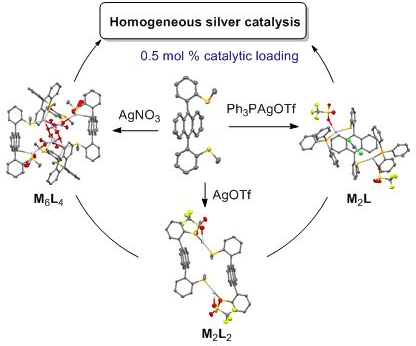
- reactions of alkynes. Results and Discussion Synthesis of silver(I) complexes Ligand 1 was synthesized in one step, from commercially available 9,10-dibromoanthracene and 2-(methylthio)phenylboronic acid, using a Suzuki–Miyaura cross-coupling reaction. Notably, the yield was low (26%) [55], and the X-ray
Functionalization of 4-bromobenzo[c][2,7]naphthyridine via regioselective direct ring metalation. A novel approach to analogues of pyridoacridine alkaloids
Beilstein J. Org. Chem. 2019, 15, 2304–2310, doi:10.3762/bjoc.15.222
- ]. Reaction of allyl iodide (17) with metalated 9d after addition of catalytic amounts of CuCN∙2LiCl led to the formation of the 5-allyl compound 18 in 37% yield. Metalation of 9d using TMPMgCl∙LiCl and subsequent transmetalation with ZnCl2 followed by Negishi cross-coupling reaction in the presence of Pd(dba
Norbornadiene-functionalized triazatriangulenium and trioxatriangulenium platforms
Beilstein J. Org. Chem. 2019, 15, 1815–1821, doi:10.3762/bjoc.15.175

- decomposition, which is a necessary precondition for ultra-high vacuum STM investigations. The 3-bromo-2-cyano-substituted norbornadiene 4 was synthesized as described in the literature (Scheme 1) [10][11][12]. 4 was converted to 5 with trimethylsilylacetylene (72%) in a Sonogashira cross-coupling reaction. The
- obtained in a convergent synthesis (Scheme 2). Boronic ester 9 was synthesized as described in the literature [15]. In a Suzuki cross-coupling reaction norbornadiene 4 was coupled with 9 to the extended norbornadiene 10 (38%), which was attached to the TATA platform 6 to yield the extended norbornadiene
Recent advances on the transition-metal-catalyzed synthesis of imidazopyridines: an updated coverage
Beilstein J. Org. Chem. 2019, 15, 1612–1704, doi:10.3762/bjoc.15.165

Synthesis of 9-O-arylated berberines via copper-catalyzed CAr–O coupling reactions
Beilstein J. Org. Chem. 2019, 15, 1575–1580, doi:10.3762/bjoc.15.161
- leading to the failure of direct BBRB cross coupling. 9-O-Aryl berberine scope via cross-coupling reaction. 9-O-Ph-linked berberine dimer through double cross-coupling reaction. Optimization of the reaction conditions.a Supporting Information Supporting Information File 138: Experimental details and
Diazocine-functionalized TATA platforms
Beilstein J. Org. Chem. 2019, 15, 1485–1490, doi:10.3762/bjoc.15.150

- a function of electronic coupling (conjugation) of the azo unit to gold. The para-diazocine-TATA 1 was synthesized in a 5-step synthesis route (Scheme 1). Bromotoluene 3 was synthesized as described [26]. In a Sonogashira cross-coupling reaction acetylene-substituted toluene 5 was prepared from
Selenophene-containing heterotriacenes by a C–Se coupling/cyclization reaction
Beilstein J. Org. Chem. 2019, 15, 1379–1393, doi:10.3762/bjoc.15.138
- starting material, but also ring fusion to selenophene was achieved by Cu-catalyzed C–Se cross-coupling reaction [28]. The detailed geometric structure and the packing behaviour in the solid state of triacenes 2–4 have been elucidated by single crystal X-ray structure analysis and X-ray diffraction on
- reinvestigated the synthesis of DTT 1 by using a Cu-catalyzed C–S cross-coupling reaction with potassium sulfide (K2S) as sulfur source [29]. The best results for this C–S ring-closure reaction were achieved by reacting 3,3’-diiodo-2,2’-bithiophene (5) [30] with the system K2S and copper iodide (CuI) as catalyst
- deprotection with TBAF in 66% yield, with selenourea and copper oxide nanoparticles surprisingly did not lead to any targeted DSS 4 in the attempted C–Se cross-coupling reaction. The structures of the prepared novel selenolotriacenes 2–4 and known DTT 1 were characterized by means of NMR spectroscopy
Insertion of [1.1.1]propellane into aromatic disulfides
Beilstein J. Org. Chem. 2019, 15, 1172–1180, doi:10.3762/bjoc.15.114
- opening of 1 with Grignard reagents enables the synthesis of aryl and some alkyl-substituted BCPs and a subsequent cross-coupling reaction [5][18]. To provide bicyclo[1.1.1]pentylamine as a building block in large-scale syntheses, Bunker et al. developed a synthesis of hydrazine BCP via a manganese
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- cross-coupling reaction [15][16][17][18][19] or via a Pd-catalyzed C–H activation reaction [20][21]. We have used the former reaction for the solid-phase preparation of biaryl cyclic peptides bearing a Phe-Phe, a Phe-Tyr or a Tyr-Tyr linkage [22][23]. Our approach involved the synthesis of the linear
Synthesis of C3-symmetric star-shaped molecules containing α-amino acids and dipeptides via Negishi coupling as a key step
Beilstein J. Org. Chem. 2019, 15, 371–377, doi:10.3762/bjoc.15.33
- containing unusual AAA units through cyclotrimerization and Negishi cross-coupling reaction as key steps under operationally simple reaction conditions. Here, we have used the readily available starting materials 4-iodoacetophenone (8) and L-serine (3). The C3-symmetric building blocks prepared were coupled
- , 2928, 1606, 1596, 1434, 783, 505 cm−1. Exemplar C3-symmetric peptide scaffolds reported in the literature. Preparation of compound 7 from L-serine (3). Preparation of the trimerized product 9. Synthesis of compound 11 via Negishi cross-coupling reaction. Synthesis of C3-symmetric trimers 12, 13 and 14
A novel and efficient synthesis of phenanthrene derivatives via palladium/norbornadiene-catalyzed domino one-pot reaction
Beilstein J. Org. Chem. 2019, 15, 291–298, doi:10.3762/bjoc.15.26
- and natural products. Results and Discussion We initiated our investigations by evaluating the three-component cross-coupling reaction of 2-iodotoluene (1a), ortho-bromobenzoyl chloride (2a) and norbornadiene, and we optimized the reaction conditions [21]. Firstly, the reaction took place under Pd





























































































































































































































































































































































































































































































